Qualitative and quantitative changes in gut microbiota play a very important role in cirrhosis. Humans harbour around 100 quintillion gut bacteria, thus representing around 10 times more microbial cells than eukaryotic ones. The gastrointestinal tract is the largest surface area in the body and it is subject to constant exposure to these living microorganisms. The existing symbiosis, proven by the lack of proinflammatory response against commensal bacteria, implies the presence of clearly defined communication lines that contribute to the maintenance of homeostasis of the host. Therefore, alterations of gut flora seem to play a role in the pathogenesis and progress of multiple liver and gastrointestinal diseases. This has made its selective modification into an area of high therapeutic interest.
Bacterial translocation is defined as the migration of bacteria or bacterial products from the intestines to the mesenteric lymph nodes. It follows that alteration in gut microbiota have shown importance, at least to some extent, in the pathogenesis of several complications arising from terminal liver disease, such as hepatic encephalopathy, portal hypertension and spontaneous bacterial peritonitis.
This review sums up, firstly, how liver disease can alter the common composition of gut microbiota, and secondly, how this alteration contributes to the development of complications in cirrhosis.
Los cambios cualitativos y cuantitativos en la microbiota intestinal juegan un papel muy importante en la cirrosis. El ser humano alberga cerca de 100 trillones de bacterias intestinales, representando así alrededor de 10 veces más células microbianas que eucariotas. El tracto gastrointestinal es el área de superficie más grande del cuerpo y se encuentra en constante exposición a estos microorganismos vivos. La simbiosis existente, demostrada por la falta de respuesta proinflamatoria contra bacterias comensales, implica la presencia de líneas de comunicación claramente definidas que contribuyen al mantenimiento de la homeostasis del hospedador. Así, las alteraciones en la flora intestinal parecen tener un papel en la patogénesis y la progresión de varias enfermedades hepáticas y gastrointestinales. Esto ha convertido su modificación selectiva en un área de interés terapéutico.
La traslocación bacteriana se define como el paso de bacterias y/o sus productos desde el intestino a los ganglios linfáticos mesentéricos. Por tanto, las alteraciones en la microbiota intestinal han mostrado su importancia, al menos parcialmente, en la patogénesis de varias complicaciones que surgen en la enfermedad hepática en fase terminal, tales como la encefalopatía hepática, la hipertensión portal y la peritonitis bacteriana espontánea.
En esta revisión se resume, por un lado, cómo la enfermedad hepática puede alterar la composición habitual de la microbiota intestinal, y por otro, cómo esta alteración contribuye al desarrollo de complicaciones en la cirrosis.
The large bowel is the most densely populated natural environment known. It contains roughly 1014 bacterial cells,1 which means that it has around 10 times more microbial than eukaryotic cells. The gut microbiota is a diverse population of live microorganisms, 99.1% of which is made up of approximately 1000 different bacterial species,2 which are highly susceptible to environmental and pathophysiological changes,3 while the rest consists mainly of Achaea, with only 0.1% of eukaryotic and viral origin.4 The total genome of the microorganisms that form part of the microbiota, called the microbiome (around 60,000 genes), is around 100 times larger than the coded human genome5 and provides functions that have not evolved in humans.6 We can consider our genome, therefore, as the sum of our genes and those that belong to the billions of microorganisms that form part of our microbiota.7 The distribution of microorganisms varies from 10 to 102bacteria/mL between the stomach and duodenum, from 102 to 108bacteria/mL between the jejunum and ileum, and from 1012 to 1013bacteria/mL in the colon.8
The gut microbiota plays several important roles in the health of the host, complementing nutritional needs through the breakdown and absorption of complex carbohydrates from the diet that human enzymes cannot digest, and synthesising some essential substances, such as vitamin K.9,10 They also help to maintain the integrity of the intestinal epithelial barrier by producing short-chain fatty acids, such as butyrate and propionate; short-chain fatty acids are the main source of energy for colon epithelial cells–they aid epithelial restitution11 and contribute to the maturation of the host's immune system.12 These organisms also protect the host against pathogenic microorganisms by competing for adhesion sites and nutrients, and by producing antimicrobial agents.
