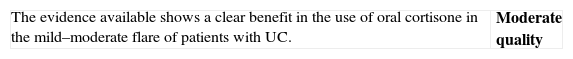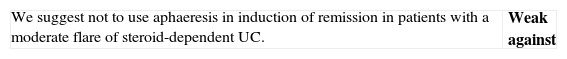Ulcerative colitis (UC) is a chronic inflammatory bowel disease of multifactorial aetiology that mainly affects the colon. There is no single pathognomonic criterion to define it, the diagnosis being based on a series of clinical, endoscopic and histological criteria, as well as on the exclusion of infectious diseases with similar symptoms.1 Both its extension and severity vary in time for all patients; hence, the definition of a specific clinical scenario demands taking both the severity and the extension of the disease into account, in accordance with the definitions suggested by the World Gastroenterology Organization (WGO) in the “Montreal Classification”.2 Moreover, the disease can remain in a situation of clinical remission, requiring the application of different treatment strategies in situations of activity or remission. It has been established that certain maintenance treatments quite significantly reduce the probability of new flares of activity.3 Even though it was described at the end of the XIX century, its frequency over different populations has changed notably, especially during the second half of the XX century, together with other immune diseases and with a clear sustained tendency to increase with social and economic development. Because it frequently starts in youth or even in childhood, and its mortality is low, its prevalence is considerably higher (at last 20 or 30 times its incidence).4 In Spain, epidemiological data suggest that up to the 1980s, it was an uncommon disease. However, over the past 30 years, it has reached incidence rates that are very similar to the ones described previously in north European countries and, in fact, in the most recent studies, data are completely similar.5
This disease has been treated with drugs, surgery or both. In the first half of the XX century, treatment procedures, such as sulfasalazine, were introduced empirically; but already in 1955 the first clinical randomized controlled trial was published and showed that hydrocortisone was superior to placebo in the treatment of moderate or severe UC flares.6 Up to 1980, observational studies of varying quality were predominant. Henceforth, numerous controlled clinical trials, epidemiological analyses, assessments of diagnostic measures and even complex genetic studies7 have been carried out with an ever more solid scientific methodology. Currently, there are numerous nutritional, pharmacological, surgical and monitoring and follow-up alternatives available.8,9 Their application over the broad range of clinical scenarios with such diverse individual and social circumstances is not always easy and is one of the reasons that justify the creation of these guidelines.
2Need for guidelinesAs this is a common, chronic disease that affects mostly young people and that has a increasing prevalence, the health, economic and social repercussions perfectly justify the need for tools to systematize the treatment of UC. However, we will point out other reasons for its creation in more detail.
In the first place, the professionals involved in this entity are quite numerous. A partial list would include a gastroenterologist, a surgeon, a primary care physician, a nutritionist, a psychologist, a nurse, a stomatherapist, a rheumatologist, an ophthalmologist and a dermatologist. The need for coordination between these professionals and the patient also justifies the need for a Clinical Practice Guidelines (CPG).
There is great evidence10 that the usual variability of clinical practice affects patients with inflammatory bowel disease (IBD) quite significantly, with important clinical, economical, and social repercussions. In fact, a recent European collaborative study reflected enormous differences in the total cost of the management of the disease, regardless of the country's per capita income, and, what is clinically most significant, they reflected great differences in the distribution of this cost: the proportion of patients treated surgically was very variable (in UC between 10 and 40%).11 This variability also justifies the need for a CPG.
There are already several publications bearing the word “guidelines” in their title, being the most important among them for their rigour and diffusion the British guidelines (British Society of Gastroenterology),12 the American guidelines13 (American College of Gastroenterology), the guidelines of the World Gastroenterology Organization,14 the guidelines of the “Asian Pacific” consensus group15 and the European guidelines16,17 (European Crohn and Colitis Organization). Although these publications, especially the latter, are of great quality and are based on the clinical evidence available, they are actually consensus documents that are more or less elaborate. To our knowledge, there is no CPG in the world created based on a structured and systematic methodology.18
One of the main objectives of the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (GETECCU) is to improve the care of patients with inflammatory bowel disease, and strategically, it has established the creation of CPGs that serve as an aid for healthcare professionals, patients, researchers and payers. In the first phase of the project, we focus on the treatment of inflammatory bowel diseases.
3Definitions- -
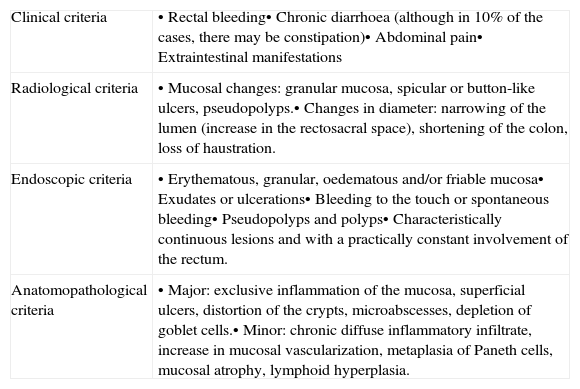
Target population: Patients of any gender and age who are diagnosed with UC according to the internationally accepted criteria by Lennard-Jones1 (Table 1). These criteria are accepted by both the WGO14 (World Gastroenterology Organization) and the ECCO16 (European Crohn and Colitis Organization).
- ∘
Complementary guidelines will be developed in the future to specify independent aspects that are relevant to the paediatric population, as well as special clinical scenarios.
