The appropriate selection of hepatocellular carcinoma (HCC) patients who are eligible for transarterial chemoembolization (TACE) remains a challenge. The ART score has recently been proposed as a method of identifying patients who are eligible or not for a second TACE procedure.
ObjectiveTo assess the validity of the Assessment for Retreatment with TACE (ART) score in a cohort of patients treated with drug-eluting bead TACE (DEB-TACE). Secondary objective: to identify clinical determinants associated with overall survival (OS).
MethodA retrospective, multicentre study conducted in Spain in patients with HCC having undergone two or more DEB-TACE procedures between January 2009 and December 2014. The clinical characteristics and OS from the day before the second DEB-TACE of patients with a high ART score (ART≥2.5) and a low ART score (ART 0–1) were compared. Risk factors for mortality were identified using Cox's proportional hazards model.
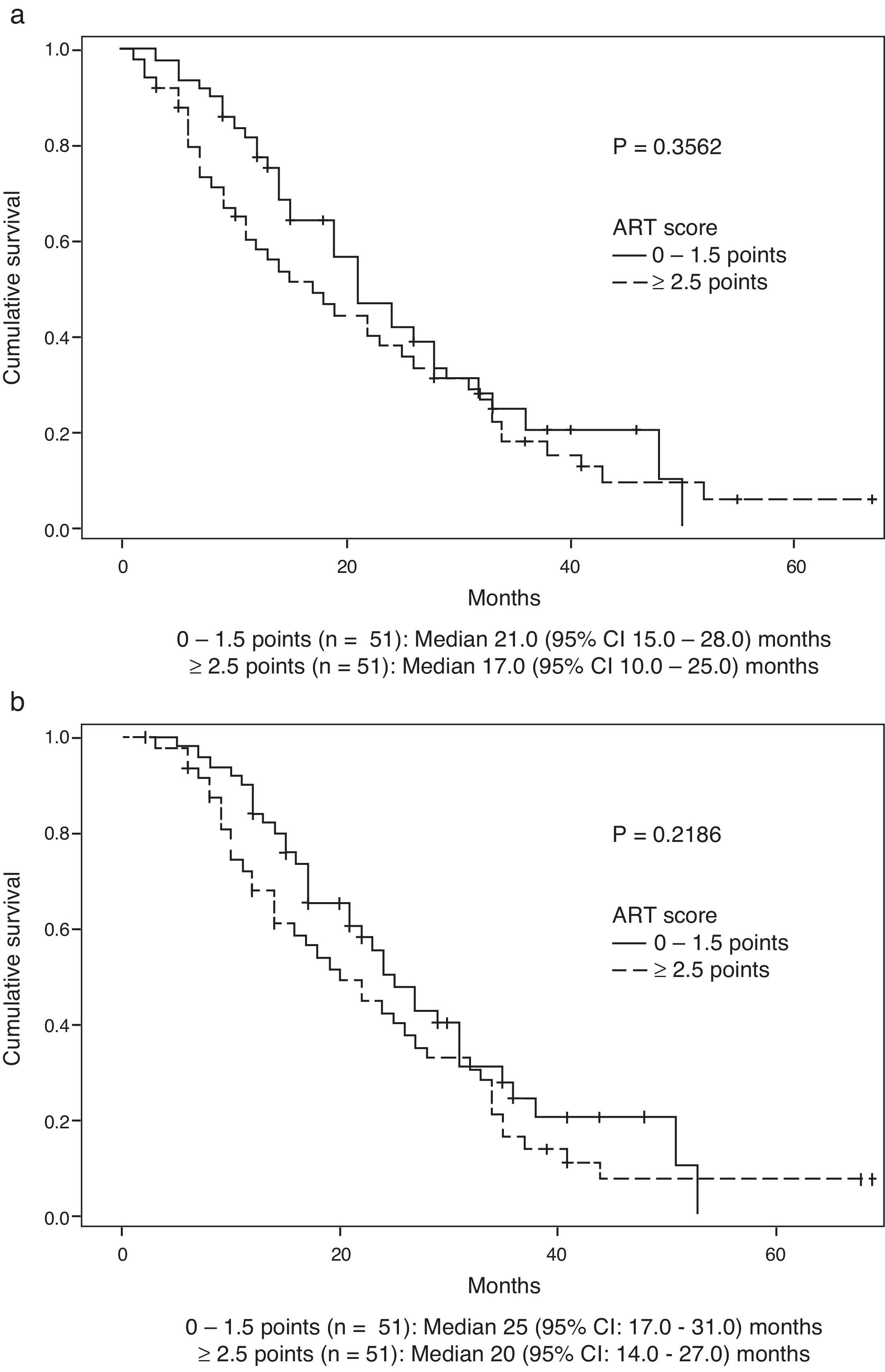
ResultsOf the 102 patients included, 51 scored 0–1.5 and 51 scored ≥2.5. Hepatitis C was more frequent in patients scoring ≥2.5. Median OS from the day before the second DEB-TACE was 21 months (95% CI, 15–28) in the group scoring 0–1.5, and 17 months (95% CI, 10–25) in the group scoring ≥2.5 (P=0.3562). Platelet count and tumour size, but not the ART score, were independent baseline predictors of OS.
ConclusionsThe ART score is not suitable for guiding DEB-TACE retreatment according to Spanish clinical practice standards.
La selección de los candidatos ideales con carcinoma hepatocelular (CHC) que más se benefician de realizar quimioembolización transarterial (TACE) sigue siendo un reto. Recientemente se ha propuesto el índice ART para seleccionar a aquellos pacientes tributarios o no de realizar un segundo procedimiento de TACE.