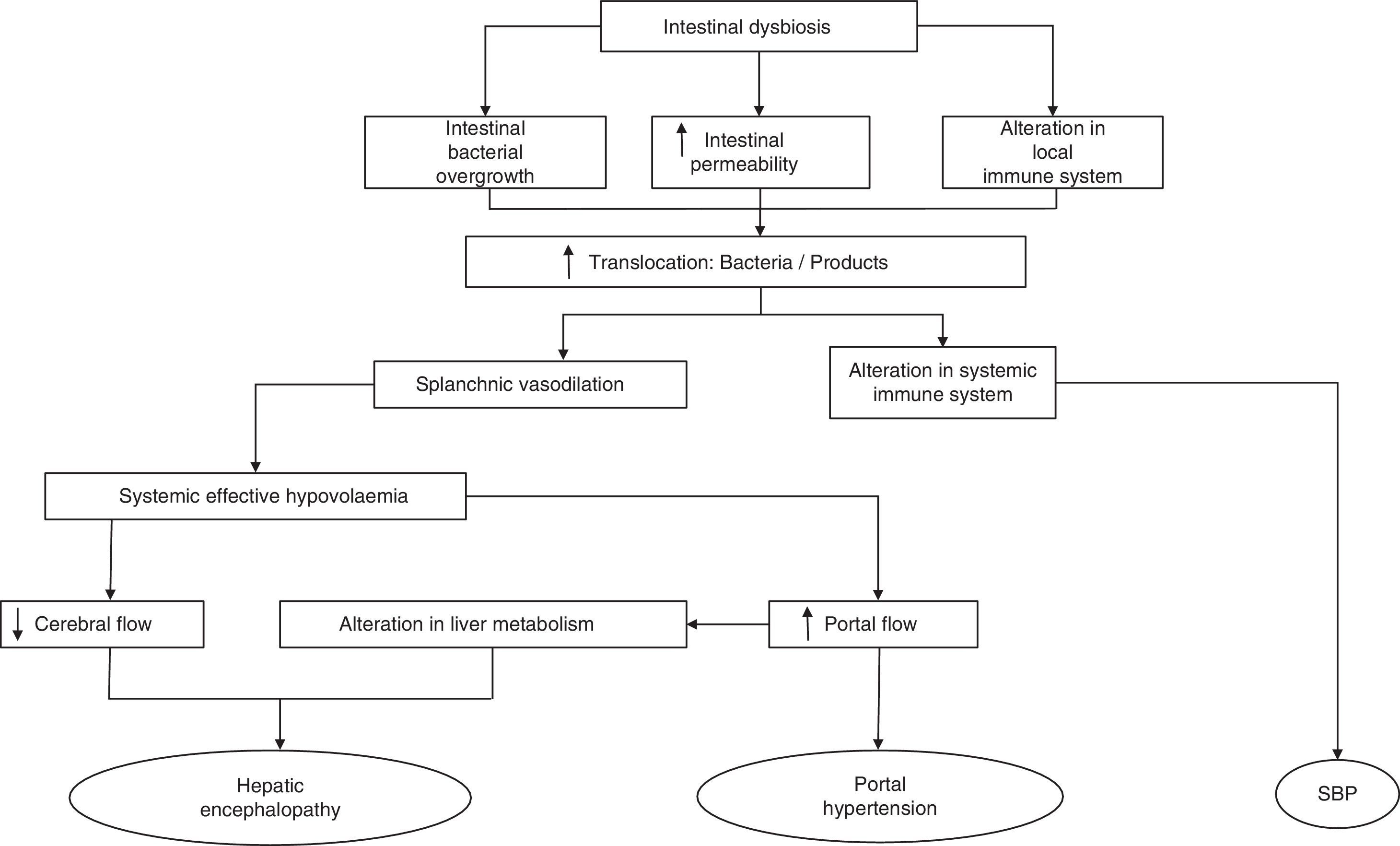
Cirrhosis is characterised by histological distortion of the liver with the presence of regenerative nodules that cause portal hypertension. This in turn alters small bowel motility, inducing small intestinal bacterial overgrowth (SIBO).13 Specifically, cirrhotic patients present an overgrowth of potentially pathogenic bacteria (e.g. gram-negative species) and a decrease in autochthonous bacterial families. The close relationship between the most common complications that arise in cirrhotic patients and the gut microbiota has come under much scrutiny in recent years, and has highlighted the importance of constant communication between the intestine and the liver in the management of patients with cirrhosis.14 Complications like hepatic encephalopathy (HE), spontaneous bacterial peritonitis (SBP) and variceal bleeding in cirrhotic patients are directly caused or exacerbated by the translocation of enteric flora or their products to extra-intestinal territories.
