Diagnosis of severe hepatitis C recurrence is based on analytical and histological criteria but there is little information about their correlation.
AimTo assess the accuracy of laboratory criteria for the diagnosis of fibrosing cholestatic hepatitis (FCH).
Patients and methodsRetrospective analysis of prospectively collected data form HCV positive patients who underwent liver transplantation (LT) between 2000 and 2014 in two European university hospitals. Patients were classified according to laboratory criteria such as FCH, cholestatic hepatitis (CH) and non-cholestatic acute hepatitis (NCAH). Histological characteristics were also evaluated.
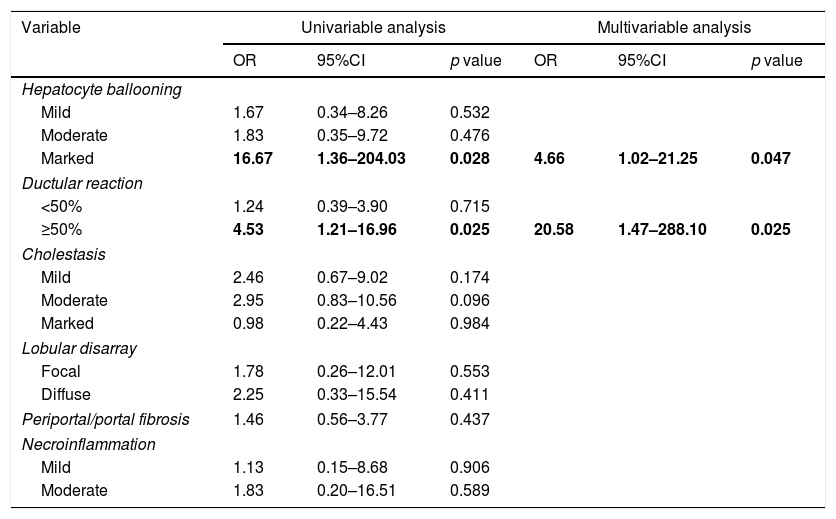
ResultsSeventy patients with acute HCV recurrence within the first year after LT with an available liver biopsy were included in the study. Most patients were male (70%) with a median age of 58 years (50–64) and infected with genotype 1b (71.4%). Median time from LT to diagnosis of recurrence was 2.96 months (2.1–5.3). Thirty-nine patients were classified as FCH, 21 as CH and 10 as NCAH. Marked hepatocyte ballooning and ductular reaction were associated with the presence of FCH with an OR of 4.66 (p=0.047) and 20.58 (p=0.025), respectively. Considering liver biopsy as the gold standard, the sensitivity, specificity, positive and negative predictive values of the analytical criteria were 0.8, 0.5, 0.3 and 0.9, respectively. However, correlation between histological and analytical criteria was poor (k=0.033).
DiscussionAnalytical criteria may be used to rule out the presence of FCH, but a biopsy is mandatory to confirm the diagnosis. Ductular reaction and hepatocyte ballooning were independent predictors of FCH.
El diagnóstico de la recurrencia grave de la hepatitis C se basa en criterios histológicos y analíticos. Sin embargo, existe poca información respecto su correlación.
ObjetivoEvaluar la precisión de los criterios analíticos el diagnóstico de la hepatitis colestásica fibrosante (HCF).
Pacientes y métodosAnálisis retrospectivo de pacientes con una recidiva grave precoz del virus de la hepatitis C (VHC) tras el trasplante hepático (TH) en 2 hospitales universitarios europeos entre 2000-2014. Los pacientes se clasificaron según criterios analíticos en HCF, hepatitis colestásica (HC) y hepatitis aguda no colestásica (HANC). Las características histológicas también fueron evaluadas.
ResultadosSe incluyeron 70 pacientes que desarrollaron una recurrencia grave del VHC en el primer año tras TH con una biopsia hepática disponible. La mayoría eran varones (70%) con mediana de edad de 58 años (50-64) y genotipo 1b (71,4%). La mediana de tiempo desde el TH hasta el diagnóstico de la recurrencia fue de 2,96 meses (2,1-5,3). Treinta y nueve pacientes fueron clasificados como HCF, 21 como HC y 10 como HANC. La balonización intensa y reacción ductular se asociaron con HCF con una OR de 4,66 (p=0,047) y 20,58 (p=0,025), respectivamente. Considerando la biopsia hepática como gold standard, la sensibilidad, especificidad y valores predictivos positivo y negativo de los criterios analíticos fueron 0,8, 0,5, 0,3 y 0,9, respectivamente. Sin embargo, la correlación entre ambos fue escasa (k=0,033).
DiscusiónLos criterios analíticos podrían utilizarse para descartar la presencia de HCF, pero la biopsia sigue siendo obligatoria para el diagnóstico. La reacción ductular y la balonización son predictores de HCF.