ObjetivoEvaluar la validez del índice ART en una cohorte tratada con TACE con partículas cargadas (DEB-TACE). Objetivo secundario: identificar los factores clínicos asociados con la supervivencia global.
MétodoEstudio retrospectivo multicéntrico español en pacientes con CHC tratados con≥2 DEB-TACE entre enero del 2009 y diciembre del 2014. Se compararon las características clínicas y la supervivencia global desde el día previo a la segunda DEB-TACE entre los pacientes con ART alto (ART≥2,5) y bajo (ART 0-1). Los factores de riesgo de mortalidad se identificaron usando el modelo de riesgos proporcionales de Cox.
ResultadosDe los 102 pacientes incluidos, 51 obtuvieron puntuación de 0-1,5 y 51 ≥ 2,5. La hepatitis C fue más frecuente en pacientes con puntuación ≥ 2,5. La supervivencia global mediana desde el día previo a DEB-TACE-2 fue de 21 meses (IC del 95%, 15-28) y de 17 meses (IC del 95%, 10-25) en los pacientes con ART 0-1,5 y ≥ 2,5, respectivamente (p=0,3562). Los factores basales predictores independientes de supervivencia fueron el recuento de plaquetas y el tamaño del tumor, pero no el índice ART.
ConclusionesEl índice ART no es adecuado para guiar el retratamiento con DEB-TACE según los estándares de práctica clínica española.
Despite the development of screening and surveillance programmes, more than 70% of cases of hepatocellular carcinoma (HCC) are not detected at initial stages.1 Asymptomatic patients with compensated liver disease and multifocal HCC are commonly classified as intermediate (B) stage according to the widely used Barcelona-Clinic Liver Cancer (BCLC) staging system,2 and are therefore ineligible for potentially curative treatments. Current guidelines recommend transarterial chemoembolization (TACE) as first-line treatment in this setting.3,4 However, BCLC-B patients are a highly heterogeneous population with different tumour burden and liver function,5 resulting in wide variation in the clinical benefit achieved from this treatment.5–7 Consecutive TACE procedures may induce deterioration of liver function. TACE retreatment should therefore be carefully balanced against radiologic tumour response.3 This is especially important since up to 90% of HCC develop in cirrhotic patients.8 Selection of candidates for repeated TACE treatment becomes therefore of utmost importance.
With this aim in mind, Sieghart et al.9 developed the Assessment for Retreatment with TACE (ART) score in 2013, based on results in a cohort of 102 Austrian HCC patients with HCC (hereafter, Austrian study). This score classifies HCC patients into two groups with different prognosis and likelihood of benefiting from a second TACE within a 90-day period, based on radiologic tumour response and impairment of liver function following the first TACE. The ART score was further validated prior to a third and fourth TACE.10 Despite the potential advantages of this tool, subsequent studies in Italian11 and Japanese12 cohorts have failed to demonstrate a prognostic impact, which suggests that factors associated with country-specific clinical practices and changes in tumour assessment and allocation procedures since 2013 are likely to affect the prognostic value of the ART-score.
Our study was designed to evaluate the prognostic value of the ART score according to current Spanish clinical practice standards, which includes allocating patients to treatment according to the BCLC staging system, homogeneous use of transarterial embolization with drug-eluting beads (DEB-TACE), and assessment of tumour response to locoregional therapy using modified Response Evaluation Criteria in Solid Tumours (mRECIST),13,14 as per current EASL-EORTC guidelines.3 To further understand the basis of this score, we analyzed the differences between both ART-based prognostic groups according to patient demographic and disease characteristics. As secondary objective, patient and disease characteristics associated to a greater risk of mortality after a second DEB-TACE were also analyzed.
MethodsDesignObservational, retrospective, multicentre study conducted in 12 Referral Hospitals in Spain on consecutive patients diagnosed with HCC (biopsy or by current non-invasive diagnosis criteria) who had undergone at least two DEB-TACE between January 2009 and December 2014. The study was approved by the Institutional Review Board of the Clinical University Hospital of Santiago de Compostela, Spain. All patients signed an informed consent to participate in the study.
Sample size calculationThe sample size for this study has been calculated taking into account that, as was done in the original study,9 it is desired to obtain a minimum sample size necessary to detect an effect on the HR (Hazard Ratio). The variables that were predictive in the aforementioned study were the tumour radiological response (HR=1.7), the AST increase greater than 25% (HR=8.4), and the increase in the Child–Pugh score in 1 (HR=2) or 2 points (HR=4.4), which were independently associated with overall survival in Sieghart's article. Thus, if it is desired to detect a HR=2 (sufficient to detect a change in the independent variable), with a confidence level of 95% with a power of 80% and estimating a proportion of 20% of censors, the minimum sample size which was obtained would be 102 patients.