Bacterial translocation in cirrhosisBacterial infections are a common complication in patients with decompensated cirrhosis, with an incidence at admission or during hospitalisation of approximately 32% in the last prospective study.15 Of these, SBP is the most common, induced in up to 70–80% of cases by gram-negative aerobic bacteria of enteric origin, mainly Escherichia coli (E. coli) and Klebsiella pneumoniae.16–18
The most common mechanism to explain the passage of bacteria or their products from the intestinal lumen to the mesenteric lymph nodes (MLN)19 and other extra-intestinal locations is defined as bacterial translocation (BT).19,20 Our knowledge of the pathogenesis of BT is based primarily on studies conducted in experimental models, given the obvious difficulty in obtaining MLNs from cirrhotic patients. BT is defined as the presence of a positive MLN culture. Based on this criterion, one study found it to be present in approximately 50–60% of rats with CCl4-induced cirrhosis and presence of ascites and in only 0–10% of control animals.21 In recent years, the concept of BT has been extended to include not only the passage of bacteria20,22–25 but also of bacterial products across the intestinal barrier, being also capable of generating an immune response.23,26–28 One of the experiments that most clearly demonstrates the translocation of bacteria to MLNs was described by Teltschik et al.29 using E. coli marked with green fluorescent protein and administered orally to rats with cirrhosis. The bacteria could be observed not only in the intestinal lumen, but also in the MLNs and ascitic fluid (AF).
The mechanisms that affect the pathogenesis of BT are mainly SIBO, local and systemic immunological changes and increased intestinal permeability.30,31 Any of these alterations disrupt the clearance of endogenous bacteria from the portal and systemic circulation, making the intestine the main source of bacterial complications in cirrhosis (Fig. 1). Therefore, understanding the physiology of the interactions of the gut bacteria and the pathogenesis of BT may lead to new therapeutic strategies focused on modulating the gut microbiota and aimed at improving or preventing episodes of BT and inflammation in cirrhosis.
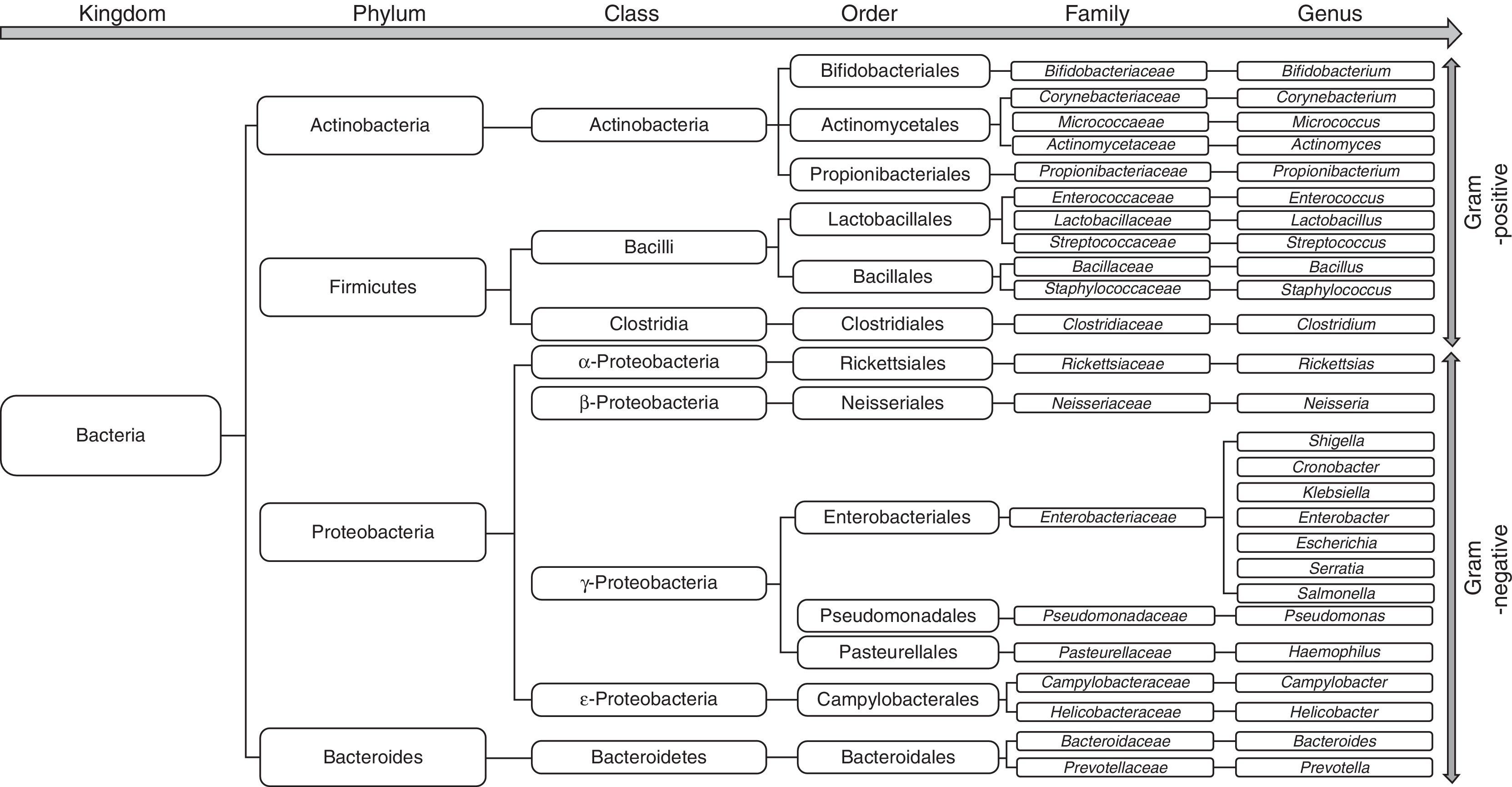
Changes in the gut microbiota in cirrhosisThe bacterial species that make up the microbiota mostly belong to the phyla Firmicutes, Bacteroidetes, Proteobacterias and Actinobacterias,32 the first 2 being the predominant phyla in all vertebrates33 (Fig. 2). Gram-negative aerobic bacteria translocate more often to the MLNs, even across a histologically-intact intestinal epithelium34,35, especially E. coli, K. pneumoniae, Pseudomonas aeruginosa and other enterobacteria and enterococci.35 Species such as E. coli most often cause bacterial infections in cirrhotic patients.36–39 Nevertheless, anaerobes exceed the number of aerobes by 100:1, thereby limiting the colonisation and excessive growth of other potentially invasive or pathogenic microorganisms. In fact, selective elimination of anaerobic bacteria facilitates SIBO and translocation of facultative bacteria.40