The use of direct acting antivirals (DAAs) for the treatment of hepatitis C virus infection (HCV) has revolutionized the landscape of liver transplantation (LT). However, in our region HCV infection still ranks as the second indication for LT after alcoholic cirrhosis.1,2 Hepatitis C recurrence is universal when patients undergo LT with detectable viremia.3 Among the spectrum of hepatitis C recurrence, fibrosing cholestatic hepatitis (FCH) is the most aggressive form, observed in 5–10% of HCV positive LT recipients. It was first described in 1991 in hepatitis B positive patients undergoing LT4,5 but it has been also reported in other immunosuppressive states, including kidney or hematopoietic-cell transplantation.6–9
Fibrosing cholestatic hepatitis is characterized by an early onset (within the first year after LT) and a poor prognosis due to a rapidly progressive allograft dysfunction. Clinically, FCH manifests with the presence of jaundice, coagulopathy and encephalopathy. Laboratory tests usually show cholestasis (increase in alkaline phosphatase [AP], γ-glutamyl transpeptidase [GGT], and bilirubin levels) associated with high HCV viral load. Histopathology a plays a major role in the diagnosis of the disease,10 and 3 of the following criteria are required for the diagnosis of cholestatic hepatitis: (1) prominent ductular reaction in the absence of biliary obstruction, (2) cholestasis, (3) prominent hepatocyte ballooning with lobular disarray, (4) any degree of periportal/perisinusoidal fibrosis.11 Despite the widespread use of DAAs has diminished the relevance of the problem, there are still some patients (severe decompensated cirrhosis, renal failure, comorbidities) in whom antiviral therapy is deferred after LT and therefore at risk of developing severe hepatitis C recurrence.12–14 Therefore, it is still mandatory to rapidly diagnose severe forms of the disease in order to preserve liver function by avoiding the delay in the initiation of antiviral therapy.
The diagnosis of FCH still relies in the liver biopsy but the commonly used criteria have several pitfalls because of the wide range of differential diagnoses, including T-cell mediated reaction, hepatotoxicity or biliary obstruction, that sometimes delay the diagnosis of FCH.15–18 To overcome this issue, it would be interesting to early identify patients with severe hepatitis C recurrence early and based on laboratory data. Therefore, the aim of the current study was to evaluate the accuracy of laboratory criteria for the diagnosis of early severe hepatitis C recurrence, and its correlation with histological criteria.
Patients and methodsPatientsThis is a retrospective analysis of prospectively collected data from all HCV positive patients who underwent LT between 2000 and 2014 in two European university hospitals (Hospital Clínic Barcelona and Padua University Hospital). The inclusion criteria of the study were: (1) 18 years old and (2) signs of early severe hepatitis C recurrence defined by an increase in AST and/or ALT of at least two times the upper normal limit (UNL) within the first year after LT in the absence of other causes of acute hepatitis. The exclusion criteria were: (1) HIV coinfection, (2) histological signs of chronic hepatitis, T-cell mediated rejection, plasma cell hepatitis, and/or chronic rejection, and (3) biliary or vascular complications.
Patients were classified into three groups, according to the type of hepatitis C recurrence using laboratory data (within 3 months of liver biopsy) in addition to the increase in AST and/or ALT levels>2 UNL: (1) fibrosing cholestatic hepatitis defined following the ILTS definition: bilirubin>6mg/dL, AP and/or GGT>5 times the upper normal limit (UNL) and high HCV viral load (HCV-RNA≥107IU/mL), (2) cholestatic hepatitis (CH) defined by AP and/or GGT>5 times the upper normal limit, not fulfilling all the other previous criteria, and (3) non-cholestatic acute hepatitis (NCAH) if the patient had an increase in AST and/or ALT>2UNL but was not included in the previous groups in the absence of other causes of liver dysfunction.10,19,20
VariablesDemographic data such as gender, age, time from the LT to hepatitis C recurrence, cytomegalovirus (CMV) infection, T-cell mediate rejection, immunosuppressive therapy, and laboratory data (transaminases levels, AP, GGT, bilirubin, INR, HCV viral load) were collected. All patients had had a liver biopsy (percutaneous or transjugular, depending on the medical criteria at the time).
Histological analysisLiver specimens were formalin fixed and paraffin embedded. Three-micron slides stained with haematoxylin and eosin, Masson trichrome and Perls stains were retrieved from the pathology files. A pathologist from each centre, blinded to the analytical classification of the patients, evaluated local biopsies.
The following data were recorded and graded semi-quantitatively as follows: (1) degree of hepatocellular damage/ballooning [0, absent; 1, mild (<5% of hepatocytes); 2, moderate (6–50%); 3, marked (>50%)]; (2) degree of lobular disarray (0, absent; 1, focal; 2, diffuse); (3) presence of ductular reaction (0, absent; 1, focal and affecting less than 50% of triads; 2, affecting the whole circumference of portal tracts or more than 50% of triads); (4) degree of necroinflammatory activity [0, absent; 1, mild (<2 foci per 10×); 2, moderate (2–5 foci per 10×); 3, marked (>5 foci per 10×)]; (5) localization of necroinflammatory activity (1, lobular; 2, portal/periportal; 3, mixed); (6) degree of periportal/pericellular fibrosis (0, no fibrosis; 1, focal expansion of collagen deposition from the portal tract towards the surrounding sinusoids, affecting less than 50% of triads; 2, expansion of focal collagen deposition from the portal tract towards the surrounding sinusoids, affecting more than 50% of triads; 3, expansive and generalized sinusoidal collagen deposition involving the sinusoids around the entire portal tract; (7) fibrosis stage according to METAVIR and (8) presence of cholestasis (0, absent; 1, mild; 2, moderate; 3, marked).