Table 1.Lennard-Jones diagnostic criteria.
Clinical criteria • Rectal bleeding• Chronic diarrhoea (although in 10% of the cases, there may be constipation)• Abdominal pain• Extraintestinal manifestations Radiological criteria • Mucosal changes: granular mucosa, spicular or button-like ulcers, pseudopolyps.• Changes in diameter: narrowing of the lumen (increase in the rectosacral space), shortening of the colon, loss of haustration. Endoscopic criteria • Erythematous, granular, oedematous and/or friable mucosa• Exudates or ulcerations• Bleeding to the touch or spontaneous bleeding• Pseudopolyps and polyps• Characteristically continuous lesions and with a practically constant involvement of the rectum. Anatomopathological criteria • Major: exclusive inflammation of the mucosa, superficial ulcers, distortion of the crypts, microabscesses, depletion of goblet cells.• Minor: chronic diffuse inflammatory infiltrate, increase in mucosal vascularization, metaplasia of Paneth cells, mucosal atrophy, lymphoid hyperplasia. Source: Lennard-Jonnes.1
- ∘
- -
Ulcerative colitis (UC):
- ∘
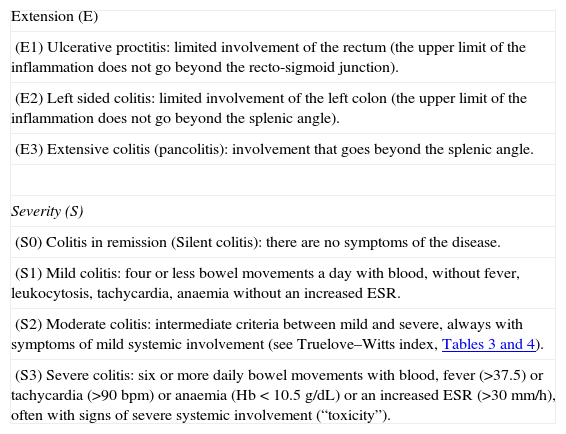
Severity criteria: The ones defined by the Montreal Classification,2 whose validity has recently been ratified by a group of experts19 (Table 2):
▪Ulcerative colitis
- •
Remission
- •
Mild
- •
Moderate
- •
Severe
- •
▪Confusing terms such as fulminant colitis or “very severe” will be avoided.
▪The differences between moderate-severe flare in the studies found in literature, especially pharmacological–biological studies, are not always well established (Box 1).
▪We will use the severity scale used in the Montreal Classification,2 which has actually been extrapolated from the index that has been used longer, the Truelove–Witts index6 (Table 3) that in some publications, particularly Spanish publications, has been modified in a semi-quantitative manner (Table 4). Actually, this index has not been validated formally in any study, and its use may be controversial20 (Box 2). In fact, there is no reference clinical index of activity, that is why it is necessary to be familiar with many of them in order to interpret the results of the studies.20,21 We provide the most important ones:
- -
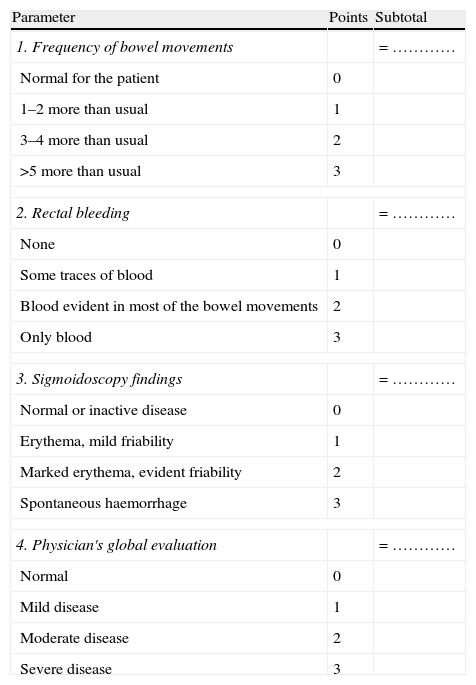
The Mayo Index (DAI)22 (Table 5)
Table 5.Mayo index (DAI).
Parameter Points Subtotal 1. Frequency of bowel movements = ………… Normal for the patient 0 1–2 more than usual 1 3–4 more than usual 2 >5 more than usual 3 2. Rectal bleeding = ………… None 0 Some traces of blood 1 Blood evident in most of the bowel movements 2 Only blood 3 3. Sigmoidoscopy findings = ………… Normal or inactive disease 0 Erythema, mild friability 1 Marked erythema, evident friability 2 Spontaneous haemorrhage 3 4. Physician's global evaluation = ………… Normal 0 Mild disease 1 Moderate disease 2 Severe disease 3 Global Mayo index assessment Symptoms Endoscopy Physician Total sum Mild 1–3 1 1 3–5 Moderate 3–6 1–2 2 6–10 Severe >6 >2 3 >10 - -Table 6.
Seo index.
Variable Assessment No. of bowel movements ×13= ≤4=1 5–7=2 ≥8=3 Blood in stools ×60= None or scarce=0 Present=1 ESR (mm/h) ×0.5= Total A (sum of previous) = Haemoglobin (g/dL) ×4= Albumin (g/dL) ×15= Total B (sum of previous) = Assessment of Seo index Score Remission or mild activity <150 Moderate-severe 150–220 Severe >220 Seo activity index; total A−total B+200=…………
Source: Seo et al.23
- -Table 7.
