Drug-induced liver injury (DILI) is a leading cause of liver failure and an important safety issue in drug development. Thalidomide is nowadays used for the treatment of several conditions including multiple myeloma (MM). Several adverse effects have been described but liver toxicity was seldom reported. We describe a case of thalidomide-induced hepatitis in a man treated for MM. The clinical setting and temporal association between the start of the drug and liver injury allowed the assumption of the causative role of thalidomide. As its clinical indications expand we wish to increase awareness of a new potential side effect of thalidomide. A short review on thalidomide-induced liver injury is also presented.
Las lesiones hepáticas inducidas por medicamentos (LHIM) son una importante causa de insuficiencia hepática y un problema de seguridad importante en el desarrollo de fármacos. Talidomida se utiliza en la actualidad para el tratamiento de varias patologías, entre ellas el mieloma múltiple (MM). Se han descrito varios efectos adversos de este fármaco, pero la toxicidad hepática es un efecto rara vez notificado. Se describe un caso de hepatitis inducida por talidomida en un hombre tratado por MM. El entorno clínico y la asociación temporal entre el inicio del fármaco y el daño hepático permitieron sospechar el papel causal de la talidomida. Puesto que se expanden las indicaciones clínicas de este fármaco, deseamos concienciar sobre el posible nuevo efecto secundario del mismo.
También presentamos una breve revisión bibliográfica sobre lesiones hepática inducidas por talidomida.
Used by pregnant women to treat morning sickness and also marketed as a sedative, thalidomide was the first medicine discovered to be highly teratogenic.1–3 Thought to exert its therapeutic effect through the modulation of cytokines, particularly tumor necrosis factor-alpha (TNF-α), its immunomodulatory, anti-inflammatory and antiangiogenic properties are currently under study in different clinical conditions.3 In 1998, thalidomide was approved for the treatment of erythema nodosum leprosum in Hansen's disease. It has also been used in the treatment of HIV-related wasting syndrome and tuberculosis-associated weight loss.3,4 Other diseases associated with increased TNF-α such as Rheumatoid Arthritis, Lupus, Multiple Sclerosis, Behcet's Disease and Crohn's Disease may benefit from the treatment with thalidomide.3,4 High-dose chemotherapy with subsequent autologous or allogeneic stem cell transplant has improved the outcome and survival of multiple myeloma (MM) patients, being this the standard of care for newly diagnosed patients under 65 years of age and good performance status.5,6
For elderly patients or those not suitable for transplant, the oral combination melphalan–prednisone (MP) was, for many years, the treatment of choice. The introduction of immunomodulatory drugs and proteasome inhibitor operated a change in the management of these patients, with melphalan–prednisone–thalidomide (MPT) being nowadays the standard for treatment.5–8 Thalidomide use is associated with several side effects. Somnolence, constipation, deep vein thrombosis and peripheral neuropathy are some of the most frequent.9 Liver failure is an unexpected event for thalidomide with increased liver function tests reported in 2.8 to 12.5% of HIV-seropositive patients submitted to this therapy in controlled clinical trials (n=68).10 Other hepatic events reported from uncontrolled investigational use of thalidomide in HIV and in other indications have included cholestatic jaundice, hepatitis, bilirubinaemia and bile duct obstruction. Nevertheless hepatotoxicity is considered to be very rare, with only 7 cases described in the literature (Table 1).