SIBO is described as a quantitative increase in the gut microbiota, and can refer to both the overall population and to a bacterial species in particular. SIBO has been arbitrarily defined as >105CFU/mL and/or the presence of colonic bacteria in upper jejunal aspirate.41 On this basis, the prevalence of SIBO in patients with cirrhosis varies from 48% to 73%.42–46 In cirrhosis, SIBO is one of the main factors that promotes BT, and the presence of BT in MLNs in experimental models is routinely associated with SIBO.30,47 Experimental studies have shown that SIBO is more common in cirrhotic rats with ascites and BT than in animals without BT.47 In contrast, BT rarely occurs in rats with absence of SIBO (0–11%), and is comparable to that observed in control rats. Another study—on this occasion in mice—showed a direct relationship between the density and composition of the bacteria present in a bowel segment and the number of viable bacteria of this strain present in MLNs.48
SIBO is more common in patients with severe liver damage49 than in healthy individuals, especially in those with more severe liver dysfunction50 and history of SBP.51In these studies, however, SIBO was estimated using the breath test, which is not a wholly reliable method of diagnosing this condition.30,44,46 Furthermore, the fact that BT was present in 50% of rats without SIBO shows that other factors as well as SIBO are involved in its pathogenesis. For example, in experimental ethanol-induced liver injury, BT increases before changes in the gut flora occur.52
SIBO in cirrhosis has traditionally been attributed to a decrease in small bowel motility and an increase in bowel transit time43,51,53–55 due, in part, to increased activity of nitric oxide (NO) synthesis51 and oxidative stress in the intestinal wall. Regardless of the aetiology, the transit defect seems to be relevant in these patients, as the administration of prokinetics, such as cisapride, considerably reduces the incidence of SIBO in patients and experimental animals.46 Other factors such as gastric acidity, defects in the local immune activity of the intestinal mucosa and a decrease in pancreatobiliary secretions in the colon of patients with cirrhosis seem to trigger a failure to control intestinal bacterial growth.56–58 The clinical implications of this failure are exemplified by the existence of pathological BT, a high risk of bacterial infections and decompensation of liver damage.24 The recently proposed association between proton pump inhibitors (PPI) and the development of SIBO44,59 and SBP60,61 has been called into question in a large cohort of cirrhotic patients.62 Hypoglycaemia and achlorhydria, however, have been observed in patients with cirrhosis, even without PPI treatment, resulting in a higher pH in the small intestine which, under these circumstances, has been associated with SIBO.63