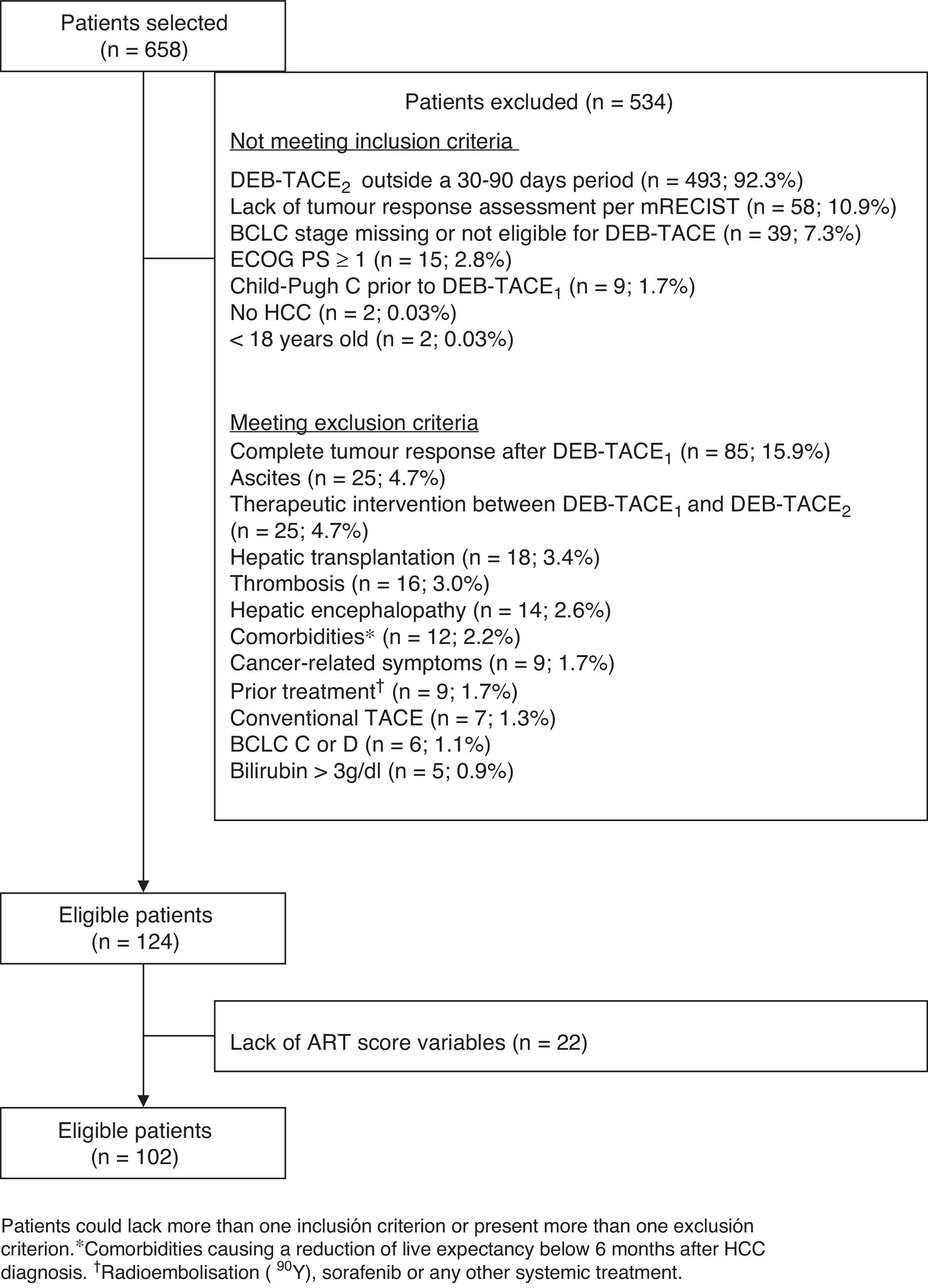
EligibilityPatients were eligible if they were aged ≥18 years at the time of diagnosis, had been classified as BCLC-B stage (or as BCLC-0 or BCLC-A when not eligible for ablation, resection or liver transplantation or experienced recurrence or failure after ablation or resection), with preserved liver function (Child–Pugh A or B prior to first TACE), were asymptomatic (Eastern Cooperative Oncology Group [ECOG] performance status 015), had undergone at least two DEB-TACE (hereafter DEB-TACE1 and DEB-TACE2) within a period of 30–90 days and had a radiologic assessment of tumour response after DEB-TACE1. Patients were excluded if prior to DEB-TACE1 procedure they presented comorbidities likely to reduce life expectancy <6 months after HCC diagnosis; had received prior conventional TACE, radioembolization (90Y), sorafenib or any other systemic treatment; had received any therapeutic intervention between DEB-TACE1 and DEB-TACE2; had achieved complete tumour response after DEB-TACE1 or presented any of the following: history of liver transplantation; BCLC stage C or D, poor liver function (Child–Pugh C), clinically evident ascites despite diuretic treatment, hepatic encephalopathy, bilirubin values ≥3g/dl, symptoms associated with cancer, portal vein thrombosis (or its branches) or hepatofugal flow. Among eligible patients, only those having all parameters needed to calculate the ART score were analyzed.
Data collectionData were retrospectively obtained from assessments routinely performed in clinical practice. Data collected at the time of diagnosis and prior to DEB-TACE1 (one day before) is displayed in Tables 1 and 2, respectively. Patient evaluation was performed 30±7 days after DEB-TACE, and included radiologic response assessment according to mRECIST13,14 using multidetector computed tomography (MDCT) or magnetic resonance imaging (MRI), laboratory tests and clinical assessment. Local data were obtained from clinical records and external monitoring was performed to review inconsistencies.
