The incidence of bacteremia after endoscopic ultrasonography (EUS) or EUS-guided fine-needle aspiration (EUS-FNA) is between 0% and 4%, but there are no data on this topic in cirrhotic patients.
AimTo prospectively assess the incidence of bacteremia in cirrhotic patients undergoing EUS and EUS-FNA.
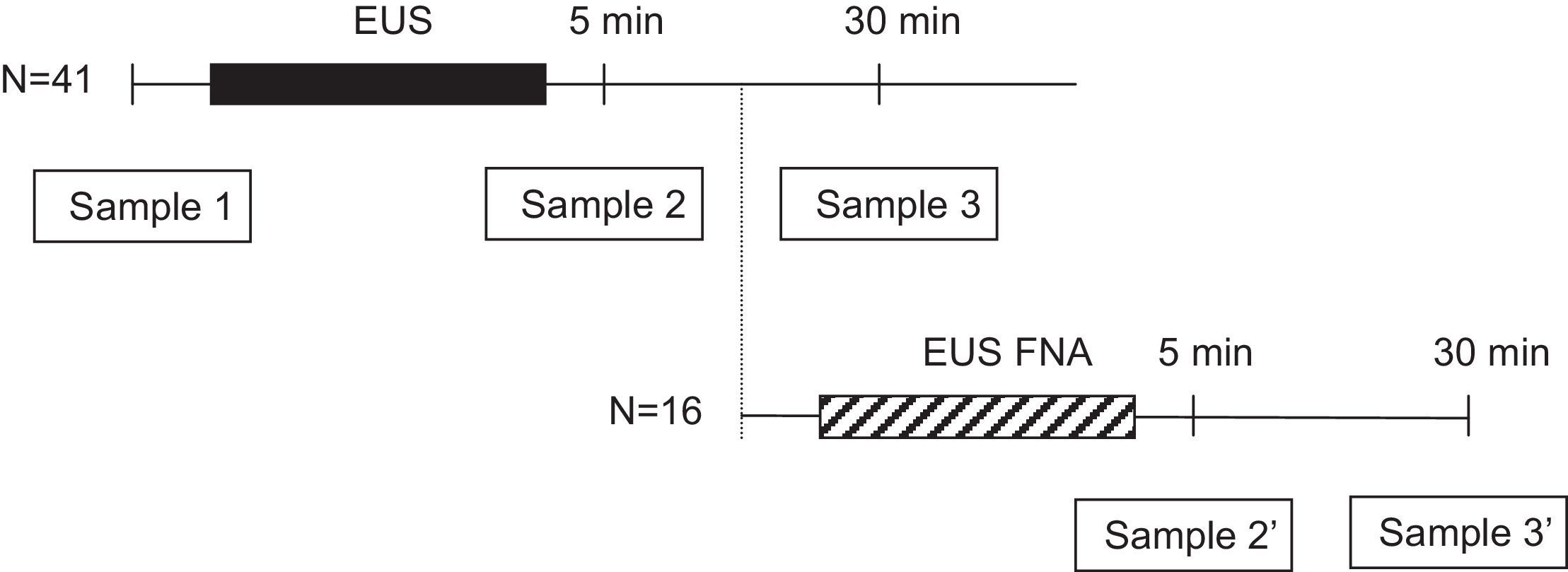
Patients and methodsWe enrolled 41 cirrhotic patients. Of these, 16 (39%) also underwent EUS-FNA. Blood cultures were obtained before and at 5 and 30min after the procedure. When EUS-FNA was used, an extra blood culture was obtained after the conclusion of radial EUS and before the introduction of the sectorial echoendoscope. All patients were clinically followed up for 7 days for signs of infection.
ResultsBlood cultures were positive in 16 patients. In 10 patients, blood cultures grew coagulase-negative Staphylococcus, Corynebacterium species, Propionibacterium species or Acinetobacterium Lwoffii, which were considered contaminants (contamination rate 9.8%, 95% CI: 5.7–16%). The remaining 6 patients had true positive blood cultures and were considered to have had true bacteremia (15%, 95% CI: 4–26%). Blood cultures were positive after diagnostic EUS in five patients but were positive after EUS-FNA in only one patient. Thus, the frequency of bacteremia after EUS and EUS-FNA was 12% and 6%, respectively (95% CI: 2–22% and 0.2–30%, respectively). Only one of the patients who developed bacteremia after EUS had a self-limiting fever with no other signs of infection.