For the purpose of the histological analysis and definition of FCH, we used the criteria proposed by Verna et al.11: (1) prominent ductular reaction, (2) cholestasis defined as cannalicular bile plugs and/or intracellular bile pigment, (3) hepatocyte ballooning, and (4) periportal sinusoidal/pericellular fibrosis. The presence of 3 out of the 4 criteria defined FCH. The number of histological criteria (Hepatitis Aggressiveness Score) as defined by Moreira et al.21 of FCH was also analyzed.
Statistical analysisCategorical variables are expressed as number and percentage. Continuous variables were expressed as median (interquartile range, IQR). Differences in proportions were compared by χ2 test or Fischer test, as appropriate. Continuous variables were compared by Kruskal–Wallis non-parametrical testing. A multivariable analysis using a logistic regression model was performed to evaluate the association between histological characteristics and the presence of FCH. Results are expressed as odds ratio (OR) and 95% confidence interval (95%CI). A p value <0.05 was considered statistically significant. SPSS software version 20 (SPSS, Chicago, IL, USA) was used to perform statistical analysis.
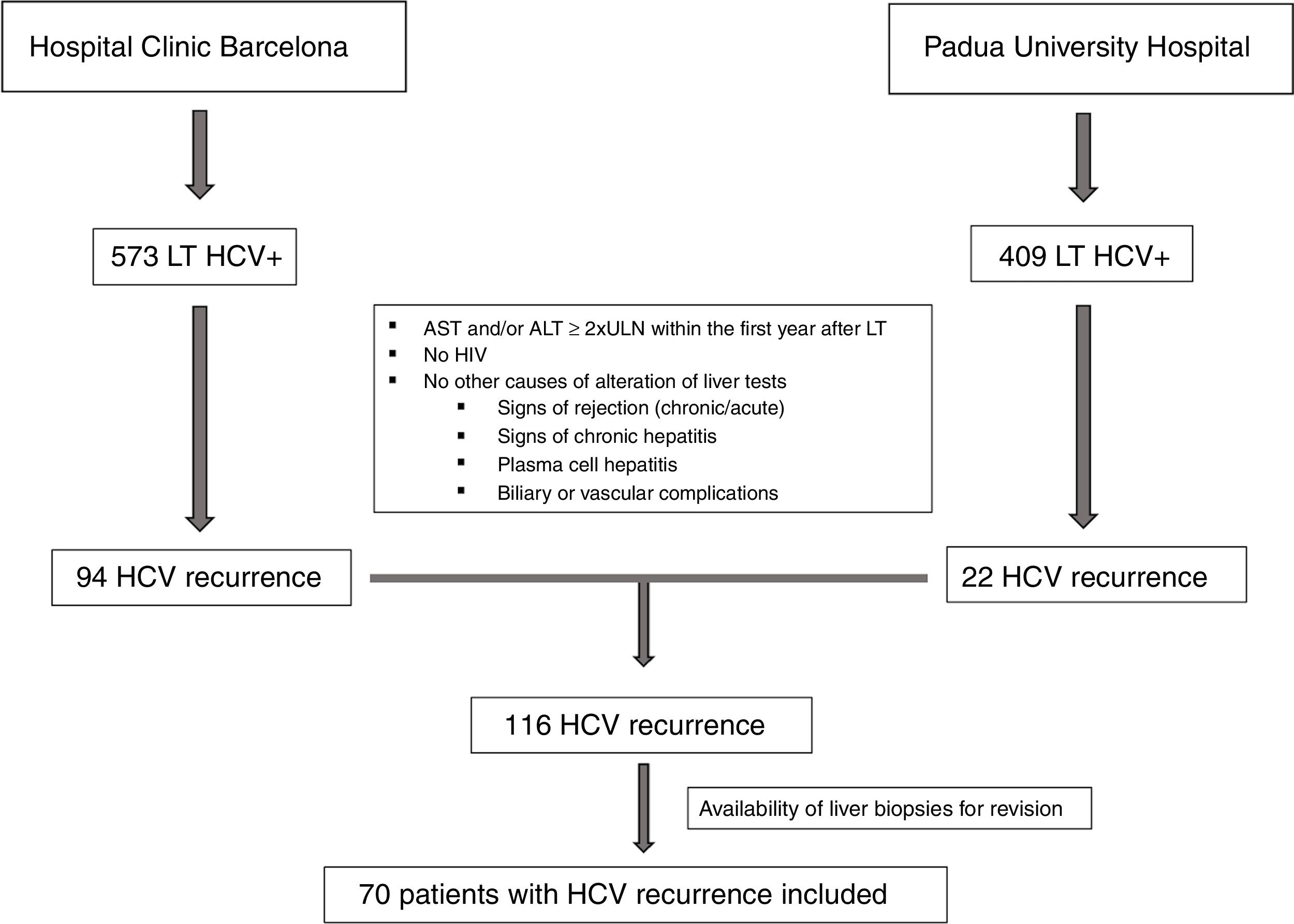
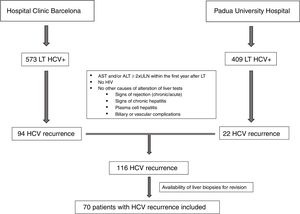
ResultsClinical and laboratory dataAs shown in Fig. 1, between 2000 and 2014, a total of 982 HCV positive patients underwent LT. One-hundred sixteen patients developed signs of severe acute hepatitis C recurrence (AST and/or ALT≥2 UNL) within the first year after LT (22 in Padua University Hospital and 94 in Hospital Clinic Barcelona). Seventy of these patients (60%) had an available liver biopsy and were included in the current study. As per protocol and based on laboratory tests at the time of recurrence, patients were classified as having FCH (n=39, 56%), CH (n=21, 30%) and NCAH (n=10, 14%).