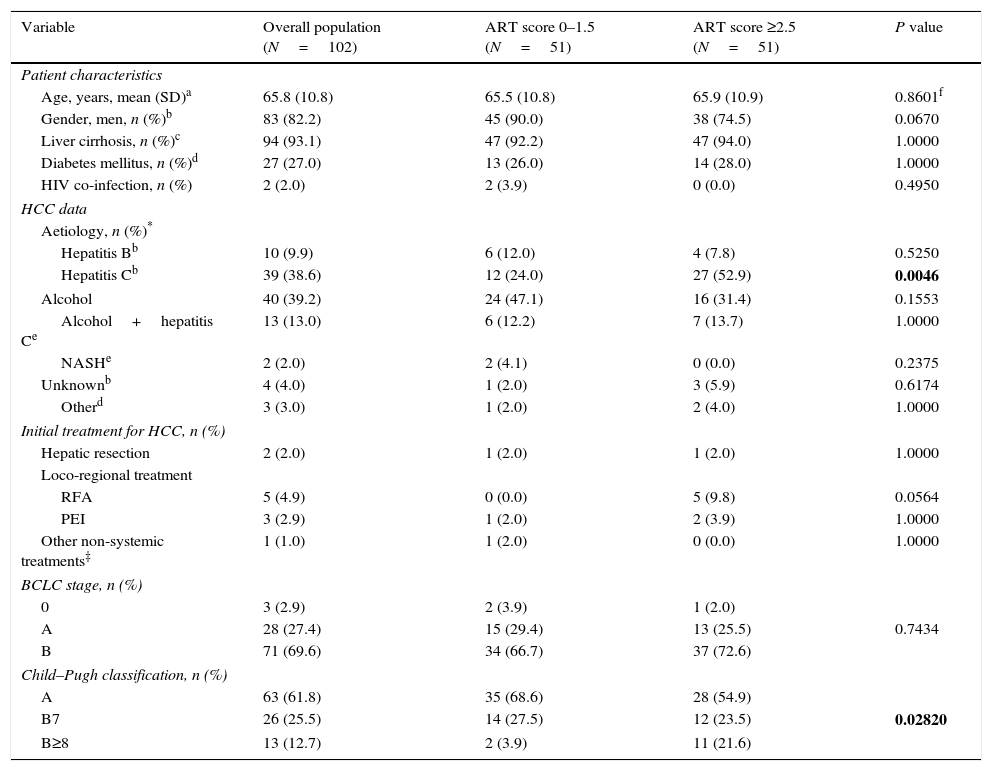
Patient characteristics and HCC data at the time of diagnosis and Child–Pugh classification prior to DEB-TACE1 (overall and according to ART score).
| Variable | Overall population (N=102) | ART score 0–1.5 (N=51) | ART score ≥2.5 (N=51) | P value |
|---|---|---|---|---|
| Patient characteristics | ||||
| Age, years, mean (SD)a | 65.8 (10.8) | 65.5 (10.8) | 65.9 (10.9) | 0.8601f |
| Gender, men, n (%)b | 83 (82.2) | 45 (90.0) | 38 (74.5) | 0.0670 |
| Liver cirrhosis, n (%)c | 94 (93.1) | 47 (92.2) | 47 (94.0) | 1.0000 |
| Diabetes mellitus, n (%)d | 27 (27.0) | 13 (26.0) | 14 (28.0) | 1.0000 |
| HIV co-infection, n (%) | 2 (2.0) | 2 (3.9) | 0 (0.0) | 0.4950 |
| HCC data | ||||
| Aetiology, n (%)* | ||||
| Hepatitis Bb | 10 (9.9) | 6 (12.0) | 4 (7.8) | 0.5250 |
| Hepatitis Cb | 39 (38.6) | 12 (24.0) | 27 (52.9) | 0.0046 |
| Alcohol | 40 (39.2) | 24 (47.1) | 16 (31.4) | 0.1553 |
| Alcohol+hepatitis Ce | 13 (13.0) | 6 (12.2) | 7 (13.7) | 1.0000 |
| NASHe | 2 (2.0) | 2 (4.1) | 0 (0.0) | 0.2375 |
| Unknownb | 4 (4.0) | 1 (2.0) | 3 (5.9) | 0.6174 |
| Otherd | 3 (3.0) | 1 (2.0) | 2 (4.0) | 1.0000 |
| Initial treatment for HCC, n (%) | ||||
| Hepatic resection | 2 (2.0) | 1 (2.0) | 1 (2.0) | 1.0000 |
| Loco-regional treatment | ||||
| RFA | 5 (4.9) | 0 (0.0) | 5 (9.8) | 0.0564 |
| PEI | 3 (2.9) | 1 (2.0) | 2 (3.9) | 1.0000 |
| Other non-systemic treatments‡ | 1 (1.0) | 1 (2.0) | 0 (0.0) | 1.0000 |
| BCLC stage, n (%) | ||||
| 0 | 3 (2.9) | 2 (3.9) | 1 (2.0) | |
| A | 28 (27.4) | 15 (29.4) | 13 (25.5) | 0.7434 |
| B | 71 (69.6) | 34 (66.7) | 37 (72.6) | |
| Child–Pugh classification, n (%) | ||||
| A | 63 (61.8) | 35 (68.6) | 28 (54.9) | |
| B7 | 26 (25.5) | 14 (27.5) | 12 (23.5) | 0.02820 |
| B≥8 | 13 (12.7) | 2 (3.9) | 11 (21.6) | |
Percentages refer to the valid population. Missing values:
n=2 for ART score ≥2.5. BCLC: Barcelona Clinic Liver Cancer; cTACE: conventional transarterial chemoembolization; HCC: hepatocellular carcinoma; HIV: human immunodeficiency virus; MELD: Model for end-stage liver disease; NASH: nonalcoholic steatohepatitis; PEI: percutaneous alcoholization; RFA: radiofrecuency ablation; SD: standard deviation