Lichtiger's index.
Symptoms Points 1. Diarrhoea 0–2 0 3–4 1 5–6 2 7–9 3 10 4 2. Nocturnal diarrhoea No 0 Yes 1 3. Visible blood in stools (%) 0 0 <50% 1 >50% 2 100% 3 4. Incontinence No 0 Yes 1 5. Abdominal pain or cramping None 0 Mild 1 Moderate 2 Severe 3 5. Performance status Perfect 0 Very good 1 Good 2 Acceptable 3 Poor 4 Very poor 5 6. Peritoneal reaction None 0 Mild and localized 1 Mild-moderate and diffuse 2 Severe or rebound 3 7. Need for an anti-diarrhoeal agent No 0 Yes 1 Assessment of the index:
Maximum score: 21 points.
Score <10 during 2 consecutive days indicates a clinical response (in the original reference to cyclosporine, severe refractory flare).
Source: Lichtiger et al.24
- -
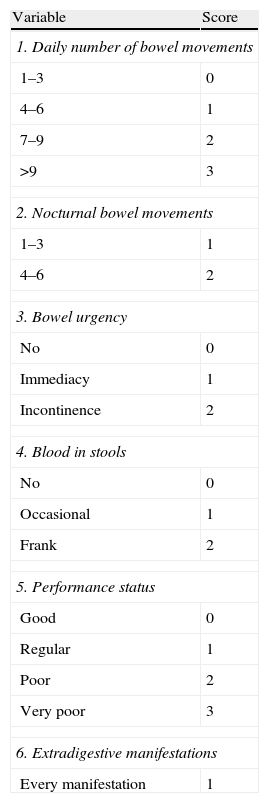
The Walmsley Index, known as the Simple Activity Index25 (Table 8)
Table 8.Simple activity index (Walmsley index).
Variable Score 1. Daily number of bowel movements 1–3 0 4–6 1 7–9 2 >9 3 2. Nocturnal bowel movements 1–3 1 4–6 2 3. Bowel urgency No 0 Immediacy 1 Incontinence 2 4. Blood in stools No 0 Occasional 1 Frank 2 5. Performance status Good 0 Regular 1 Poor 2 Very poor 3 6. Extradigestive manifestations Every manifestation 1 Assessment of the index:
Simple, clinical index; its interpretation and correlation to more complex indices, such as Power-Tuck, are adequate.
Source: Walmsley et al.25
- -
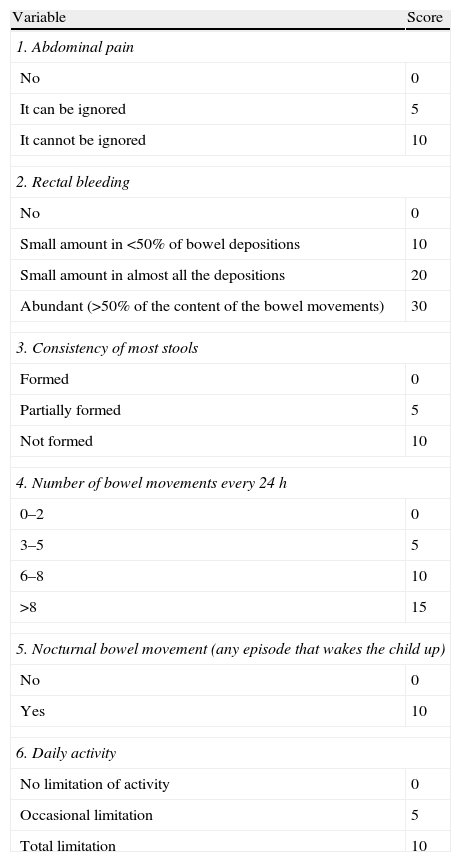
PUCAI (Paediatric Ulcerative Colitis Activity Index)26 (Table 9). Actually, this is the only index with a correct methodological validation, but it has only been used in paediatric population.
Table 9.PUCAI index.
Variable Score 1. Abdominal pain No 0 It can be ignored 5 It cannot be ignored 10 2. Rectal bleeding No 0 Small amount in <50% of bowel depositions 10 Small amount in almost all the depositions 20 Abundant (>50% of the content of the bowel movements) 30 3. Consistency of most stools Formed 0 Partially formed 5 Not formed 10 4. Number of bowel movements every 24h 0–2 0 3–5 5 6–8 10 >8 15 5. Nocturnal bowel movement (any episode that wakes the child up) No 0 Yes 10 6. Daily activity No limitation of activity 0 Occasional limitation 5 Total limitation 10 Assessment of the index:
Severe flare: ≥65 points.
Moderate flare: 35–64 points.
Mild flare: 10–34 points.
Remission (without activity): <10.
Source: Turner et al.26
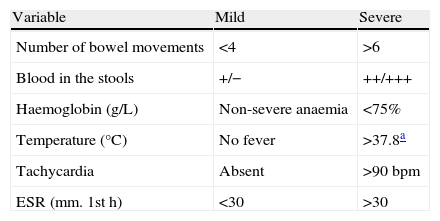
Table 3.Original Truelove–Witts index.