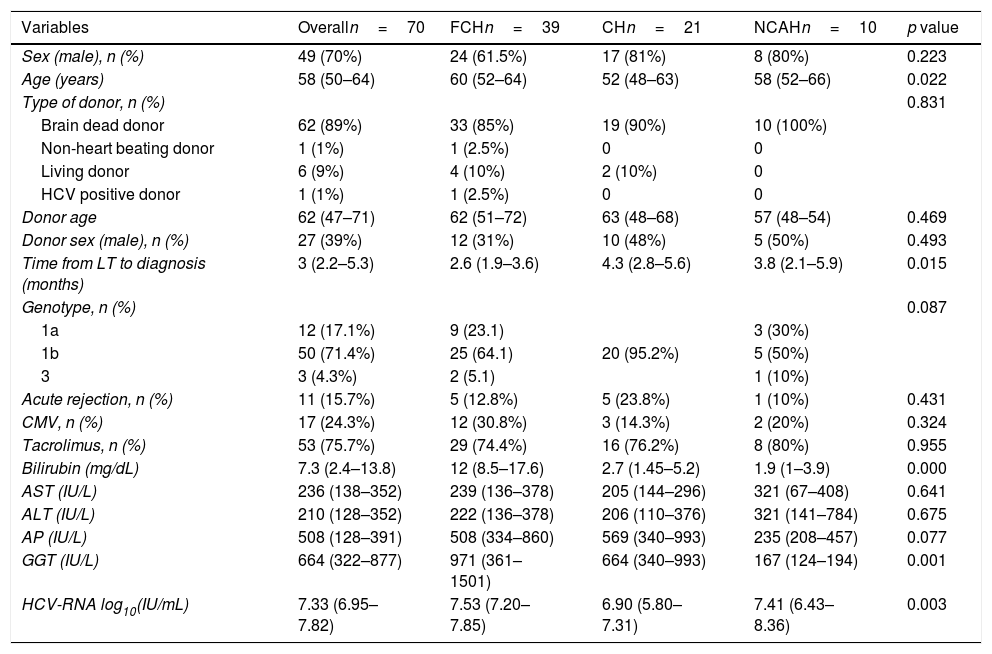
The baseline characteristics of the patients included in this study are shown in Table 1. Briefly, most patients were male (49, 70%), with a median age of 58 years (50–64) and infected with genotype 1b (71.4%). Most of the patients received a liver from a deceased liver donor (n=62, 89%), and one patient received a liver from an HCV positive donor. Median donor age was 62 years old (49–71). Most patients (n=53, 76%) received a tacrolimus-based immunosuppressive therapy. The median time from LT to the diagnosis of hepatitis C recurrence was 2.96 months (2.1–5.3). At the time of severe hepatitis C recurrence diagnosis, median bilirubin, AST, ALT, AP and GGT levels were 7.3mg/dL, (2.4–13.8), 236IU/mL (138–352), 210IU/mL (128–352), 508IU/mL (128–391), and 664IU/mL (322–877), respectively. Median HCV-RNA was 7.3 log10 IU/mL (6.9–7.8).
Clinical and laboratory data at diagnosis of severe hepatitis C recurrence.
| Variables | Overalln=70 | FCHn=39 | CHn=21 | NCAHn=10 | p value |
|---|---|---|---|---|---|
| Sex (male), n (%) | 49 (70%) | 24 (61.5%) | 17 (81%) | 8 (80%) | 0.223 |
| Age (years) | 58 (50–64) | 60 (52–64) | 52 (48–63) | 58 (52–66) | 0.022 |
| Type of donor, n (%) | 0.831 | ||||
| Brain dead donor | 62 (89%) | 33 (85%) | 19 (90%) | 10 (100%) | |
| Non-heart beating donor | 1 (1%) | 1 (2.5%) | 0 | 0 | |
| Living donor | 6 (9%) | 4 (10%) | 2 (10%) | 0 | |
| HCV positive donor | 1 (1%) | 1 (2.5%) | 0 | 0 | |
| Donor age | 62 (47–71) | 62 (51–72) | 63 (48–68) | 57 (48–54) | 0.469 |
| Donor sex (male), n (%) | 27 (39%) | 12 (31%) | 10 (48%) | 5 (50%) | 0.493 |
| Time from LT to diagnosis (months) | 3 (2.2–5.3) | 2.6 (1.9–3.6) | 4.3 (2.8–5.6) | 3.8 (2.1–5.9) | 0.015 |
| Genotype, n (%) | 0.087 | ||||
| 1a | 12 (17.1%) | 9 (23.1) | 3 (30%) | ||
| 1b | 50 (71.4%) | 25 (64.1) | 20 (95.2%) | 5 (50%) | |
| 3 | 3 (4.3%) | 2 (5.1) | 1 (10%) | ||
| Acute rejection, n (%) | 11 (15.7%) | 5 (12.8%) | 5 (23.8%) | 1 (10%) | 0.431 |
| CMV, n (%) | 17 (24.3%) | 12 (30.8%) | 3 (14.3%) | 2 (20%) | 0.324 |
| Tacrolimus, n (%) | 53 (75.7%) | 29 (74.4%) | 16 (76.2%) | 8 (80%) | 0.955 |
| Bilirubin (mg/dL) | 7.3 (2.4–13.8) | 12 (8.5–17.6) | 2.7 (1.45–5.2) | 1.9 (1–3.9) | 0.000 |
| AST (IU/L) | 236 (138–352) | 239 (136–378) | 205 (144–296) | 321 (67–408) | 0.641 |
| ALT (IU/L) | 210 (128–352) | 222 (136–378) | 206 (110–376) | 321 (141–784) | 0.675 |
| AP (IU/L) | 508 (128–391) | 508 (334–860) | 569 (340–993) | 235 (208–457) | 0.077 |
| GGT (IU/L) | 664 (322–877) | 971 (361–1501) | 664 (340–993) | 167 (124–194) | 0.001 |
| HCV-RNA log10(IU/mL) | 7.33 (6.95–7.82) | 7.53 (7.20–7.85) | 6.90 (5.80–7.31) | 7.41 (6.43–8.36) | 0.003 |
Data expressed as median (interquartile range).