ConclusionAsymptomatic Gram-positive bacteremia developed in cirrhotic patients after EUS and EUS-FNA at a rate higher than in non-cirrhotic patients. However, this finding was not associated with any clinically significant infections.
La incidencia de bacteriemia después de una ultrasonografía endoscópica (USE) o USE con punción aspirativa con aguja fina (USE-PAAF) se sitúa entre el 0-4%. No existen datos acerca de la incidencia en pacientes con cirrosis hepática.
ObjetivoEvaluar prospectivamente la incidencia de bacteriemia en pacientes cirróticos sometidos a USE y USE-PAAF.
Pacientes y métodosSe incluyeron un total de 41 pacientes. Dieciséis (39%) fueron sometidos también a USE-PAAF. Se realizaron hemocultivos antes y a los 5 y 30 minutos después del procedimiento. Cuando se practicó USE-PAAF se obtuvo una muestra de sangre adicional después de acabar la USE radial y antes de la introducción del ecoendoscopio sectorial. Todos los pacientes fueron seguidos durante 7 días.
ResultadosLos hemocultivos fueron positivos en 16 pacientes. En 10 pacientes crecieron gérmenes que fueron considerados contaminantes (tasa de contaminación 9,8%, IC 95%: 5,7-16%). Los 6 pacientes restantes tuvieron hemocultivos positivos por gérmenes no contaminantes y fueron considerados verdaderas bacteriemias (15%, IC 95%: 4-26%). En 5 pacientes los hemocultivos fueron positivos después de la USE diagnóstica y solo en uno después de la USE-PAAF. Por lo tanto, la frecuencia de bacteriemia asociada a USE y USE-PAAF fue 12 y 6%, respectivamente (IC 95%: 2-22% y 0,2-30%, respectivamente). Solo uno de los pacientes presentó bacteriemia sintomática tras la USE que consistió en fiebre autolimitada sin otros signos de infección.
ConclusiónLos pacientes cirróticos presentan una incidencia de bacteremia asintomática por gérmenes gram-positivos después de la USE (con o sin PAAF) mayor que los pacientes sin esta patología. Sin embargo, este hecho no se asocia a una mayor incidencia de infecciones clínicamente significativas.
The risk of bacteremia after EUS of the upper GI tract in non-cirrhotic patients is low (0–4%),1,2 and it is not increased by the performance of EUS-guided fine-needle aspiration (EUS-FNA).1–3 Moreover, preliminary results suggest that bacteremia and associated complications are uncommon after EUS-FNA of solid rectal and perirectal lesions.4 As a result, antibiotic prophylaxis may not be warranted for spontaneous bacterial endocarditis in the general population undergoing EUS and/or EUS-FNA.
As it is well known, a high risk of bacteremia and septic complications has been described for patients with advanced liver diseases undergoing endoscopic procedures other than EUS.5–7 Colonoscopy seems not to induce bacteremia in cirrhotic patients with or without ascites in the absence of GI bleeding8 whereas sclerotherapy of esophageal varices has been associated with bacteremia rates as high as 31%.9 It has been suggested that this fact could be related to an impaired ability to clear blood-circulating bacteria from the blood due to the compromised immune function and the portal systemic shunting typical in cirrhosis.10 Nevertheless, no data exists on the risk of bacteremia and other infectious complications in cirrhotic patients undergoing EUS or EUS-FNA and, therefore, there is a lack of information on the need of antibiotic prophilaxis for endocarditis and spontaneous bacterial peritonitis.
This prospective study was undertaken to specifically assess the incidence of bacteremia in cirrhotic patients undergoing upper EUS with or without FNA.
Patients and methodsPatientsDuring a 12-month period, consecutive cirrhotic patients undergoing upper EUS with or without FNA were enrolled in this prospective study. Exclusion criteria were the following: (1) age less than 18 years; (2) bacterial infection and/or antibiotic treatment within the previous 2 weeks; (3) clinical evidence of any intercurrent complication such as GI bleeding or encephalopathy within 2 weeks of EUS; (4) esophageal stricture dilation or sclerotherapy of esophageal varices within 2 weeks of EUS; (5) treatment with albumin within the previous two weeks; (6) need for antibiotic prophylaxis according to American Society for Gastrointestinal Endoscopy (ASGE)11; and (7) lack of informed consent. No patient was included more than once. This study was approved by the Ethics Committee of Hospital Clinic, Barcelona, and informed consent was obtained from all patients.