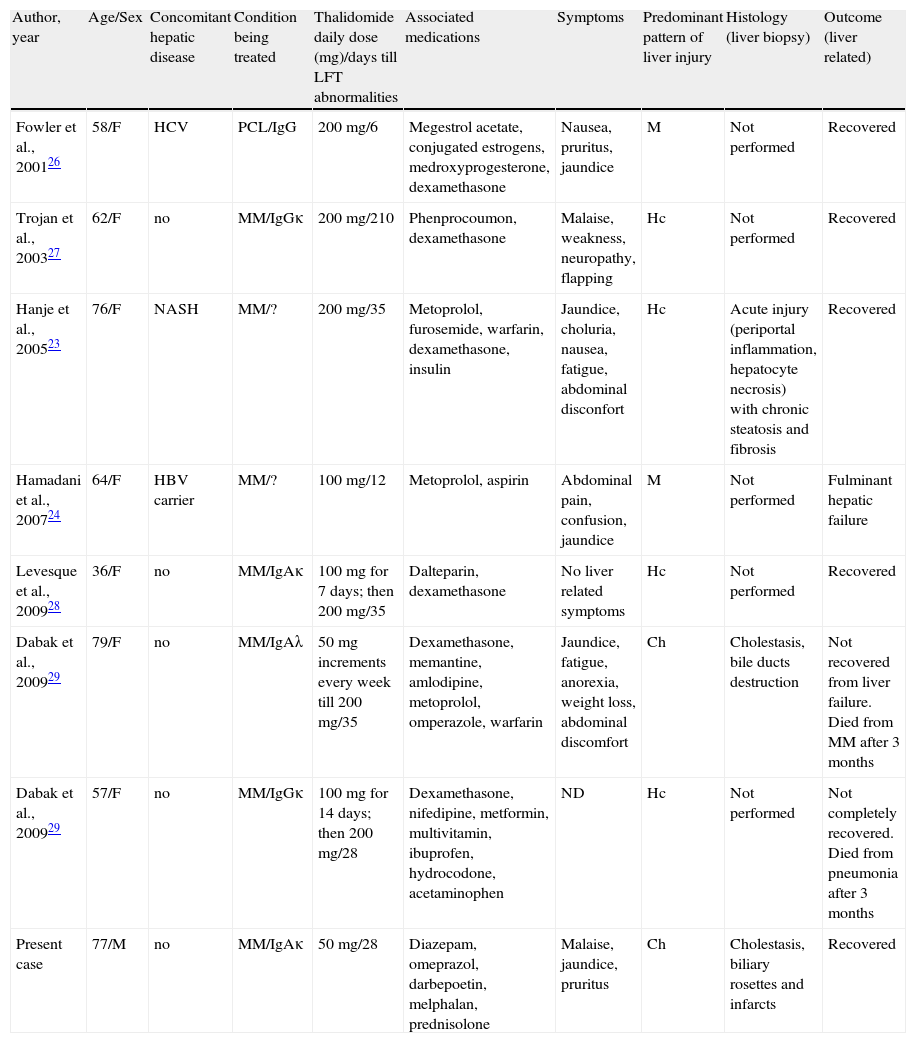
Summary of 7 previously reported cases of thalidomide-induced liver injury.
| Author, year | Age/Sex | Concomitant hepatic disease | Condition being treated | Thalidomide daily dose (mg)/days till LFT abnormalities | Associated medications | Symptoms | Predominant pattern of liver injury | Histology (liver biopsy) | Outcome (liver related) |
| Fowler et al., 200126 | 58/F | HCV | PCL/IgG | 200mg/6 | Megestrol acetate, conjugated estrogens, medroxyprogesterone, dexamethasone | Nausea, pruritus, jaundice | M | Not performed | Recovered |
| Trojan et al., 200327 | 62/F | no | MM/IgGκ | 200mg/210 | Phenprocoumon, dexamethasone | Malaise, weakness, neuropathy, flapping | Hc | Not performed | Recovered |
| Hanje et al., 200523 | 76/F | NASH | MM/? | 200mg/35 | Metoprolol, furosemide, warfarin, dexamethasone, insulin | Jaundice, choluria, nausea, fatigue, abdominal disconfort | Hc | Acute injury (periportal inflammation, hepatocyte necrosis) with chronic steatosis and fibrosis | Recovered |
| Hamadani et al., 200724 | 64/F | HBV carrier | MM/? | 100mg/12 | Metoprolol, aspirin | Abdominal pain, confusion, jaundice | M | Not performed | Fulminant hepatic failure |
| Levesque et al., 200928 | 36/F | no | MM/IgAκ | 100mg for 7 days; then 200mg/35 | Dalteparin, dexamethasone | No liver related symptoms | Hc | Not performed | Recovered |
| Dabak et al., 200929 | 79/F | no | MM/IgAλ | 50mg increments every week till 200mg/35 | Dexamethasone, memantine, amlodipine, metoprolol, omperazole, warfarin | Jaundice, fatigue, anorexia, weight loss, abdominal discomfort | Ch | Cholestasis, bile ducts destruction | Not recovered from liver failure. Died from MM after 3 months |
| Dabak et al., 200929 | 57/F | no | MM/IgGκ | 100mg for 14 days; then 200mg/28 | Dexamethasone, nifedipine, metformin, multivitamin, ibuprofen, hydrocodone, acetaminophen | ND | Hc | Not performed | Not completely recovered. Died from pneumonia after 3 months |
| Present case | 77/M | no | MM/IgAκ | 50mg/28 | Diazepam, omeprazol, darbepoetin, melphalan, prednisolone | Malaise, jaundice, pruritus | Ch | Cholestasis, biliary rosettes and infarcts | Recovered |
LFT=liver function tests; Ch =Cholestatic; Hc=Hepatocellular; M=Mixed (hepatocellular plus cholestatic injury); HCV=hepatitisC virus infection; NASH=nonalcoholic steatohepatitis; HBV carrier=Chronic carrier state for hepatitis B; MM=multiple myeloma; PCL=plasma cell leukemia; ND=no data available.