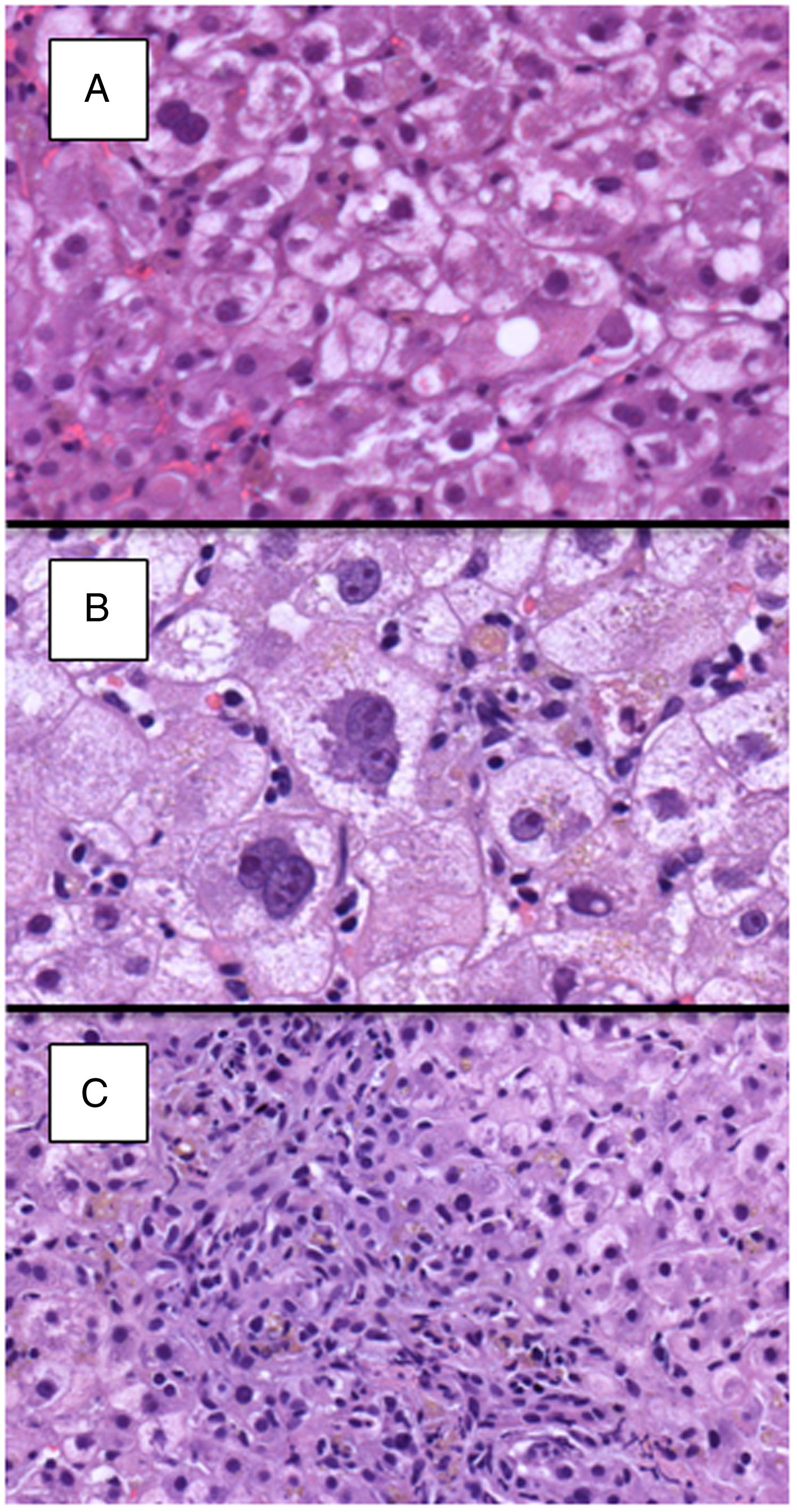
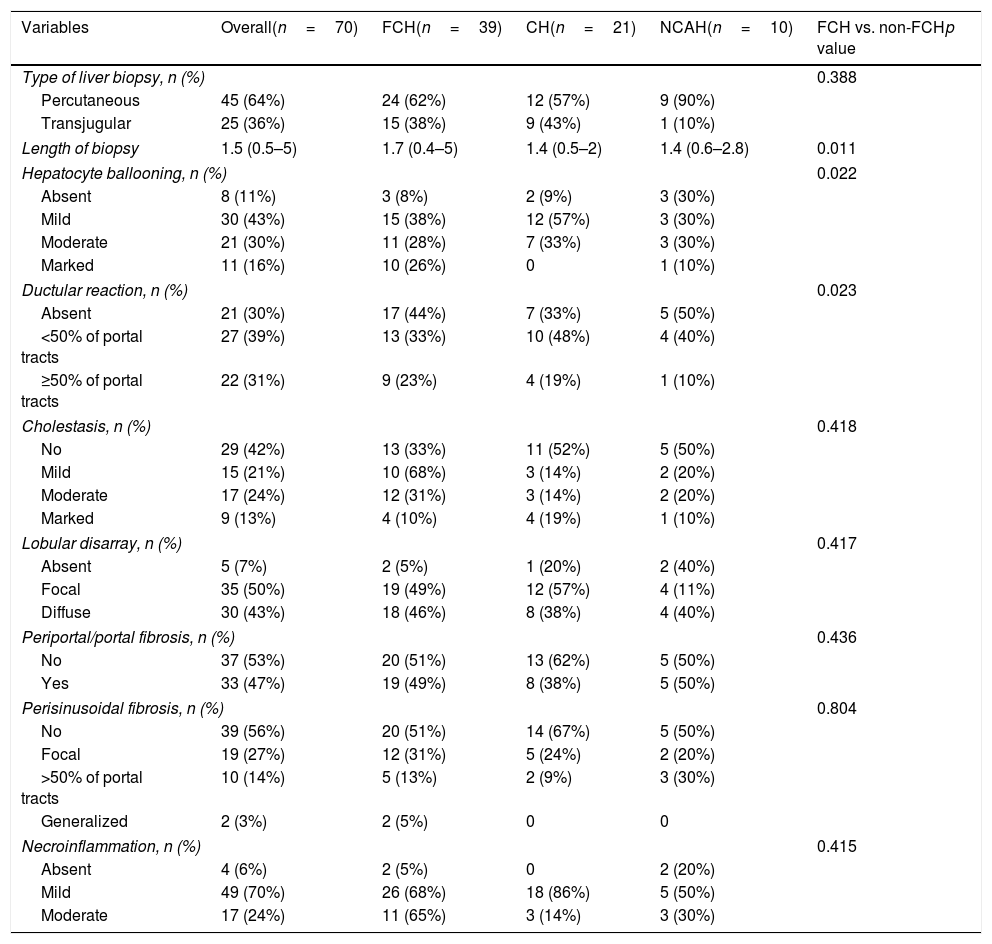
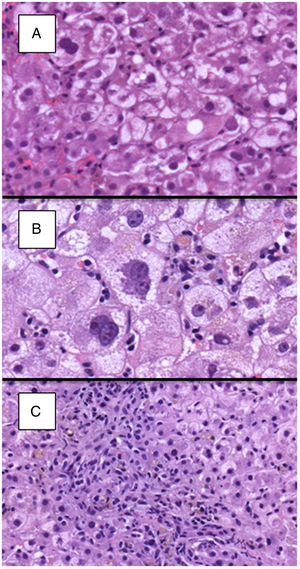
Detailed histological characteristics are shown in Table 2. Most of the patients (65, 93%) showed some degree of lobular disarray. Thirty-three patients (47%) had portal/periportal fibrosis, 32 (46%) moderate/marked hepatocyte ballooning, 31 (47%) periportal/pericellular fibrosis, 26 (27%) moderate/marked cholestasis, and 22 (31%) ductular reaction in more than 50% of the portal tracts (Fig. 2). Of notice, necroinflammatory foci were absent or mild in most patients, being moderate in only 17 (24%) of them.
Histological analysis of patients included in the study according to analytical classification.