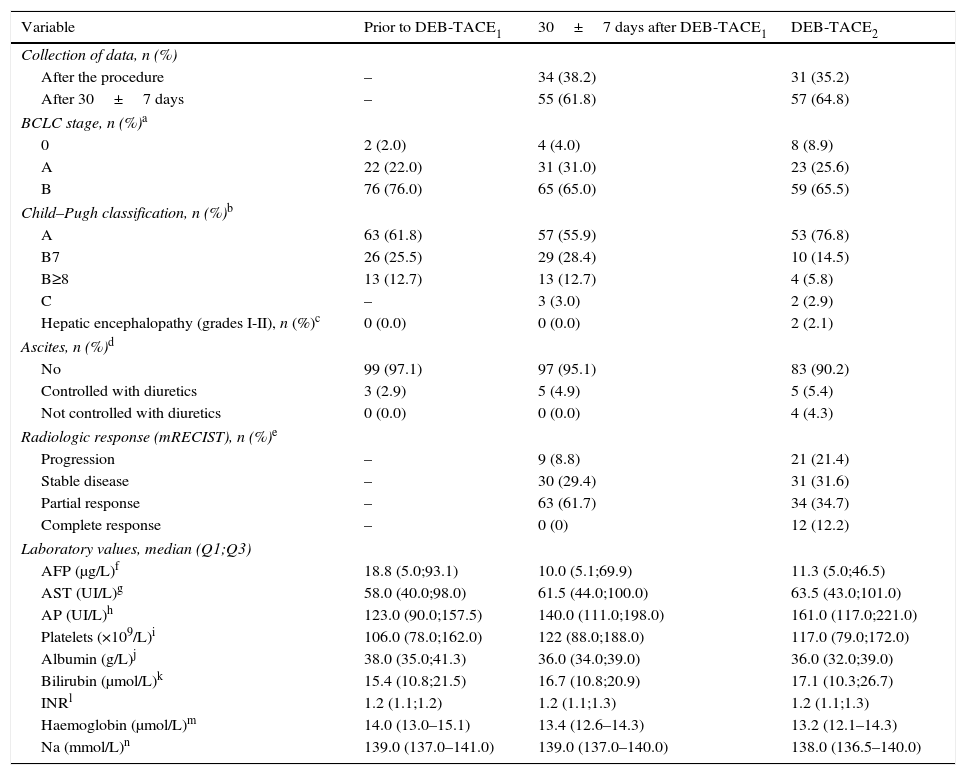
Patient and disease characteristics prior to DEB-TACE1 and after DEB-TACE1 and DEB-TACE2 (overall population).
| Variable | Prior to DEB-TACE1 | 30±7 days after DEB-TACE1 | DEB-TACE2 |
|---|---|---|---|
| Collection of data, n (%) | |||
| After the procedure | – | 34 (38.2) | 31 (35.2) |
| After 30±7 days | – | 55 (61.8) | 57 (64.8) |
| BCLC stage, n (%)a | |||
| 0 | 2 (2.0) | 4 (4.0) | 8 (8.9) |
| A | 22 (22.0) | 31 (31.0) | 23 (25.6) |
| B | 76 (76.0) | 65 (65.0) | 59 (65.5) |
| Child–Pugh classification, n (%)b | |||
| A | 63 (61.8) | 57 (55.9) | 53 (76.8) |
| B7 | 26 (25.5) | 29 (28.4) | 10 (14.5) |
| B≥8 | 13 (12.7) | 13 (12.7) | 4 (5.8) |
| C | – | 3 (3.0) | 2 (2.9) |
| Hepatic encephalopathy (grades I-II), n (%)c | 0 (0.0) | 0 (0.0) | 2 (2.1) |
| Ascites, n (%)d | |||
| No | 99 (97.1) | 97 (95.1) | 83 (90.2) |
| Controlled with diuretics | 3 (2.9) | 5 (4.9) | 5 (5.4) |
| Not controlled with diuretics | 0 (0.0) | 0 (0.0) | 4 (4.3) |
| Radiologic response (mRECIST), n (%)e | |||
| Progression | – | 9 (8.8) | 21 (21.4) |
| Stable disease | – | 30 (29.4) | 31 (31.6) |
| Partial response | – | 63 (61.7) | 34 (34.7) |
| Complete response | – | 0 (0) | 12 (12.2) |
| Laboratory values, median (Q1;Q3) | |||
| AFP (μg/L)f | 18.8 (5.0;93.1) | 10.0 (5.1;69.9) | 11.3 (5.0;46.5) |
| AST (UI/L)g | 58.0 (40.0;98.0) | 61.5 (44.0;100.0) | 63.5 (43.0;101.0) |
| AP (UI/L)h | 123.0 (90.0;157.5) | 140.0 (111.0;198.0) | 161.0 (117.0;221.0) |
| Platelets (×109/L)i | 106.0 (78.0;162.0) | 122 (88.0;188.0) | 117.0 (79.0;172.0) |
| Albumin (g/L)j | 38.0 (35.0;41.3) | 36.0 (34.0;39.0) | 36.0 (32.0;39.0) |
| Bilirubin (μmol/L)k | 15.4 (10.8;21.5) | 16.7 (10.8;20.9) | 17.1 (10.3;26.7) |
| INRl | 1.2 (1.1;1.2) | 1.2 (1.1;1.3) | 1.2 (1.1;1.3) |
| Haemoglobin (μmol/L)m | 14.0 (13.0–15.1) | 13.4 (12.6–14.3) | 13.2 (12.1–14.3) |
| Na (mmol/L)n | 139.0 (137.0–141.0) | 139.0 (137.0–140.0) | 138.0 (136.5–140.0) |
Percentages refer to the valid population. Missing values: Prior to DEB-TACE:
n=12. bn=33. cn=7. dn=10. en=4. fn=21. gn=8. hn=16. in=7. jn=9. kn=8. ln=28. mn=7. nn=7.