We here report a detailed clinical, biological and histological case of hepatotoxicity in a MM patient treated with thalidomide.
Case reportA 77 year-old Caucasian male with unremarkable medical history was referred to our institution in mid-March 2011 with acute renal failure. Clinical investigation revealed stage IB/ISS-3 multiple myeloma (IgAκ) and regular hemodialysis was started due to continued renal failure. By the end of March, the patient was discharged and referred to the Hematology outpatient clinic taking diazepam 5mg/day, omeprazol 20mg/day and darbepoetin alfa 0.45μg/kg per week. Liver function tests were by this time normal.
First-line treatment for MM with MPT (scheduled 28-day cycles) was started two weeks later on April 15th 2011. Melphalan 4mg/day was given for 7 days, prednisolone 80mg/day for 7 days and thalidomide 50mg/day continuously without dose increments for four weeks. No adjustments to his preexisting drug therapy were made.
Viral serological markers of infection for HIV 1 and 2, HBV and HCV were confirmed negative before the start of MPT treatment. An outpatient consultation was performed on May 4th, two weeks after the beginning of MPT treatment. At that time, patient discarded any significant side effects from the treatment. Immunoglobulin quantification, serum and urine paraprotein measurements confirmed good response to the treatment.
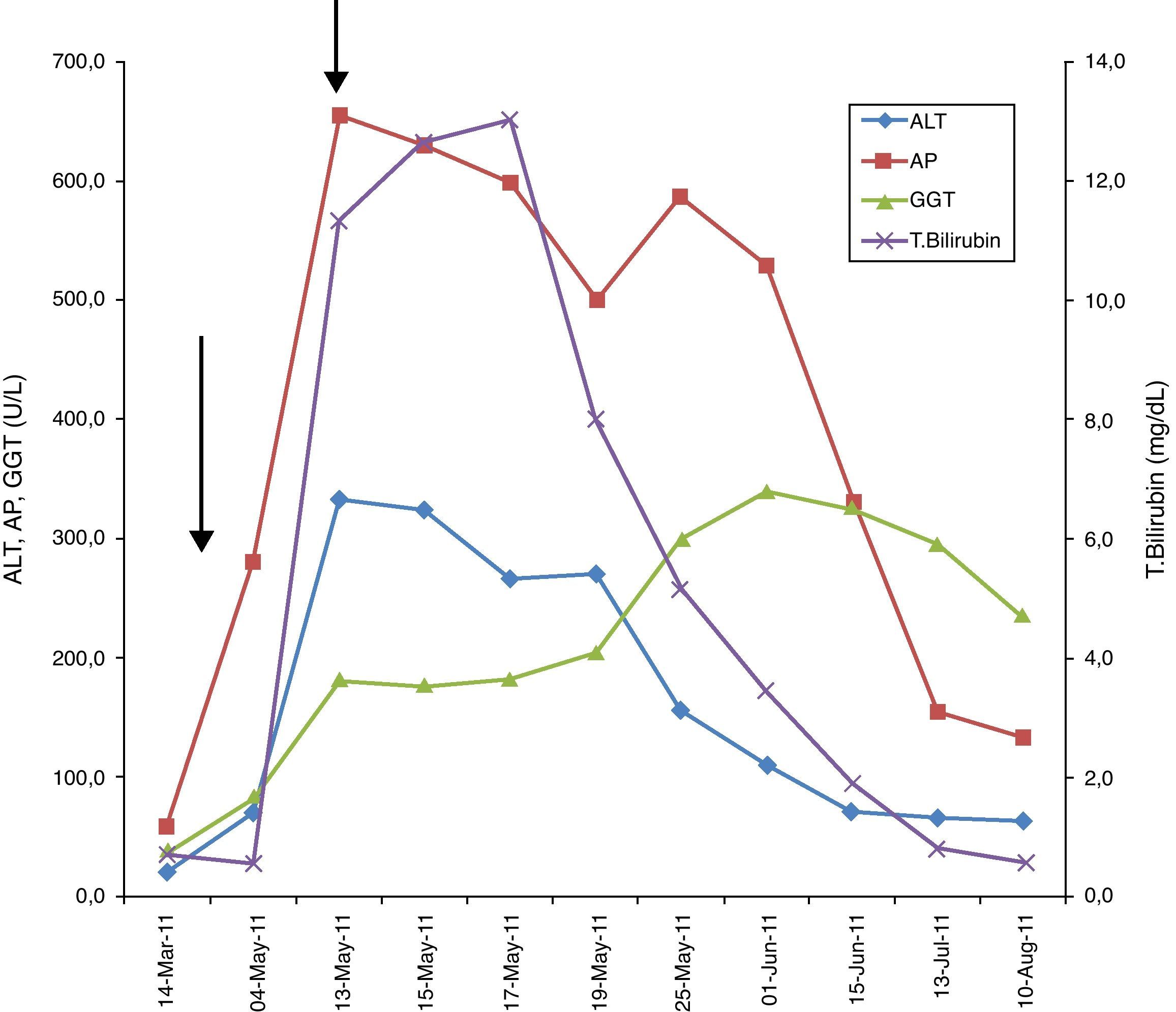
Laboratory workup at that time (two weeks after the beginning of MPT treatment) showed elevated ALT and AST (both 2× upper limit of normal [ULN]) and cholestasis (Alkaline phosphate 2× ULN and γ-glutamiltransferase 1.5× ULN). Bilirrubin levels were normal. On May 13th, four weeks after the start of thalidomide, the patient came to the emergency department with malaise and pruritus. The symptoms have been worsening progressively in the previous two