Variable Mild Severe Number of bowel movements <4 >6 Blood in the stools +/− ++/+++ Haemoglobin (g/L) Non-severe anaemia <75% Temperature (°C) No fever >37.8a Tachycardia Absent >90bpm ESR (mm. 1sth) <30 >30 Assessment of the index:
Mild flare: when all the variables are in the “mild” category.
Severe flare: when all the variables are in “severe”.
When there are variables in both categories, it is a moderate flare.
Abbreviations: bpm, beats per minute.
Source: Truelove and Witts.6
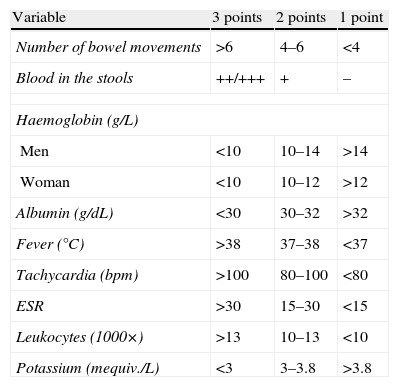
Table 4.Modified Truelove–Witts index.
Variable 3 points 2 points 1 point Number of bowel movements >6 4–6 <4 Blood in the stools ++/+++ + – Haemoglobin (g/L) Men <10 10–14 >14 Woman <10 10–12 >12 Albumin (g/dL) <30 30–32 >32 Fever (°C) >38 37–38 <37 Tachycardia (bpm) >100 80–100 <80 ESR >30 15–30 <15 Leukocytes (1000×) >13 10–13 <10 Potassium (mequiv./L) <3 3–3.8 >3.8 Assessment of the index:
Inactive: <11.
Mild flare: 11–15.
Moderate flare: 16–21.
Severe flare: 22–27.
Abbreviation: bpm: beats per minute.
- -
Table 2.Montreal Classification for ulcerative colitis.
Extension (E) (E1) Ulcerative proctitis: limited involvement of the rectum (the upper limit of the inflammation does not go beyond the recto-sigmoid junction). (E2) Left sided colitis: limited involvement of the left colon (the upper limit of the inflammation does not go beyond the splenic angle). (E3) Extensive colitis (pancolitis): involvement that goes beyond the splenic angle. Severity (S) (S0) Colitis in remission (Silent colitis): there are no symptoms of the disease. (S1) Mild colitis: four or less bowel movements a day with blood, without fever, leukocytosis, tachycardia, anaemia without an increased ESR. (S2) Moderate colitis: intermediate criteria between mild and severe, always with symptoms of mild systemic involvement (see Truelove–Witts index, Tables 3 and 4). (S3) Severe colitis: six or more daily bowel movements with blood, fever (>37.5) or tachycardia (>90bpm) or anaemia (Hb<10.5g/dL) or an increased ESR (>30mm/h), often with signs of severe systemic involvement (“toxicity”). Abbreviations: Hb, haemoglobin; bpm, beats per minute.
Source: Silverberg et al.2
- ∘
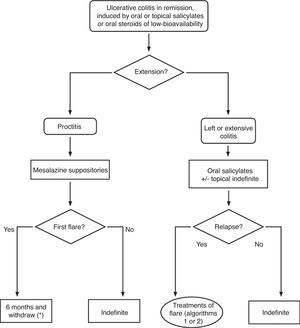
Extension criteria: The ones defined in the Montreal Classification.2 We must admit the fundamental limit of this classification: the extension may change over time and, in fact, a variable part of proctitis cases will evolve to a more extensive colitis19; however, for the analysis of the therapeutic effect, this is of a minor importance, because the colitis is analyzed at the extension it has at the moment the study is carried out:
▪Extensive UC: involving the rectum beyond the splenic angle.
▪Left-sided UC: up to the splenic angle.
▪Ulcerative proctitis: limited involvement of the rectum (the upper limit of the inflammation does not go beyond the recto-sigmoid junction).
- ∘
Aside from the fundamental definitions, it is necessary to know other terms of regular use in medical literature about UC. As the definition is arbitrary, it would probably be most adequate follow a consensus, and the most accepted international consensus is the one of the ECCO:16
▪Remission: full resolution of symptoms, accompanied by mucosal healing (this aspect is not evaluated in many studies). Not all the trials use exactly the same definition of remission, and when this is relevant, we will specifically point it out in the text (Box 1).
▪Response: a significant improvement of clinical and/or endoscopic situation (severe to moderate, severe to mild, moderate to mild) without reaching remission.
▪Relapse: a new flare in a patient with an established UC after a remission has been achieved, either spontaneously or after medical treatment.
▪Steroid dependence:
- -
the impossibility of reducing the dose of steroids under 10mg/day of prednisone (or its equivalent) after 3 months of starting the steroid treatment or
- -
a relapse within the first 3 months after having discontinued the treatment with steroids.
- -
▪Steroid resistance: A situation of clinical activity in spite of a 4-week treatment at full doses (0.75mg/kg/day of prednisolone or 1mg/kg/day of prednisone, or its equivalent). This definition is very controversial and will most likely be modified in the future. In fact, in the context of a severe flare, the majority of clinicians will define steroid-resistance as the lack of clinical response after the intravenous administration of steroids at full doses for 7 days. Moreover, there is a growing tendency to place the definition in 5 or even 3 days. To the effects of this CPG, we will use the concept of a month for mild or moderate flares, and 7 days for severe flares.
▪Pouchitis: inflammation of the ileal pouch created to maintain the intestine-anus continuity after a total colectomy. In many patients it behaves as a chronic illness.