After DEB-TACE1: an=2, fn=14, hn=10, in=1, ln=18, mn=2, nn=4.
After DEB-TACE2: an=2, fn=19, hn=9, in=1, ln=20, mn=1, nn=4.
AFP: α-fetoprotein; AP: alkaline phosphatase; AST: aspartate aminotransferase; BCLC: Barcelona Clinic Liver Cancer; DEB-TACE: Drug eluting beads transarterial chemoembolization; INR: international normalized ratio; SD: standard deviation.
In accordance with EASL-EORTC guidelines,3 patients were allocated to DEB-TACE according to the BCLC staging system2 after multidisciplinary committees reviewed the cases. Local anaesthesia was administered. No antibiotic prophylaxis was used. All patients underwent baseline angiography of the celiac trunk, superior mesenteric and hepatic arteries using a peripheral arterial approach. Supraselective catheterization of segmental and non-segmental HCC arteries was performed with a 2.7–2.8 Fr microcatheter (inner diameter of 0.025–0.027 inches) to achieve complete obstruction of the nourishing arteries and avoid damage to the liver. The day before the procedure, DEB (BioCompatibles Ltd., UK) with a diameter ranging between 500 and 700μm were loaded with 75mg doxorubicin (Pharmacia-UpJohn, Barcelona, Spain) and mixed with 4ml of excipient. Prior to the procedure, contrast media was added (final volume was 10ml). The maximum dose of doxorubicin administered in a single DEB-TACE session was 150mg. Additional unloaded spheres were used to complete the embolization procedure if necessary.
Outcome variablesAccording to Sieghart et al.,9 deterioration of liver function was defined as an increase in Child–Pugh score (0, 1 and ≥2 points increase being scored as 0, 1.5 and 3, respectively) and in aspartate aminotransferase (AST) >25% (0 points for no increase; 4 points for increase). Radiologic tumour response was scored as 0 (lack of response, defined as “stable disease” SD or “disease progression” DP) or 1 (response, defined as “partial response”). Under this scoring system, two groups of patients are defined (0–1.5 points and ≥2.5 points), the former with a better prognosis than the latter and therefore benefiting from further DEB-TACE sessions. Differences in variables analyzed at the time of diagnosis between both ART groups were assessed. Overall survival (OS) was defined as the time from the day prior to the second DEB-TACE session until death or last follow-up. OS was calculated for both ART groups. Independent risk factors for mortality among patients and disease characteristics (baseline and pre-DEB-TACE) were also investigated.
Statistical analysisContinuous variables are expressed as mean (standard deviation [SD]) or as median with first and third quartiles (Q1; Q3) or interquartile range (IQR) when not normally distributed. Comparisons were made using the paired t test/ANOVA for parametric data or the U-Mann Whitney or Kruskal Wallis tests for non-parametric data. Categorical variables were expressed as n (%) and compared using Fisher's exact test. Median OS times and their 95% confidence intervals (CIs) were calculated. Survival curves were estimated using Kaplan–Meier analysis and compared using log-rank tests. All P-values were two-sided, with a P-value <0.05 being considered statistically significant. The effects of patient and disease characteristics (baseline and prior to DEB-TACE1) and ART criteria on OS were analyzed with Cox's proportional hazards models using a forward stepwise selection (entrance criterion P<0.1 and exit criterion P<0.05). Only data from patients where all variables were available were included in this analysis.
For these analyses, continuous variables were categorized, with laboratory values being grouped according to their quartiles. All statistical analyses were performed using SAS v. 9.4.
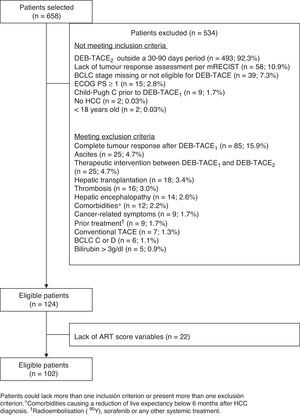
ResultsOf the 658 patients selected, 102 met all inclusion criteria and no exclusion criteria, and had all the variables required to calculate the ART-score (fig. 1). The main exclusion criterion among non-eligible patients (n=534) was not having had the DEB-TACE2 within 30 and 90 days after DEB-TACE1 (n=493; 88.7%). Patients included (82.2% of whom were men) had a mean age of 65.7±10.8 years. Most patients (93.1%) presented with liver cirrhosis and two thirds (69.6%) had BCLC stage B. Hepatitis C (38.6%) and alcohol consumption (39.2%) were the most frequent aetiologies (Table 1). Fifty-one (50.0%) patients had an ART score ≤1.5 and 51 (50.0%) had an ART score ≥2.5. Hepatitis C was more frequent in patients with an ART score ≥2.5 (52.9% vs. 24.0%; P=0.0046). No other differences were observed (Table 1).