weeks. The patient denied any fever. Physical exam was unremarkable except for jaundice. No rash was noted and we found no signs of chronic liver disease. Blood tests performed at the emergency department revealed elevated transaminases (aspartate aminotransferase, 271U/L (normal range (NR): 7–37); alanine aminotransferase 333U/L (NR: 7–37)) and bilirrubin (total, 11.35mg/dL (NR<1.4); direct, 6.36mg/dL (NR<0.4). Alkaline phosphate and γ-glutamiltransferase were both increased (4 times the upper normal value). Hepatic synthetic function was only minimally impaired with albumin of 27.8g/L (NR 35–50) and protrombin time 96%. No serum eosinophilia was found.
The diagnosis of acute hepatitis was established and the patient was admitted. Thalidomide was immediately discontinued because of suspected drug induced liver injury (DILI). All other medication remained unchanged.
The calculated R value (Council for International Organizations of Medical Sciences/Roussel Uclaf Causality Assessment Method – CIOMS/RUCAM system) based on the pattern of serum enzymes (first analytical test showing elevations above normal) defined the type of liver injury as cholestatic (R=2).11 He denied any history of alcohol intake, over the counter medications or herbal supplements use or extramarital sexual contacts. Abdominal doppler ultrasonography revealed neither hepatomegaly nor biliary duct dilation. There were no signs of portal vein thrombosis. Serology for hepatotrophic virus including hepatitis A (IgM negative; IgG positive), B (HBsAg, HBcAb and HBsAb were negative) and C (negative HCV antibodies) virus, Epstein–Barr (IgG positive; IgM negative), Herpes Simplex virus 1 and 2 (IgM negative; IgG negative for both) and Cytomegalovirus (IgM negative; IgG positive) were negative. Hepatitis B DNA was undetectable on polymerase chain reaction as were also hepatitis C and hepatitis E RNA.