EUS procedureEUS was performed using radial and linear echoendoscopes for diagnostic EUS and EUS-FNA, respectively (GF UM160 and GF UC140P, Olympus America Inc., Melville, NY, USA). For EUS-FNA, a 22 gauge-needle (Wilson-Cook Medical Inc., Winston-Salem, NC, USA) was used in all patients. FNA could be repeated with the same needle several times during one session after flushing the system with saline solution. Endosonographic examinations and EUS-FNA were performed according to the standard techniques described elsewhere.12
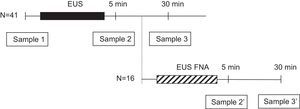
Microbiologic examinationBefore starting the procedure, two separate 22-gauge angiocatheters, each in a different arm, were placed by using aseptic techniques. One intravenous line was used for intravenous administration of sedative medications and the other was used to collect blood cultures. The intravenous wound site was initially cleaned with 70% alcohol followed by air drying for 30s. The area was then cleaned with 10% povidone iodine solution for 60s and allowed to air dry for another 60s. Three blood samples (20mL each) were obtained from every patient, just before EUS (sample 1) and at 5 and 30min of withdrawal of the radial echoendoscope (samples 2 and 3, respectively). In patients undergoing FNA, the sample at 30min after diagnostic EUS (sample 3) was not obtained, but two additional samples were obtained at 5 and 30min of withdrawal of the sectorial echoendoscope (samples 2′ and 3′, respectively) (Fig. 1). Blood cultures were processed by the Bactec 9240 System (Becton-Dickinson, Sparks, MD, USA). The incubation protocol was of 5 days, and the isolated microorganisms were identified and tested for antibiotics sensitivity by standard techniques.13 Bacteria with a priori high likelihood of being contaminants (such as coagulase-negative staphylococci, Bacillus species, Corynebacterium species, and Propionibacterium acnes) were considered to be contaminants unless isolated from 2 or more separate blood culture sets.14–16 The isolation of other bacteria was considered a positive culture and defined as an episode of bacteremia.
Follow-upPatients were closely monitored (axillary temperature, presence of chills, and onset of symptoms like abdominal pain after the examination were checked every 12h) in the first 72h after the procedure to detect infectious complications. Hospitalized patients were evaluated by a physician whereas outpatients were interviewed by telephone call. Furthermore, patients were instructed to return to the hospital if any of the symptoms mentioned above appeared within one week after EUS. In addition, one week later, a research fellow not involved in the study contacted patients and inquired to the development of any of these symptoms.
Statistical analysisThe frequency of bacteremia, including the 95% confidence interval (CI), was calculated.
The following factors that potentially could influence the occurrence of bacteremia were recorded: patient characteristics (age, gender, etiology of cirrhosis, severity of liver disease measured by Child–Pugh classification), indication for EUS/EUS-FNA, other endoscopic procedures performed before EUS (upper endoscopy and/or routine biopsies), duration of the procedure, type and localization of the lesion, number of needle passes through the lesion.
The Mann–Whitney U was used for statistical comparison of groups, as appropriate.
Results are expressed as mean±SD.
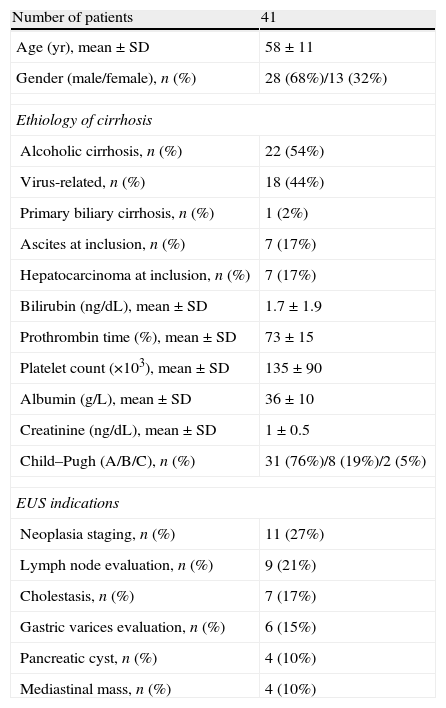
ResultsThe clinical features of the patients included in the study and EUS indications are shown in Table 1. In 11 cases (26%) an upper endoscopy was performed before EUS and routine biopsies were obtained in 6 of them. One patient presented with esophageal stenosis that was traversed with the blind echoendoscope without previous dilation. EUS-FNA was performed in 16/41 patients (39%). Lesions sampled were intraabdominal lymphadenopathies (44%), mediastinal lymphadenopathies (19%), pancreatic mass (19%) and miscellanea (18%). In 6 patients, more than 1 site was aspirated (mean 1.4±0.6 sites/patient, range 1–3). Mean number of passes was 2.6±1.6 per patient, range 1–7. Mean examination time was 43±26min (range, 12–128).