| Variables | Overall(n=70) | FCH(n=39) | CH(n=21) | NCAH(n=10) | FCH vs. non-FCHp value |
|---|---|---|---|---|---|
| Type of liver biopsy, n (%) | 0.388 | ||||
| Percutaneous | 45 (64%) | 24 (62%) | 12 (57%) | 9 (90%) | |
| Transjugular | 25 (36%) | 15 (38%) | 9 (43%) | 1 (10%) | |
| Length of biopsy | 1.5 (0.5–5) | 1.7 (0.4–5) | 1.4 (0.5–2) | 1.4 (0.6–2.8) | 0.011 |
| Hepatocyte ballooning, n (%) | 0.022 | ||||
| Absent | 8 (11%) | 3 (8%) | 2 (9%) | 3 (30%) | |
| Mild | 30 (43%) | 15 (38%) | 12 (57%) | 3 (30%) | |
| Moderate | 21 (30%) | 11 (28%) | 7 (33%) | 3 (30%) | |
| Marked | 11 (16%) | 10 (26%) | 0 | 1 (10%) | |
| Ductular reaction, n (%) | 0.023 | ||||
| Absent | 21 (30%) | 17 (44%) | 7 (33%) | 5 (50%) | |
| <50% of portal tracts | 27 (39%) | 13 (33%) | 10 (48%) | 4 (40%) | |
| ≥50% of portal tracts | 22 (31%) | 9 (23%) | 4 (19%) | 1 (10%) | |
| Cholestasis, n (%) | 0.418 | ||||
| No | 29 (42%) | 13 (33%) | 11 (52%) | 5 (50%) | |
| Mild | 15 (21%) | 10 (68%) | 3 (14%) | 2 (20%) | |
| Moderate | 17 (24%) | 12 (31%) | 3 (14%) | 2 (20%) | |
| Marked | 9 (13%) | 4 (10%) | 4 (19%) | 1 (10%) | |
| Lobular disarray, n (%) | 0.417 | ||||
| Absent | 5 (7%) | 2 (5%) | 1 (20%) | 2 (40%) | |
| Focal | 35 (50%) | 19 (49%) | 12 (57%) | 4 (11%) | |
| Diffuse | 30 (43%) | 18 (46%) | 8 (38%) | 4 (40%) | |
| Periportal/portal fibrosis, n (%) | 0.436 | ||||
| No | 37 (53%) | 20 (51%) | 13 (62%) | 5 (50%) | |
| Yes | 33 (47%) | 19 (49%) | 8 (38%) | 5 (50%) | |
| Perisinusoidal fibrosis, n (%) | 0.804 | ||||
| No | 39 (56%) | 20 (51%) | 14 (67%) | 5 (50%) | |
| Focal | 19 (27%) | 12 (31%) | 5 (24%) | 2 (20%) | |
| >50% of portal tracts | 10 (14%) | 5 (13%) | 2 (9%) | 3 (30%) | |
| Generalized | 2 (3%) | 2 (5%) | 0 | 0 | |
| Necroinflammation, n (%) | 0.415 | ||||
| Absent | 4 (6%) | 2 (5%) | 0 | 2 (20%) | |
| Mild | 49 (70%) | 26 (68%) | 18 (86%) | 5 (50%) | |
| Moderate | 17 (24%) | 11 (65%) | 3 (14%) | 3 (30%) | |
FCH: fibrosing cholestatic hepatitis, CH: cholestatic hepatitis, NCAH: non-cholestatic acute hepatitis.
We then evaluated these histological characteristics in the different groups of patients classified according to laboratory data. There were no significant differences in histological variables among patients with NCAH and CH. There was an lineal association between the grade of hepatocyte ballooning and ductular reaction both with the presence of FCH (p=0.022 and p=0.023, respectively) which was not observed in patients with CH or AH. These variables (hepatocyte ballooning and ductular reaction) were re-examined in a univariable and multivariable logistic regression model (Table 3), and both, marked hepatocyte ballooning and ductular reaction (>50% of the portal tracts) were independently associated with the presence of FCH pre-defined by laboratory criteria with an OR of 4.66 (p=0.047) and 20.58 (p=0.025), respectively. There were no other significant differences among patients with or without FCH in the remaining histological characteristics.
Multivariable analysis of predictors of FCH.
| Variable | Univariable analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | p value | OR | 95%CI | p value | |
| Hepatocyte ballooning | ||||||
| Mild | 1.67 | 0.34–8.26 | 0.532 | |||
| Moderate | 1.83 | 0.35–9.72 | 0.476 | |||
| Marked | 16.67 | 1.36–204.03 | 0.028 | 4.66 | 1.02–21.25 | 0.047 |
| Ductular reaction | ||||||
| <50% | 1.24 | 0.39–3.90 | 0.715 | |||
| ≥50% | 4.53 | 1.21–16.96 | 0.025 | 20.58 | 1.47–288.10 | 0.025 |
| Cholestasis | ||||||
| Mild | 2.46 | 0.67–9.02 | 0.174 | |||
| Moderate | 2.95 | 0.83–10.56 | 0.096 | |||
| Marked | 0.98 | 0.22–4.43 | 0.984 | |||
| Lobular disarray | ||||||
| Focal | 1.78 | 0.26–12.01 | 0.553 | |||
| Diffuse | 2.25 | 0.33–15.54 | 0.411 | |||
| Periportal/portal fibrosis | 1.46 | 0.56–3.77 | 0.437 | |||
| Necroinflammation | ||||||
| Mild | 1.13 | 0.15–8.68 | 0.906 | |||
| Moderate | 1.83 | 0.20–16.51 | 0.589 | |||
95%CI: 95% confidence interval; OR: odds ratio. p value <0.05 was considered statistically significant.
Subsequently, patients were compared according to the number of histological criteria defining FCH.21 Whilst, 25 (64%) patients with FCH (defined by biochemical and viral load criteria as previously described), had 2 or more histological criteria compatible with the diagnosis, only 5 (24%) patients with CH and 3 (30%) patients with NCAH had 2 or more criteria (p=0.005). Interestingly, 12 (31%) patients classified as FCH by analytical criteria had only one of the histological criterion required for the diagnosis of the disease. On the other hand, among the 15 patients with ≥3 histological criteria of FCH, 12 (80%) had analytical criteria of FCH, 2 (13%) of CH and 1 (7%) of NCAH. In these 15 patients with histological definition of FCH median bilirubin, AP and GGT were 12.8mg/dL, 508IU/ml and 493IU/mL, respectively. Among the 39 patients classified as FCH by analytical criteria, there were no significant differences in liver tests in patients with or without histological definition of FCH (≥3 histological criteria).