Patients with cirrhosis are at high risk of dysbiosis due to the pathological interactions between the liver and gastrointestinal (GI) tract. When the gastric acid barrier is altered, increased numbers of oropharyngeal bacteria, including mainly gram-positive bacteria (Streptococcus spp., Staphylococcus spp., Micrococcus spp., Lactobacillus spp., Neisseria spp., Veillonella spp., Corynebacterium spp., Actinomyces spp., Fusobacterium spp.) are found in the stomach, duodenum and proximal jejunum. At the same time, when intestinal clearance is impaired due to reduced bowel motility, the concentration of colonic microbiota (including Enterobacteriaceae, Enterococcus spp., Pseudomonas spp., and Bacteroides spp.) increases in the small intestine.43 Furthermore, the decrease in bile acids in the intestine contributes to overgrowth of pathogenic intestinal microbiome species, including Porphyromonadaceae and Enterobacteriaceae.64 In fact, a recent study by Kakiyama et al. provides evidence that low bile acid levels entering the intestine could cause dysbiosis in patients with cirrhosis.65
Using culture techniques and pyrosequencing of faecal contents, a qualitative change has been identified in the gut microbiota in both animal models of cirrhosis and in human cirrhosis.66,67 The microbiome in cirrhotic patients has been associated with a decrease in the phylum Lachnospiraceae (particularly Clostridiae)67,68 and Bacteroidetes (mainly the Bacteroidaceae family)67 and an increase in the phylum Proteobacteria (mainly Gammaproteobacteria, and particularly Enterobacteriaceae).67,68 Curiously, the decrease in Clostridiae gave rise to an increase in the pro-inflammatory response68 and was negatively correlated with the Child–Pugh score.67
In a recent study, Fouts et al.66 investigated the dynamics of liver disease, BT and changes in the gut microbiome in a model of cholestasis induced by ligation of the common bile duct and toxic liver injury induced by CCl4 in mice. The authors observed an increase in the intestinal permeability and very early BT after the liver injury, as well as SIBO of aerobic and anaerobic bacteria, evident early after common bile duct ligation. The post-cholestasis microbiome was qualitatively similar to that of the control mice, while an increase in Firmicutes and Actinobacteria was observed in the model of CCl4-induced cirrhosis. This study also showed that BT precedes qualitative and quantitative changes in the gut microflora in a model of CCl4-induced toxic liver injury. The same was found to be true in a model of alcoholic liver disease.52
In a recent study, Qin et al.69 drew up a catalogue of genes of the gut microbiota in samples from patients with cirrhosis and healthy controls. Bacteroidetes and Firmicutes were the most common phyla in both study groups. However, patients with cirrhosis presented fewer Bacteroides and more Veillonella, Streptococcus, Clostridium and Prevotella compared to controls, raising the possibility that alteration of the microbiota could be a cause of cirrhosis. In the same study, the authors selected 15 microbial genes as biomarkers to create a discrimination index between patients with cirrhosis and controls.