Clinical characteristics of the patients included in the study.
| Number of patients | 41 |
| Age (yr), mean±SD | 58±11 |
| Gender (male/female), n (%) | 28 (68%)/13 (32%) |
| Ethiology of cirrhosis | |
| Alcoholic cirrhosis, n (%) | 22 (54%) |
| Virus-related, n (%) | 18 (44%) |
| Primary biliary cirrhosis, n (%) | 1 (2%) |
| Ascites at inclusion, n (%) | 7 (17%) |
| Hepatocarcinoma at inclusion, n (%) | 7 (17%) |
| Bilirubin (ng/dL), mean±SD | 1.7±1.9 |
| Prothrombin time (%), mean±SD | 73±15 |
| Platelet count (×103), mean±SD | 135±90 |
| Albumin (g/L), mean±SD | 36±10 |
| Creatinine (ng/dL), mean±SD | 1±0.5 |
| Child–Pugh (A/B/C), n (%) | 31 (76%)/8 (19%)/2 (5%) |
| EUS indications | |
| Neoplasia staging, n (%) | 11 (27%) |
| Lymph node evaluation, n (%) | 9 (21%) |
| Cholestasis, n (%) | 7 (17%) |
| Gastric varices evaluation, n (%) | 6 (15%) |
| Pancreatic cyst, n (%) | 4 (10%) |
| Mediastinal mass, n (%) | 4 (10%) |
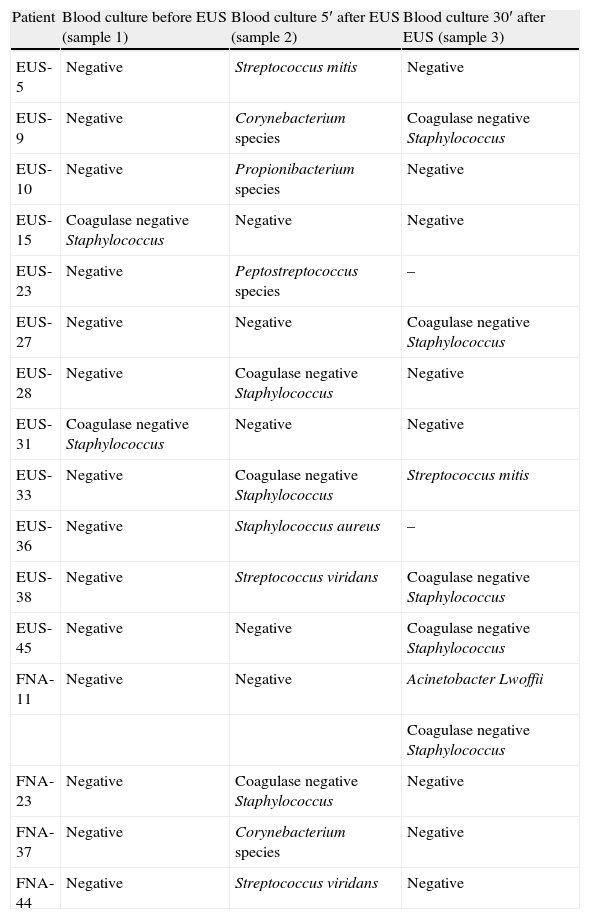
There were a total of 133 sets of blood cultures drawn. Blood cultures were positive in 16 of the 41 patients. Culture of blood in 10 patients grew coagulase negative Staphylococcus, Corynebacterium species, Propionibacterium species or A. lwoffii which was considered contaminants. Therefore, the contamination rate of blood cultures was 9.8% (13 of 133, 95% CI: 5.7–16%) (see Table 2). The other 6 patients had true positive blood cultures and were considered to have had veritable bacteremia (15%, 95% CI: 4–26%): 5 after diagnostic EUS (12%, 95% CI: 2–22%) and only 1 after EUS-FNA (6%, 95% CI: 0.2–30%). Only one patient with a positive blood culture (2%) developed clinical signs of infection (fever within the first 24h) who recovered with antibiotic treatment without further complications.