Cohen's κ was run to determine whether there was a good correlation between analytical and histological criteria in the diagnosis of FCH. Interestingly, the correlation between the histological and analytical criteria was poor, κ=0.193, p=0.033. Considering liver biopsy as the gold standard for the diagnosis of FCH, the sensitivity, specificity, positive and negative predictive values of analytical criteria were 0.8, 0.5, 0.3 and 0.9, respectively.
Allograft outcomeTwenty-seven (39%) patients lost their graft due to hepatitis C recurrence after a median time of 18 months (1–110) since the diagnosis of the recurrence. The frequency of graft loss was higher (albeit not statistically significant) in the group of patients with FCH (18 of 39, 46%) as compared to those with non-FHC recurrence (9 of 31, 29%). Neither the number of FCH histological criteria, nor the presences of ballooning or ductular reaction were significantly associated with the outcome of the liver allograft. However, the availability of interferon-free regimens in the last 2 years of the inclusion period might have influenced graft outcomes by allowing the treatment of patients with severe hepatitis C recurrence.
DiscussionThe widespread use of DAAs to treat hepatitis C has allowed the treatment of patients with advanced liver disease awaiting liver transplantation. This strategy is associated with: (1) a delisting probability due to clinical improvement of up to 20%22,23 and (2) an increase in post-transplant survival due to a significant reduction in the number of patients with hepatitis C recurrence after LT.12 However, there are still some patients who are not candidates to receive antiviral therapy before transplant (those with MELD score>18–20 and/or renal dysfunction24) and therefore at risk of severe hepatitis C recurrence. In these patients, it is essential to early and accurately diagnose the presence of FCH in order to start antiviral therapy without delay. Currently, the diagnosis of FCH relies on the combination of analytical and histological criteria not well validated.
Here, we evaluated the accuracy of laboratory criteria for the diagnosis of FCH based on its correlation with histological criteria. Thirty-nine (58%) patients met the analytical criteria10 and 15 (21%) the histological criteria (presence of ≥3 out of 4 characteristics).11,21 Only 12 patients met both analytical and histological criteria indicating a poor correlation between both criteria (κ=0.193). It is interesting to note, however, that the absence of analytical criteria (total bilirubin>6mg/dL, increase in AP and GGT>5 times UNL and HCV viral load≥107IU/mL) rule out the presence of FCH with a negative predictive value of 90%. Among the 39 patients classified as FCH by analytical criteria, there were no significant differences in liver tests in patients with or without histological criteria. Therefore, we were not able to identify different cut-off points for liver tests that help to increase the specificity and positive predictive value of analytical criteria for the diagnosis of FCH.
Focusing on histological criteria, only the presence of marked ballooning and ductular reaction in more than 50% of the portal tracts, were significantly associated with FCH according to laboratory results. This is similar to what was found by Salomao et al.17 in a study aiming to identify the histological useful to differentiate FCH and biliary obstruction. In that study, the authors found a significantly higher frequency of hepatocyte ballooning (85% vs. 15%, p<0.001) and ductular reaction CK7 negative (74% vs. 8%, <0.001) in patients with FCH as compared with those with biliary obstruction. In contrast to the study by Salomao we did not observe an increase in the frequency of periportal perisinusoidal fibrosis that was present in all patients with FCH in their study.
In the current study, 27 patients lost their graft due to hepatitis C recurrence, most of them in the group of patients with FCH defined by analytical criteria (n=18, 66%). However, we were not able to find a significant association between the presence of hepatocyte ballooning or ductular reaction (independent predictors of FCH) and graft outcomes. In 2013, Moreira et al.21 defined the Hepatitis Aggressiveness Score (HAS) in which the number of FCH histological criteria were highly predictive of fibrosis progression and the best predictor of graft loss. In that study, the presence of 3 or 4 histological criteria of FCH increased by 5.5 times the risk of graft loss as compared to patients with ≤2 criteria (HAS 1 and 2). In contrast to Moreira et al., we were not able to find a significant association between the number of FCH defining criteria and the outcome of the liver allograft. The reason for this difference is unknown but it could be related to the low number of patients with histological criteria of FCH or how different treatment strategies could have influenced the outcome, since some patients underwent treatment with DAAs because of the length of the study period.
Our study has several limitations: (1) it is a retrospective analysis with the limitations typical of this type of studies and (2) the widespread use of DAA that could decrease the number of patients with this severe form of hepatitis C recurrence. However, there are still some patients that are not candidates to receive antiviral therapy while awaiting LT and therefore at risk of developing FCH. An early and correct diagnosis of this complication would lead to a rapid initiation of antiviral therapy improving grafts and patients’ outcomes.
In conclusion, we found a poor correlation between analytical and histological criteria of FCH. Analytical criteria may be used to rule out the presence of FCH due to its elevated negative predictive value, but in patients with laboratory data compatible with FCH, a biopsy is mandatory to confirm the diagnosis. Ductular reaction in more than 50% of the portal tracts and hepatocyte ballooning, were the histological characteristics independently associated with the analytical definition of FCH but did not predict graft outcomes in the current study. Altogether these data indicate that the definition of the 3 entities (FCH, CH, and NCAH) is arbitrary and all probably represent a continuum of the same complication that require a prompt antiviral therapy.
FundingXF: Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement, grant 2017_SGR_1753. LPL: Resident Award “Clínic-La Pedrera” granted by the Hospital Clínic Barcelona, Research, Innovation and Education Department.
Conflict of interestXF received unrestricted grant support from Abbvie and acted as advisor for Abbvie and Gilead.