Gamaglobulines including IgG and IgM were within the normal limits. IgA levels were elevated as expected because of the MM. Test for autoimmune liver disease including antinuclear antibodies (ANAs), ds-DNA, ANCAs, anti-smooth muscle (SM), anti-LKM and anti-mitochondrial (M2, M4 and M9) was found negative.
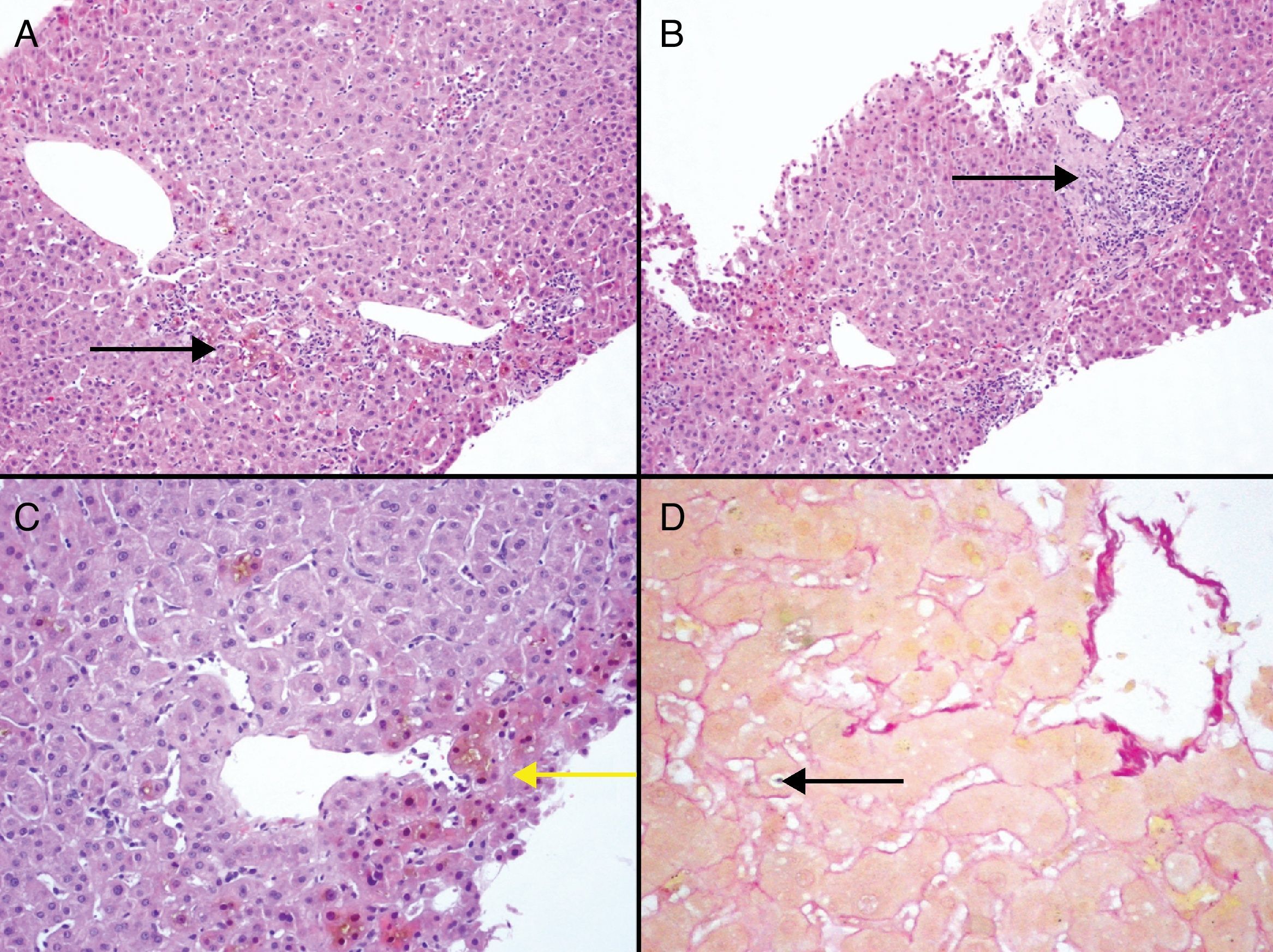
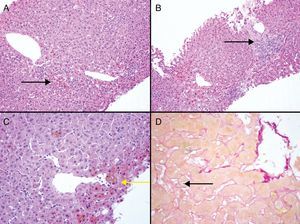
During the first week after thalidomide discontinuation, the patient's symptoms were improving but laboratory workup revealed aggravated hyperbilirubinemia (Fig. 1). With this fact, we decided to perform a liver biopsy that showed canalicular and hepatocellular cholestasis, mostly in acinar zone 3 with the formation of biliary rosettes and infarcts. At intralobular level, necroinflammatory lesions were identified (Fig. 2). After the peak of hyperbilirubinemia at May 17th, a gradual improvement in bilirrubin levels ensued. Transaminase levels were also decreasing but continued high (AST 7× ULN; ALT 5× ULN). By the time patient was discharged on May 25th, a clear trend to normalization was observed. (Fig. 1)
Several causality assessment methods based on specifically developed instruments for adverse drug reactions are indicated for establishing the diagnosis of DILI. Two of these are the Maria and Vitorino12 and the Naranjo scales.13
Our case totalized a score of 7 in Naranjo adverse drug reaction probability scale and 15 in the scale by Maria and Vitorino defining the association as probable. These two scales present important drawbacks which have recently been reported in the literature,14 when trying to generate categories of probabilities.
The CIOMS/RUCAM scale11 is currently the standard instrument for causality assessment of hepatotoxicity. The calculated CIOMS/RUCAM score for this case was 8 – probable adverse drug reaction.
The patient was kept without medication for MM in the month following discharge. He continued taking diazepam 5mg/day, omeprazol 20mg/day and darbepoetin alfa 0.45μg/kg per week.