Bacteria isolated in patients with positive blood culture.
| Patient | Blood culture before EUS (sample 1) | Blood culture 5′ after EUS (sample 2) | Blood culture 30′ after EUS (sample 3) |
| EUS-5 | Negative | Streptococcus mitis | Negative |
| EUS-9 | Negative | Corynebacterium species | Coagulase negative Staphylococcus |
| EUS-10 | Negative | Propionibacterium species | Negative |
| EUS-15 | Coagulase negative Staphylococcus | Negative | Negative |
| EUS-23 | Negative | Peptostreptococcus species | – |
| EUS-27 | Negative | Negative | Coagulase negative Staphylococcus |
| EUS-28 | Negative | Coagulase negative Staphylococcus | Negative |
| EUS-31 | Coagulase negative Staphylococcus | Negative | Negative |
| EUS-33 | Negative | Coagulase negative Staphylococcus | Streptococcus mitis |
| EUS-36 | Negative | Staphylococcus aureus | – |
| EUS-38 | Negative | Streptococcus viridans | Coagulase negative Staphylococcus |
| EUS-45 | Negative | Negative | Coagulase negative Staphylococcus |
| FNA-11 | Negative | Negative | Acinetobacter Lwoffii |
| Coagulase negative Staphylococcus | |||
| FNA-23 | Negative | Coagulase negative Staphylococcus | Negative |
| FNA-37 | Negative | Corynebacterium species | Negative |
| FNA-44 | Negative | Streptococcus viridans | Negative |
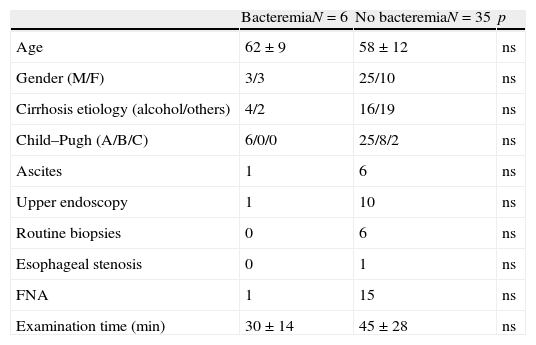
Data from patients with significant bacteremia (n=6) and those with negative or contaminated blood cultures (n=35) were compared for potential factors that might predispose to bacteremia. All the parameters evaluated (patient characteristics, indication for EUS/EUS-FNA, other endoscopic procedures performed before EUS, duration of the procedure, punctured lesion characteristics or number of needle passes through lesion) did not show statistically significant differences between both groups (Table 3).
Predictive risk factors for EUS associated bacteremia.
| BacteremiaN=6 | No bacteremiaN=35 | p | |
| Age | 62±9 | 58±12 | ns |
| Gender (M/F) | 3/3 | 25/10 | ns |
| Cirrhosis etiology (alcohol/others) | 4/2 | 16/19 | ns |
| Child–Pugh (A/B/C) | 6/0/0 | 25/8/2 | ns |
| Ascites | 1 | 6 | ns |
| Upper endoscopy | 1 | 10 | ns |
| Routine biopsies | 0 | 6 | ns |
| Esophageal stenosis | 0 | 1 | ns |
| FNA | 1 | 15 | ns |
| Examination time (min) | 30±14 | 45±28 | ns |
All subjects were successfully contacted by phone. No other complications were identified.
DiscussionThe risk of bacteremia after gastrointestinal instrumentation and the usefulness of prophylactic antibiotics have been matters of great controversy and the origin of several guidelines provided by Scientific Societies.11,17
In the more recent guidelines published by the ASGE, prophylactic antibiotics against infective endocarditis are less required and, for instance, antibiotics are not indicated in patients undergoing colonoscopy (even in patients who have prosthetic heart valves, previous history of endocarditis, and surgically constructed systemic–pulmonary shunts).11 However, antibiotic prophylaxis is still recommended for certain endoscopic procedures associated with a high risk of bacteremia, including esophageal stricture dilation,18–20 ERCP in patients with biliary obstruction21–23 and drainage of a pancreatic pseudocyst24 or to prevent cyst infection in EUS-FNA of any cystic lesion.25
There is limited information on the risk of bacteremia after EUS innon-cirrhotic patients and, moreover, the reported incidence varies widely (0–6%).1–3 A possible explanation for such variability is the timing of blood sampling. Several prospective studies in patients with bacteremia after endoscopic procedures have shown that peak bacteremia rates occur during and within 5min of the end of the procedure.8,9,26 These data could explain the low incidence of bacteremia after EUS (0%) reported by Barawi et al.3 since blood samples were withdrawn at 30 and 60min after EUS-FNA.