▪Cuffitis: inflammation of the rectal mucosa cuff that remains in the anal area in some ileo-anal anastomoses.
- ∘
- -
Pharmacological index
- ∘
Systemic drugs:
▪Salicylates: sulfasalazine, mesalazine
▪Oral steroids:
- -
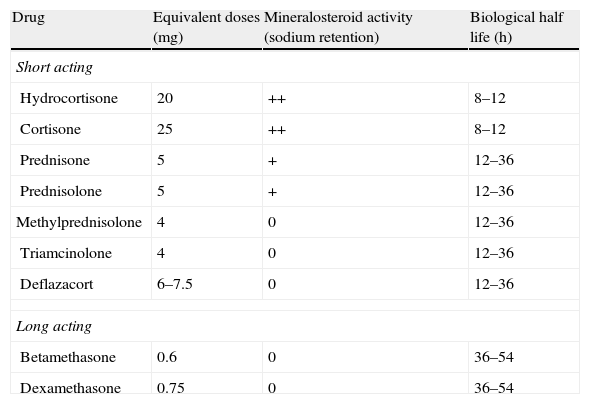
Systemic steroids: hydrocortisone, prednisone, methylprednisolone, deflazacort, betamethasone, dexamethasone.
- -
Topical action: beclomethasone dipropionate (BDP), budesonide
- -
▪Immunomodulating drugs (immunosuppressants):
- -
Thiopurine agents: azathioprine, mercaptopurine, thioguanine
- -
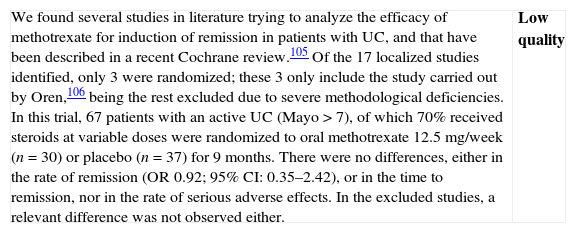
Methotrexate
- -
Calcineurinics: cyclosporine, tacrolimus
- -
▪Biological agents: infliximab, adalimumab.
▪Heparin.
- ∘
Topical drugs
▪Mesalazine
▪Steroids
- -
Oral administration: beclomethasone, budesonide
- -
Rectal administration:
- •
High systemic bioavailability: hydrocortisone, prednisolone, triamcinolone, methylprednisolone and betamethasone
- •
Low systemic bioavailability: budesonide, beclomethasone and prednisolone-metasulphobenzoate
- •
- -
- ∘
Other treatment techniques:
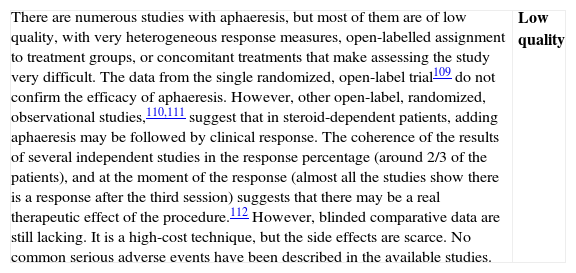
▪Aphaeresis: leucocyte aphaeresis, granulocyte aphaeresis
- ∘
The objectives were defined by the Elaboration Committee (see Appendices), following the GRADE working methodology.27 In summary, after choosing the three clinical scenarios to which recommendations may be applied (induction of remission in severe colitis, induction of remission in mild-to-moderate colitis, and maintenance of remission); in each of the scenarios, the possible variables of result were explained and defined in a numeric scale from 1 to 9 (1–3: are not included; 4–6: important but not critical; and 7–9: critical for decision-making) by the nine members of the Elaboration Committee. After being classified in order of importance, variables with an average score above 4 were included as result variables. In all the critical variables, the degree of internal agreement of the Committee was excellent, with unanimity in the score or with a maximum variability of one point. After this previous process, the following were defined as CPG objectives:
▪To establish recommendations based on available tests for the induction treatment in the severe UC flare prioritizing the following assessment variables:
▪To establish recommendations for the induction treatment in the mild–moderate UC flare prioritizing the following assessment variables:
▪To establish recommendations for the maintenance treatment of UC in remission prioritizing the following assessment variables:
- ∘
For the elaboration of the CPG, AGREE methodology was followed, which is described in detail on the web page: www.agreecollaboration.org. Briefly, an interdisciplinary working team was formed, structured in a Design Committee (general for the different CPG promoted by GETECCU), an Elaboration Committee (for the three first CPG), a Working Committee (specific for this CPG), an Internal Review Committee (for the three first CPG) and an External Review Committee (specific for this CPG). The External Review Committee included gastroenterologists, surgeons, primary care physicians, nurses and patients. A more detailed composition of all these committees is presented in the guidelines’ Appendices. Throughout the entire process, both in the formation of the team as well as in the areas in which it was necessary, the methodological support of the specialized Cochrane Collaboration staff was available. After the objectives were defined by the Elaboration Committee, the Working Committee carried out a Systematic Literature Review, specifically directed at answering the key questions posed previously. After the information was obtained, it underwent an evaluation process, following the GRADE methodology.28 Thus, the quality of the studies was evaluated considering the study's design, their consistency and the direct and indirect evidence. Considering a combination of the four components, quality was defined as High (very unlikely that new studies change the estimation), Moderate (it is likely that new studies change the reliability of the result), Low (it is very likely that new studies will have an impact on the result's reliability and that they might modify it) and Very low (any estimated results are very doubtful). Aside from the essential factors mentioned, for the overall estimation of quality of the evidence, other factors such as the number of patients studied, the strength of the associations that were found, the presence of dose–response gradients (in pharmacological studies) and the acknowledgement or not of possible confounding factors identified by the evaluators were taken into account. In any case, all of these factors are considered cumulatively and with a specific methodology that may be consulted in the references. In order to establish recommendations, we considered not only the quality of the evidence, but we also carried out a weighing between the potential benefits of the intervention, its risks, and its applicability in the population that will be treated and, finally, its costs. Even without having analyzed formal cost-effectiveness analyses, costs are always considered from a global standpoint and are compared, not only considering direct costs, but also the indirect costs and the context (regarding the costs of alternatives). The recommendations issued are classified into four degrees: recommendation or we recommend, which implies strongly advising the clinician: Do it; suggestion or we suggest which means to advise the clinical probably do it; suggestion against or we do no suggest which implies the same as probably don’t do it; and we recommend avoiding or we do not recommend which strongly and clearly indicates don’t do it. This type of classification is both easily understandable and flexible, because it can be adapted to the very different clinical scenarios possible.