Clinical consequences of changes in the microbiome of cirrhotic patientsThe clinical course of cirrhosis is often complicated by the development of GI haemorrhages, HE, renal failure or SBP, resulting in a deterioration in liver function and poor patient prognosis. Alterations in the gut microbiome can also affect the development and evolution of these complications.70 A recent study reported a change in the ratio between autochthonous and non-autochthonous flora in patients with cirrhosis. This ratio was higher in the control patients (2.05), followed by patients with compensated (0.89) and decompensated cirrhosis (0.66) (p<0.0001); it was negatively correlated with the endotoxin values.71 It is therefore important to understand the profile of these microorganisms in order to develop strategies to modify the course of the disease.
Hepatic encephalopathyHE is a serious, progressive neuropsychiatric disease that presents in patients with advanced cirrhosis. It is present in 25% of patients with acute liver failure72 and is associated with lower survival.73,74
Several studies show that the gut microbiota is altered in cirrhotic patients with HE. More specifically, a quantitative change has been described in the ratio of Bacteroides/Firmicutes, with potentially pathogenic bacteria (Enterobacteriaceae and Streptococcaceae)67,75 prevailing over beneficial populations (Lachnospiraceae).75 Liu et al.76 showed significant overgrowth of potentially pathogenic E. coli and Staphylococcal spp. in the gut microbiota of cirrhotic patients with minimal HE. Other studies have shown that patients with cirrhosis and HE have a higher concentration of Enterobacteriaceae and Alcaligenaceae compared to control subjects and cirrhotic patients without HE.75 The study also shows that specific bacterial families (Alcaligenaceae, Porphyromonadaceae, Enterobacteriaceae) are strongly associated with cognitive function and inflammation in HE.75 Bajaj et al. also studied the relationship between the gut microbiota, inflammation and cognitive function in patients.75,77,78 The increase in bacteria of the Alcaligenaceae family was significantly associated with poor cognitive performance, while the increase in bacteria of the Enterobacteriaceae family was associated with worsening of inflammation in the group of patients with cirrhosis. The Alcaligenaceae group is made up of proteobacteria that degrade urea to produce ammonia, which could explain its association with poorer cognitive function.79 Finally, in patients with HE, there are immune response markers that are highly correlated with components of the gut microbiome, suggesting possible synergy between inflammation, cognitive function and changes in the microbiome.80,81
Some probiotics and lactulose have been shown to be effective in the secondary prophylaxis of HE, with a lower incidence of diarrhoea and abdominal distension when probiotics are used.82 In fact, lactulose has a prebiotic effect on the gut flora,83 as it increases the Bifidobacteria population. In a recent study,84 patients with cirrhosis who had recovered from an episode of HE the previous month were randomised to take probiotic VSL#3 or placebo for 6 months. The study showed an improvement in liver function and a reduction in the levels of proinflammatory markers in patients who received probiotics, perhaps because of a reduction in the rate of BT due to the resulting dysbiosis, although the authors did not measure any BT indicators during the study.