The results of our study show: first, that contamination of the intravenous catheter is frequent, second that the rate of bacteremia in cirrhotic patients after EUS is higher than in patients with other conditions and always due to Gram-positive bacteria and finally, and more importantly, that bacteremia has not clinical relevance. Despite we used two separated intravenous lines, the rate of contamination observed in our study is higher than other previously reported and could be explained by an inadequate manipulation of peripheral catheter.
In our study, 6 positive blood cultures, and therefore bacteremia, were observed in 6 of the 41 cirrhotic patients undergoing EUS (15%, 95% CI: 4–26%). As stated before, this rate of bacteremia is clearly higher than those previously reported after EUS in non-cirrhotic patients, but equal to that reported by Lin et al.27 in a set of cirrhotic patients treated with endoscopic variceal ligation. The organisms recovered from the blood in patients with true positive cultures were all of them Gram-positive skin and oropharyngeal commensals. This finding is consistent with the hypothesis that bacteremia and infectious complications after EUS are more likely to be related with the procedure itself than with the clinical condition of the cirrhotic patient. In fact, in our study no patients with bacteremia had impaired liver function or decompensated cirrhosis. However, there is evidence of neutrophil dysfunction and decrease phagocytic capacity even in early stages of cirrhosis.28 On the other hand, all positive cultures at 5′ after the procedure were negative at 30′ meaning that bacteremia was transient in these patients.
In our series, only one patient with a positive blood culture developed clinical signs of infection autolimited within the 24h after the procedure. This fact demonstrates that bacteremia after EUS in cirrhotic patients has no clinical relevance and, therefore, antibiotic prophylaxis to prevent it would not be indicated. The use of prophylactic antibiotics against bacteremia would only be necessary in those cirrhotic patients at high-risk of endocarditis, as described in the ASGE guidelines.11 It has to be taken into account, however, that most patients included in the present study did not have severe liver failure (67% of them were Child A and only 14% have had previous episodes of ascites or variceal hemorrhage) and so it is unclear whether or not the incidence of symptomatic bacteremia could be higher in patients with more advanced liver disease.
As previously reported, EUS-FNA did not increase the incidence of bacteremia (in 5 patients bacteremia was detected after the radial EUS examination whereas only in one patient bacteremia developed after EUS-FNA). No other risk factors for the development of bacteremia were identified, even the severity of liver disease (bacteremia only developed in Child–Pugh A patients). This is consistent with the results of previous studies performed in non-cirrhotic patients where factors such as the duration of the procedure, the performance of upper endoscopy with routine biopsies or esophageal dilation before EUS, the number of passes when performing FNA or the underlying pathology, were not related with the risk of bacteremia.1–3 However, the number of patients is relatively small and it would be possible that such factors were identified in studies with larger sample.
In summary, Gram-positive bacteremia develops in cirrhotic patients after EUS (with or without FNA) at a rate higher than that reported in patients with other conditions. However, since there is no clinical impact, antibiotic prophylaxis seems not to be necessary in all cirrhotic patients except in those at high risk for endocarditis.
Authors contributionF-E G and G A have participated in: (1) conception and design, acquisition of data, analysis and interpretation of data; (2) drafting the article; and (3) final approval of the version to be published. S O, A I, P M, A M, G-S B, L-C M, C H, S E, U H and L J have participated in: (1) analysis and interpretation of data; (2) revising the article critically for important intellectual content; and (3) final approval of the version to be published.
Core tipThis prospective study examines the incidence of bacteremia in cirrhotic patients undergoing an upper EUS or EUS-FNA and demonstrates: first, that contamination of the intravenous catheter probably due to manipulation is frequent; second, that the rate of bacteremia in cirrhotic patients after EUS is higher than in non-cirrhotic patients and in this series was always due to Gram-positive bacteria and finally, and more important, that bacteremia is transient and without clinical relevance. For this reason, the use of prophylactic antibiotics against bacteremia would only be necessary in those cirrhotic patients at high-risk of endocarditis, as described in the current guidelines
Conflict of interestThe authors have no conflict of interest to declare.