- ∘
The Systematic Literature Review was carried out with free and conditioned searches in the following databases: PUBMED, EMBASE, TRIPDATABASE, and COCHRANE COLLABORATION, as well as in textbooks and by cross-referencing several studies. A review was carried out for each one of the questions asked. Furthermore, cross references were carried out in specialty texts and in all the recent reviews. In all the cases in which a systematic review was already published, it was used if its methodology was deemed adequate by the Working Committee, following the criteria of the Cochrane Foundation. If there were doubts about the methodology, the source data were reviewed, and if there were several systematic reviews, the choice was made firstly based on methodological criteria, and secondly, on time criteria (the most recent cases were preferred in case of methodological equivalence). Only documents published completely were evaluated. Nonetheless, at the moment of the final writing of the Guidelines, the Writing Committee considered some results reported formally during the last national and international congresses on some specific points that will be specified in the text. The limit for the inclusion of studies is February 2011. In the reference list, we only include key studies, systematic reviews and quotes after these.
- ∘
This CPG will be of free and universal access. It will be published in several formats (hard and soft copy) and will be available freely on the Internet to be accessed accessible by any professional and/or patient.
- ∘
The information will be presented answering general questions posed in three formats: review of the evidence, summarized explanatory text and decision algorithms. In sum, the evidence is presented answering each one of the questions asked, whereas the explanatory texts are articulated around the six specific algorithms that have been elaborated.
- ∘
The strategic decisions about the CPG will be taken by GETECCU, represented by its Board of Directors, and by the Guidelines Elaboration Committee named to this effect.
- ∘
The costs are covered by GETECCU, who has obtained a GRANT (with no restrictions) from MSD.
- ∘
The conflicts of interest of the authors are explained in Appendix.
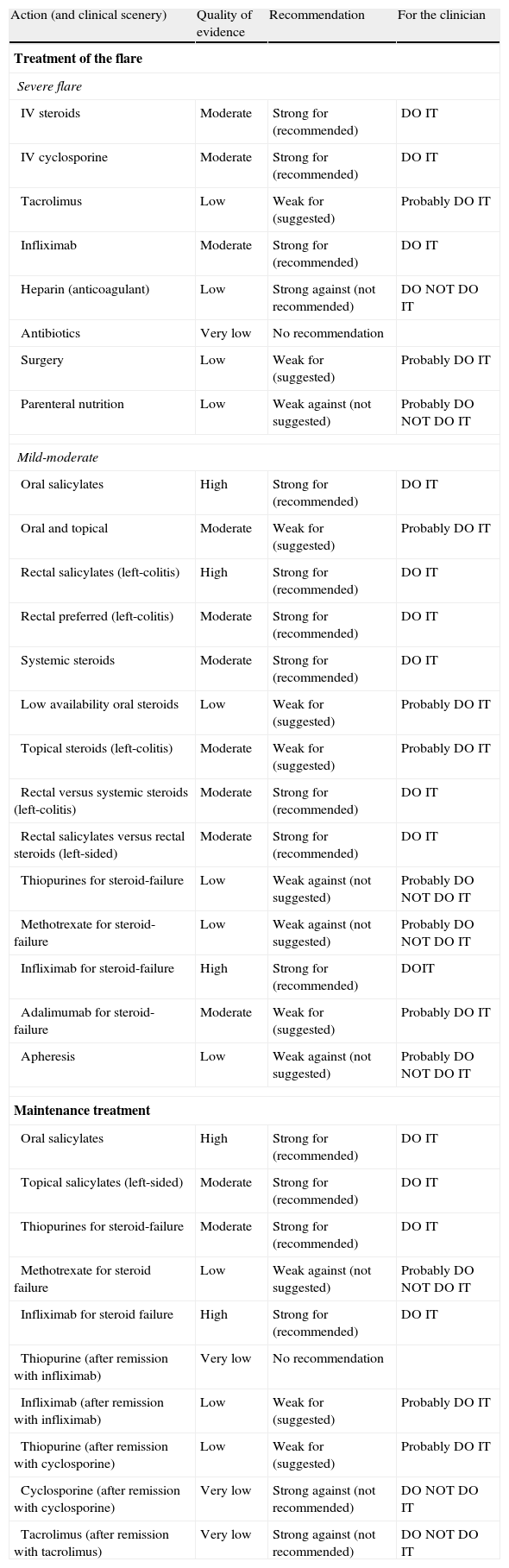
Any situation in which the patient has symptoms and/or signs indicating disease activity is considered an active colitis. In case of doubt, endoscopy and histology are reference techniques for defining the symptoms. For severity and extension we used the Montreal Classification2 (Table 2). The key information necessary for the clinician is summarized in algorithms 1, 2 and their corresponding explanatory text. The review of the evidence is presented by answering the questions elaborated by the working group.