Portal hypertensionSplanchnic arterial vasodilation is the main factor in the pathogenesis of hyperdynamic circulatory syndrome, which occurs in patients with cirrhosis and portal hypertension.85–87 It is mainly caused by an increase in the synthesis and release of NO, a potent vasodilator, by the endothelial and inducible forms of NO synthase into the splanchnic circulation. Although the activation of endothelial NO synthase is mainly due to shear stress, activation of the inducible form has been associated with exposure to bacteria and their products, and with an increase in NO release,23 which in turn further aggravates the circulatory abnormalities present in cirrhosis.88,89 Tumour necrosis factor-α (TNF-α) also appears to be involved in the pathogenesis of this syndrome.90,91 Oesophageal varices develop in response to portal hypertension and are clinically relevant due to the risk of rupture and bleeding: mortality is 30% per episode, with a 30–40% likelihood of re-bleeding within the first 6 weeks if not adequately treated.
The haemodynamic changes in cirrhosis seem to be a consequence of intestinal dysbiosis and migration of commensal bacteria to the intestinal cavity and systemic circulation.92 Pro-inflammatory cytokines contribute to hyperdynamic circulation, portal hypertension,93 impaired liver function and coagulation abnormalities.57 There is a causal relationship between BT-induced inflammation and portal hypertension, as shown in studies with animal models in which the administration of bacterial DNA or lipopolysaccharide (LPS) triggers an increase in portal pressure.94 In a study in cirrhotic patients randomised to receive selective intestinal decontamination with norfloxacin (400mg/twice daily) or placebo,95 only patients receiving antibiotic therapy showed an improvement in the hyperdynamic circulation. The ability of norfloxacin to selectively alter the gut microbiota seems to be responsible for the haemodynamic changes seen in patients. These results indirectly show the relationship between intestinal dysbiosis, the pro-inflammatory state caused by BT and worsening portal hypertension. In another recent study on rifaximin, a non-absorbable antibiotic, the authors found a similar reduction of portal pressure and fibrosis in vivo compared to the untreated group.96 In this study, the authors used a TLR4 knockout mouse model, which was therefore incapable of activating the TLR4 pathway in response to LPS. In these animals, exposure to LPS was not reflected in changes in portal pressure, angiogenesis, fibrosis or inflammatory state of the animals, showing that the effect of rifaximin on liver inflammation and portal hypertension is mediated by downregulation of the TLR4 pathway as a result of a decrease in the translocation of gram-negative bacteria.
Spontaneous bacterial peritonitisSBP is an infection of the AF in the absence of any other source of primary intra-abdominal infection, and is considered to be present if the neutrophil count in AF is >250 per microlitre,97 regardless of whether the microbiological culture is positive or negative.18,98 Patients with chronic liver damage have SIBO and a high rate of BT, evidenced by an increase in the presence of circulating bacterial DNA22 and antimicrobial antibodies.99 Bacteraemia and the increase in translocation of bacterial products to the hepatosplanchnic and systemic circulation lead to an increased pro-inflammatory response which, added to the host's increased susceptibility and failure to control BT, causes damage to organs far from the intestine. Intestinal permeability is also altered in cirrhotic patients with ascites, history of SBP and high Child–Pugh score.100
Intravenous broad spectrum therapy should be started immediately upon diagnosis of SBP in patients in whom there is clinical suspicion. SBP is generally treated with a 5-day course of third-generation cephalosporins, such as cefotaxime.101–104 In a randomised, controlled study, oral ofloxacin was as effective as intravenous cefotaxime in a sub-group of patients with SBP and absence of other risk factors.39 Patients who survive an episode of SBP have a 1-year recurrence rate of around 70%. In this respect, selective intestinal decontamination of the gram-negative microbiota with norfloxacin reduces the risk of SBP recurrence to 20% in 2 years.105 Our group has similarly shown—in both patients with cirrhosis and in experimental models—a reduction in the rate of translocation of bacterial products by norfloxacin,26,106 as well as its direct modulatory effect on the BT-associated inflammatory response.68,107 However, some authors have described an increase in the frequency of infections due to quinolone-resistant bacteria (both gram-negative and gram-positive) and multiresistant bacteria in patients treated with norfloxacin; this has been associated with failure of the antibiotic therapy to control the infection and increased mortality.108