Most patients (61.8%) were classified as Child–Pugh A before DEB-TACE1. Deterioration of liver function was greater in patients with an ART score ≥2.5 (Table 1). The median number of nodules was 2.7±1.4 (values missing: 5) and median diameter of the main nodule was 44.4±24.5mm, with no differences between both ART groups (P=0.4654 and 0.3132, respectively). The tumour was unilobar in 67.6% cases.
The reason for DEB-TACE1 was BCLC-B stage in 72% of patients (n=72; 5 values missing). Of the remaining 30 patients (BCLC-0 or A), 24 (24.0%) were not eligible for resection or ablation, and 4 (4.0%) had progressed after treatment. Patient and disease characteristics before DEB-TACE1 are shown in Table 2. From the time of diagnosis, 5 patients had progressed from BCLC-A to BCLC-B. Patient outcomes after DEB-TACE1 are shown in Table 2. Liver function of 6 (6%) Child–Pugh A patients worsened, and 3 patients progressed to Child–Pugh C. No cases of encephalopathy were observed. Two patients developed ascites, which was successfully managed with diuretics. Partial response was achieved in 63 (61.7%) patients, while 9 patients progressed. Laboratory values remained similar.
The reason for DEB-TACE2 was radiologic tumour response considered to be “partial” or “stable disease” in 90 (88.2%) patients, and treatable progression of the disease in 12 (11.7%) patients. Mean time between both DEB-TACE was 84.6±52.2 days (N=97). The evolution of the parameters analyzed is shown in Table 2. After DEB-TACE2 two cases of encephalopathy and 4 more of ascites not controlled with diuretics were reported. Complete response was achieved in 12 (12.2%) patients, while 21 (21.4%) patients progressed. Laboratory values remained similar.
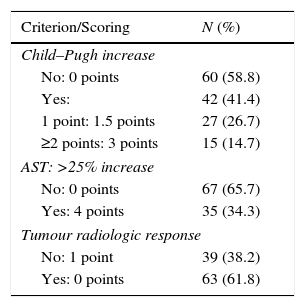
Table 3 shows the percentage of patients meeting any ART criterion and the score. Increased Child–Pugh after DEB-TACE1 was the criterion contributing to a higher score. An AST increase >25% was significantly higher among patients with hepatitis C (51.3% vs. 24.2% in those without; P=0.00941) but not in those with HCC caused by alcohol consumption (25% vs. 40.3%; P=0.13712).
Contribution of each ART criterion to the increase of the scoring (n=102).
| Criterion/Scoring | N (%) |
|---|---|
| Child–Pugh increase | |
| No: 0 points | 60 (58.8) |
| Yes: | 42 (41.4) |
| 1 point: 1.5 points | 27 (26.7) |
| ≥2 points: 3 points | 15 (14.7) |
| AST: >25% increase | |
| No: 0 points | 67 (65.7) |
| Yes: 4 points | 35 (34.3) |
| Tumour radiologic response | |
| No: 1 point | 39 (38.2) |
| Yes: 0 points | 63 (61.8) |
AST: aspartate aminotransferase.
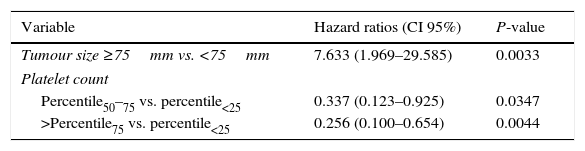
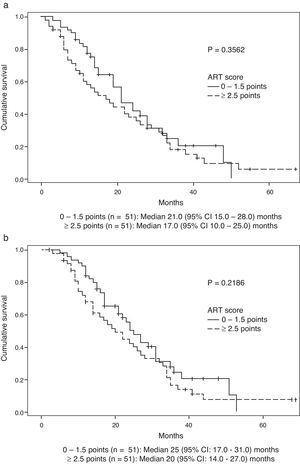
Survival data was available for 99 patients. Median (Q1:Q3) follow-up after DEB-TACE2 was 18 (9.0–29.0) months. During the observation period, 35 (71.4%) patients died in the group scoring 0–1.5 points and 42 (84.0%) in the group scoring ≥2.5 points (P=0.1530). Median OS (95% CI) was 21.0 (15.0–28.0) months in the former group and 17.0 (10.0–25.0) months in the latter (P=0.3562) (Fig. 2a). No significant differences were observed when survival was assessed from the day before DEB-TACE1: median OS (95% CI) was 25.0 (17.0–31.0) months in the former group and 20.0 (14.0–27.0) months in the latter (P=0.2189) (Fig. 2b). Multivariate analysis showed that of variables analyzed at baseline and prior to DEB-TACE1 (Tables 1 and 2), tumour size ≥75mm and platelet count below the 50th percentile (median of 103.5 [IQR 86]×109/L) were independent predictors of poor OS (Table 4).
Significant relative risk of mortality in multivariate analysis.
| Variable | Hazard ratios (CI 95%) | P-value |
|---|---|---|
| Tumour size ≥75mm vs. <75mm | 7.633 (1.969–29.585) | 0.0033 |
| Platelet count | ||
| Percentile50–75 vs. percentile<25 | 0.337 (0.123–0.925) | 0.0347 |
| >Percentile75 vs. percentile<25 | 0.256 (0.100–0.654) | 0.0044 |
Percentile50: median of 103.5 [IQR 86]×109/L.