6.1Severe UC flare (of any anatomical extension) (Algorithm 1)6.1.1Steroids- -
Are steroids effective in induction of remission in patients with a severe UC flare?
| We have a systematic review29 of great methodological quality, in which the five randomized controlled trials (RCTs) available are reviewed with a total of 149 patients; together with several observational studies with a total of 1991 patients. We only identified one subsequent relevant study30 that compares the continuous infusion administration to the bolus administration, without any relevant differences. Since the patients groups are very heterogeneous, it is incredibly notable that the outcome of the treatment with steroids is very homogenous. They are effective in inducing remission, with a mean dose of 68±13mg/day of prednisone (or its equivalent) (Box 3) (range 40–100; with only one study below 60mg/day) and a rate of remission close to 70%. On the other hand, in spite of using steroids, approximately 30% of the patients need a colectomy (44/149) (95% CI: 23–37%) in RTCs, and 537/1842 (95% CI: 27–31%) in observational studies. | Moderate quality |
| Data available have been obtained from studies that are very heterogeneous. However, the results are extraordinarily coherent. A large number of observational studies carried out in different countries and at different times agree with the clinical experience of the large sites and of the experts. Everyone among these independently confirms the final conclusion of the first clinical trial that was already published in 1955: steroids clearly reduce (from >20% to <2%) the mortality of a severe UC flare. Considered jointly, the data allow establishing the recommendation in an affirmative manner. In fact, for ethical reasons, we believe it to be highly unlikely for new trials to be carried out with a placebo group in this patient group.For the group of available data, the total dose seems to be more important than the form of administering the drug (in bolus once daily, in continuous infusion or in distributed doses). However, there are not enough comparative data, so the treatment may be decided in every scenario in terms of the concrete steroid used (Box 3, Table 17), the pharmacokinetic data, and the local experience. |
- -
Is cyclosporine effective in induction of remission in patients with a severe UC flare? and in a situation of steroid resistance?
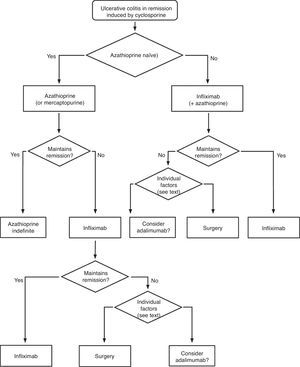
| There are two systematic reviews that evaluate the effect of cyclosporine in UC. A Cochrane review31 includes just two controlled trials with a total of only 50 patients, one versus steroids in patients who are not refractory to these drugs32 and another versus placebo in patients after failure with steroids. However, the other review33 also includes two more controlled studies (the first one comparing 4mg/kg/day versus 2mg/kg/day34 and the second study comparing cyclosporine alone versus cyclosporine and steroids35 with a total of 153 patients in both studies); and a total of 27 observational studies with 574 patients treated with cyclosporine. After these reviews, a relevant retrospective series was published.36The studies are considerably heterogeneous, both in the definition of the patients to include, as in the primary objectives. Not all the parameters defined as key in this CPG (mortality, need for colectomy) are easy to analyze globally, and only one of the studies with a small number of patients provides a direct comparison with placebo.24 Therefore, we qualified the quality of the evidence as moderate. However, the difference of absolute risk with placebo was so clear (81%) and the result in the rest of the studies was so homogeneous (a clinical response perfectly defined between 60 and 80% of the cases), that the efficacy of this treatment alternative seems evident, especially when the vast majority of patients included showed a previous failure after treatment with steroids. Recent data reported in an abstract form confirm that cyclosporine is as useful as infliximab in severe flare of steroid-resistant colitis (CYSIF study, reported in the ECCO 2011). Moreover, in this study, the rate of colectomies at 3 months was of only 20% in a group of patients with steroid resistance, very selected due to their severity.The different protocols also make it difficult to evaluate safety. However, and not surprisingly (severe patients, with extended hospitalizations, undergoing complex immunosuppression), serious side effects are common and, although they affect less than 10% of the total, they can result in mortality (approximately 1%), in spite of the use of protocols in clinical studies (a scenario that is generally more favourable for patients than daily clinical practice).As for the dose, 2mg/kg/day administered intravenously turned out to have a similar efficacy than the previously used dose of 4mg/kg/day and with fewer side effects, although as in both studied groups the doses were adjusted according to the blood levels, the final amounts received were very similar34 (Box 3). | Moderate quality |
| In a flare of steroid-refractory UC, one must always consider possible infections as a real cause of refractoriness (Cytomegalovirus (CMV) infection, Clostridium difficile infection). Furthermore, there are always different treatment options available, and the scenario must be defined in the most precise way possible before recommending a decision. The patient's individual characteristics will influence the treatment choice: thus, prior treatment or not with immunomodulators is a very important aspect. If the patient already received them when he/she experienced the severe refractory flare, the probability of maintaining remission with the same drugs, after obtaining remission with cyclosporine, is presumably low, although direct evidence is scarce. Given that cyclosporine can be administered for a limited period of time in a benign entity, due to the risk of irreversible renal toxicity, it does not seem reasonable to use it in this context. The option of cyclosporine use must always be evaluated together with the possibility of surgery, or the use of infliximab.Safety is the most important conditioning factor in the use of cyclosporine, which is why it is advisable to follow a pre-established follow-up protocol.37 It is most likely for some adverse effects to appear in extended treatments, which is why the duration of the prescription must be considered carefully. Another one of the most important factors in the appearance of side effects is co-treatment with other immunomodulators, especially steroids. The experience of the site in using the drug can also be determinant when it comes to using cyclosporine or not (facilities must be available for determining blood levels, for example). |
- -
Is tacrolimus effective in induction of remission in patients with a severe UC flare? and in a situation of steroid resistance?