Transaminase levels decreased over the next two months (June–July 2011) but not to normal laboratory reference ranges within this time period (ALT 2× ULN). Bilirrubin and alkaline phosphate were normal one month and a half after discharge but γ-glutamiltransferase continued elevated (5× ULN). As expected, immunoglobulin quantification revealed significant increase of IgA and the patient was started on oral melphalan–prednisone (MP) combination in mid-July. Thalidomide was not restarted. One month after the start of MP regimen, liver tests showed no recrudescence.
In late-August, five months after the MM diagnosis and three months passed since the episode of acute hepatitis, the patient died because of severe pneumonia.
DiscussionThalidomide is now widely used in the treatment of MM for elderly patients not suited for transplant. As stated, the adverse event profile associated with thalidomide includes numerous side effects but hepatic toxicity is extremely rare. We were able to retrieve seven case reports published in the English literature to date (Table 1) using the terms “thalidomide” AND “liver” AND “multiple myeloma”.
As in our case, all seven previous reported cases of thalidomide induced liver toxicity, occurred in patients treated for plasma cell neoplasms. There are currently no controlled data regarding the incidence of increased liver function tests or hepatic failure in oncology patients treated with thalidomide.
All previous reports of hepatotoxicity refer to female patients. This fact makes us wonder the possible involvement of a gender factor particularly if we consider that MM is slightly more frequent in men than in women. Curiously, our case report is exclusive, as it is the first concerning a male patient.
The age of patients reported, ranged from 36 to 79 years (mean 62). Three of them had concomitant liver disease (one hepatitis C chronic infection, one nonalcoholic steatohepatitis (NASH) and one chronic carrier of hepatitis B virus). The safety, efficacy and pharmacokinetics of thalidomide have not been specifically studied in patients with hepatic dysfunction.10
Our patient, like the remaining four, had no concomitant liver disease.