The ART score was designed to provide an answer to a key issue in the treatment of BCLC-B patients undergoing TACE: the selection of patients who may benefit from retreatment. Despite the potential benefits of such a prognostic tool, the ART score has been shown to fail to differentiate patients with different prognoses in our setting. This is not surprising, since the ART score's lack of validity has also been highlighted in other studies conducted in Italy11 and Japan.12
When comparing the OS rates in our study and in the Austrian study,9 it is noteworthy that despite OS rates being similar in the group scoring 0–1.5 (median OS [95% CI] 21.0 0 [15.0–28.0] vs. 23.7 [16.2–32.2] months, respectively), the OS rate in the group scoring ≥2.5 was much higher in our cohort (median OS [95% CI] 17.0 [10.0–25.0] vs. 6.6 [4.5–8.8] months, respectively), thus reducing the difference in OS between the two prognostic groups. Similarly, the same was observed in the Japanese study,12 where median OS [95% CI] was 22.4 [13.1–31.7] and 16.5 [0–44.3] months in the groups scoring 0–1.5 and ≥2.5, respectively. Unfortunately, this data is not available for the Italian study. As stressed by other authors,11,12 this observation points to the existence of remarkable differences in patients considered eligible for TACE between the Austrian and subsequent studies, including ours, which may have led to the selection of patients for whom TACE would not be recommended according to current criteria.
Comparison of patient and disease characteristics between the group scoring ≥2.5 points in both studies is impossible, as these have not been reported in the Austrian study.9 Globally, in comparison with the training cohort in the Austrian study, patients undergoing DEB-TACE in our centres were BCLC-A in a greater proportion (27.4% vs. 11%), showed smaller tumour size (44.4±24.5mm vs. 55.8±29.0mm) and had received fewer prior therapies (10.8% vs. 27%), indicating less severity. On the other hand, viral hepatitis B or C was also more frequent (48.5% vs. 33.0%) while alcohol was less frequent (39.2% vs. 46.0%). No other differences were observed.
Besides the lack of difference in OS, the higher percentage of patients scoring ≥2.5 points in our cohort compared to the Austrian study (50% vs. 38%, respectively)9 is also noteworthy, which may also be at least in part due to differences in the populations analyzed. For instance, the higher percentage of AST increase >25% (34.3% vs. 28.0%, respectively) may be related to the higher incidence of hepatitis C in our cohort, as previously commented upon. The percentage of patients with increased Child–Pugh scores was also higher (41.2% vs. 29.9%, respectively). In any event, neither of these two criteria emerged as independent mortality risk factors, which may indicate that these elevations in markers of liver injury may be transient but, in any case, were not associated with poorer OS according to the multivariate analysis. With regard to tumour response, partial response rate was lower in our series (36.6% vs. 52.3% respectively), which is likely due to differences in assessing tumour response: while the radiologic criteria of the European Association for the Study of the liver (EASL)3 was used in the Austrian study,9 mRECIST13,14 was the only criteria used in our cohort. It is known that mRECIST may be more accurate in discerning the prognosis between patients with partial response and stable disease.16 The lower rate of tumour response was not associated to poorer OS in our series.
Another important difference with the Austrian study is their lack of a lower limit of 30 days between TACE procedures, which does not reflect current clinical practice and points to some patients presenting with multifocal tumours or having undergone suboptimal TACE. A second TACE in such a short period may seriously impact OS. However, no information is given about the reasons for TACE2 or the characteristics of patients needing a second TACE before 30 days after TACE1.
Our study has revealed that a platelet count below a median of 103.5 (IQR 86)×109/L and a tumour size >75mm were independent predictors of poor survival among HCC patients undergoing two DEB-TACEs. These variables were distributed between both prognostic groups and resulted in similar OS when analyzed from the day before DEB-TACE1. Tumour size was not related to mortality in the Austrian9 or the Italian11 studies in multivariate analysis using similar entry criteria. Although the effect of a low platelet count on mortality was not analyzed in these studies, our findings are not surprising in patients presenting with liver cirrhosis and portal hypertension. Both tumour size and platelet count have been related to poor prognosis in patients with HCC.17,18
Results of our study should be interpreted with caution given the limitations derived from its retrospective nature. However, it provides data from a higher number of patients than other studies analysing the value of the ART score as a prognostic tool (51 patients in the Italian study and 44 in the Japanese study). Besides, it reflects current management standards such as the uniform use of DEB-TACE, which is associated with similar tumour response and 1- and 2-year survival compared to cTACE, but better tolerance.19 Only 19.7% patients in the Austrian study received DEB-TACE, and it is unknown how this procedure modality was distributed in both prognostic groups. The heterogeneity of TACE types was in fact a strong limitation of the Austrian study.9 Tumour response assessment was also homogeneous, following mRECIST criteria. It should be noted, however, that a central review was not performed, and treatment decisions were made according to protocols at each centre. Patient and disease characteristics of both prognostic groups are provided for the first time, allowing future comparisons, which may be relevant to understanding putative differences in OS.
In conclusion, the evidence provided by this study does not support the validity of the ART score as a prognostic tool suitable to guiding decision-making with respect to exposing patients to a second DEB-TACE, at least according to clinical practice standards in our setting. Tumour size >75mm seems to be associated with poorer OS; an adequate platelet count (above the median of 103.5×109/L) also seems to be relevant. These results highlight the importance of multidisciplinary teams for decisions regarding eligibility for DEB-TACE, appropriateness of retreatment or migration to other therapies.20–27
Conflict of interestsThe authors declare that they have no conflicts of interest.
The authors thank Beatriz Viejo, Ph.D. for medical writing assistance and David Calbet for statistical support. The study was sponsored by Bayer Healthcare Spain.