Some studies have evaluated therapeutic alternatives to the use of norfloxacin in patients with cirrhosis with a risk of suffering an episode of SBP. For example, in an experimental model of rats with CCl4-induced cirrhosis, administration of trimethoprim-sulfamethoxazole delayed the development of ascites and reduced the incidence of gram-negative BT,109 and as such is a good alternative to norfloxacin. Pro-inflammatory cytokines are involved in the process of BT. Administration of anti-TNF significantly reduces BT in a rat model of cirrhosis.110 However, this method of reducing the pro-inflammatory response did not increase the risk of infection. Pentoxifylline is also an anti-TNF drug with an efficacy similar to norfloxacin in the prevention of BT and SBP in rats with CCl4-induced cirrhosis and ascites.111 This is in line with a study on experimental CCl4-induced cirrhosis in rats, which showed that prolonged oxidative stress in the intestine, accompanied by changes in the composition of the gut microbiota, might facilitate translocation across the mucosa, resulting in complications such as SBP.112 In fact, the administration of pentoxifylline, but not norfloxacin, reduces oxidative stress in the caecal mucosa.
Numerous studies have focused on preventing or reducing the rate of BT by modifying the gut microbiota with probiotics, both in experimental models and in patients with SBP. However, the results are still contradictory. In a rat model of experimental cirrhosis and ascites,113Lactobacillus strain GG failed to prevent BT and AF infection. In another study, Lactobacillus johnsonii La1 combined with an antioxidant given to rats with CCl4-induced cirrhosis114 reduced the percentage of BT and the number of intestinal enterobacteria compared with untreated control rats, although the same group later reported that L. johnsonii La1 alone, without the antioxidant, had no effect.115 A double-blind study116 was also conducted in patients with cirrhosis who had recovered from a previous episode of SBP or who had a high risk of presenting SBP. The patients were given norfloxacin (400mg/day) with probiotic capsules (Enterococcus faecalis, Clostridium butyricum, Bacillus mesentericus JPC, Bacillus coagulans) or norfloxacin with placebo and the number of SBP episodes within a 6-month period was determined. The results were similar in both groups, calling into question the efficacy of adding probiotics. In contrast, the use of Bifidobacterium pseudocatenulatum CECT7765 has shown a beneficial effect in reducing the permeability of the intestinal barrier and BT in a model of experimental cirrhosis.117 In a recent study with a rat model of experimental CCl4-induced cirrhosis, administration of probiotic VSL#3118 decreased both BT and the pro-inflammatory state and increased ileal occludin expression, showing that the administration of the probiotic improved the integrity of the intestinal barrier.
ConclusionsThe importance of the interaction between the intestine and the liver in the development of bacterial complications in cirrhosis has been clearly demonstrated. Changes in the composition of the microbiota alter intestinal homeostasis, leading to the translocation of bacterial antigens that induce a pro-inflammatory response, damaging the host's defence mechanisms and facilitating production of soluble mediators that complicate the liver disease.
The use of antibiotics continues to be an effective strategy for the management of these complications. However, other alternatives are emerging in the attempt to prevent the adverse impact of antibiotic therapy on the composition and function of the microbiota, given its importance in maintaining intestinal homeostasis. These strategies include the use of previous formulations, probiotics or symbiotics, and experimental faecal transplantation aimed at re-establishing homeostasis in patients with decompensated cirrhosis. However, in contrast to the abundant clinical evidence for the efficacy and safety of the use of antibiotics in these patients, data on the efficacy of these other strategies come mainly from individual experimental studies.119
A deeper understanding of the alterations in the gut microbiota and their influence on the host's immunobiology will enable different strategies to be improved in the coming years that will allow implementation of more personalised treatments for the management of chronic liver disease.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Gómez-Hurtado I, Such J, Francés R. Microbioma y traslocación bacteriana en la cirrosis. Gastroenterol Hepatol. 2016;39:687–696.