It cannot be assumed, by the small number of patients reported, the weight of previous liver disease in the increasing risk of injury by thalidomide but is seems clear that concomitant liver disease is not a requisite for toxicity.
The most frequent dose of thalidomide used in previous reports of DILI was 200mg/day, usually with a period of dose increment until full dose was reached.
In our patient a lower dose was used (50mg/day with no increments). This fact raises the hypothesis that toxicity is not dose related. These unpredictable reactions are mostly either idiosyncratic or immune-mediated, or both, but may also, in some cases, have a dose-related component.15 In fact the pathogenesis of idiosyncratic DILI is thought to be unpredictable and dose-independent but recently Lammert et al.16 found that a significantly greater proportion of oral medications prescribed at daily doses greater than 50mg were reported to more frequently cause liver failure and liver failure leading to death or liver transplantation.
An unresolved issue is the identification of patients who are more susceptible to this unpredictable, idiosyncratic form of injury.17
The potential mechanism of liver toxicity by thalidomide is not known. The drug itself does not appear to be hepatically metabolized to any large extent, but undergoes non-enzymatic hydrolysis into multiple products in biological fluids.18,19
The study by Kroger et al. in animal models (mice) support the hypothesis that the hepatotoxic effects of thalidomide may involve interference with the hepatic metabolism of NAD-adenoribosylation.20
We also do not know the importance of the disease being treated in the process of toxicity development. If we could show a relationship between thalidomide dose and toxicity, a reactive metabolite hypothesis could be supported as suggested for the pathogenesis of idiosyncratic DILI.16
In previously published case reports, the mean period of time till liver toxicity development was 46 days (ranging from 6 to 210). Our patient manifested the abnormalities in this same time frame.
Clinically, biochemically and histologically, DILI can simulate all forms of acute and chronic liver injuries.21
The pattern of liver injury related to thalidomide toxicity was not fully studied yet. Information related to this subject comes only from the case reports described. Four of them identified hepatocellular injury, two mixed (hepatocellular plus cholestatic) and only one referring predominant cholestatic injury as our case report.
Liver biopsy, for a number of reasons, was performed only in two previously reported patients. In those cases, as in ours, the histological findings corroborated the patterns of biochemical injury and the proposed drug related damage. In fact, routine liver biopsy is claimed not to be mandatory if DILI is suspected, especially if rapid improvement in liver tests is demonstrated after drug cessation, as there are no specific histological findings of this insult. Despite this fact, if an underlying liver disease is suspected despite negative blood tests, a liver biopsy may be valuable to exclude other diagnoses and it is an important tool to define imputability.21,22
In the report of thalidomide-induced hepatotoxicity23 by Hanje et al., liver biopsy made possible the discovery of previously undiagnosed NASH. As the authors discuss, whether preexisting liver disease puts patients at increased risk of thalidomide liver toxicity, remains to be elucidated.23
Recovery from liver injury after discontinuation of the drug was possible in 5 out of 7 cases. Two liver failure related deaths were described, one of them with a fulminant picture.24 This latter case report fulfill “Hy's rule” which predicts an incidence no lower than 10% of liver transplantation and/or death in drug-induced hepatocellular jaundice.15,25
Our patient had an uneventful outcome from liver toxicity, with symptoms resolving by week 2 despite the fact that he had a very slow biochemical recovery from liver insult.
Recovery following the withdrawal of the offending drug is frequent in DILI, especially in less-severe cases with early recognition of the causative agent.17
The slower subside of cholestatic and mixed lesions is described for other causes of DILI.21 This was the case for our patient. In fact, this type of damage is more prone to a chronic outcome than hepatocellular-type injury, albeit the severity appears to be greater in the latter.17
ConclusionDrug-induced liver injury remains a diagnostic challenge, as patients have a wide variety of clinical, analytical and histological presentations. Several chemotherapy drugs have the potential for producing liver damage, though they occur rarely and are deemed idiosyncratic, believed to be attributable to host response and possible immunologic mechanism. As thalidomide is being increasingly used for different conditions, we expect that this case report may increase physicians’ awareness for its possible hepatotoxic potential allowing early identification and proper management.
Conflicts of interestThe authors have no conflicts of interest to declare.