| There is a single controlled trial38 in patients with active moderate to severe UC (especially moderate), hospitalized, steroid-resistant or steroid-dependant, who were randomized into three groups: placebo (n=20) or low-dose oral tacrolimus (n=22) or high-dose tacrolimus (n=21) (target concentration of 5–10 and 10–15mg/mL in blood, respectively). Efficacy was evaluated in week 2, using Mayo Index22 (DAI), the primary objective being induction of improvement (partial response or remission). In the intent-to-treat analysis, there was a clinical response (partial response or remission) in 2/20 (10%), 8/22 (36%) and 13/21 (62%), respectively. The OR for tacrolimus response versus placebo was 8.66 (95% CI: 1.79–42) with an absolute risk reduction of 0.39 and an NNT of 3. Only at high doses it is significantly higher compared to placebo, and data suggest a dose–response effect. If we focus only on analyzing remission, the differences between tacrolimus and placebo did not reach statistical significance: tacrolimus at high doses 19% (4/21), low doses 9% (2/22) and placebo 5% (1/20), with an OR of 2.27 (95% CI: 0.35–14.75). If we analyze efficacy in terms of the severity of the flare, tacrolimus was effective both in moderate as well as in severe flares. If we analyze the response in terms of the indication (steroid resistance or steroid dependence), in both cases tacrolimus is effective, and in a similar fashion. There were no colectomies or mortality, nor were there any serious adverse effects.A systematic review39 collected the different observational series available. Without being able to define how many patients were severe and/or steroid-refractory, 53% of the treated patients reached “complete response or remission”. A later series40 did not allow for a definition of severity either, but it included 40 patients with UC, 14 of them steroid-dependent and 26 steroid-refractory, treated with oral tacrolimus. The initial response rate (defined at 30 days) was 77.5% and the remission rate was 18%. In the subsequent follow-up (up to 44 months), 9 patients (22.5%) are colectomized. The duration of the treatment with tacrolimus was very variable and 77% of the patients received azathioprine concomitantly (none of them received another immunomodulator); without any common serious adverse events. These same data are collected in a Cochrane systematic review.41We have not found any controlled studies that have compared the use of tacrolimus against its alternatives, cyclosporine or infliximab. Practical data about tacrolimus are summarized in Box 3. | Low quality |
| Even if the mechanism of action is very similar to that of cyclosporine, and both drugs show similar efficacies in other clinical scenarios (transplants), the published data are clearly less numerous and of a lower methodological quality. In fact, the results of the only controlled clinical trial available are disappointing when compared to observational studies. Of course, in both drugs, the experience of the medical team in their use is absolutely necessary to minimize toxicity, as has been extensively demonstrated in the context of transplants. Only the experience of the team could lead to preferring tacrolimus, taking into account the data published to date. On the other hand, most of the previously mentioned comments in the cyclosporine section are completely applicable. |
- -
Is infliximab effective in induction of remission in patients with a severe UC flare? and in a situation of steroid resistance?
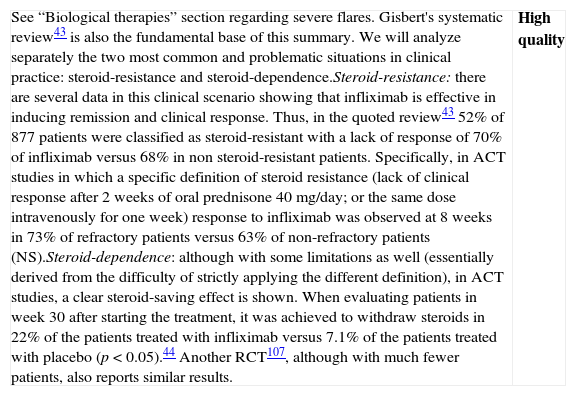
| Although we have a Cochrane review available,42 the one published by Gisbert et al.43 includes both the RCTs (7 RCTs: 5 compared to placebo, 2 compared to steroids, including a total of 555 patients) as well as non-controlled studies (27 studies, 241 patients); which is why we use it as the basis for our own analysis. In most RCTs, severe and moderate patients are mixed. In fact, the intensity of the flare is only clearly defined in 322 of the 896 patients, 207 cases of which (64.2%) were severe. In the two studies that had included most of the patients,44 the majority of the patients were outpatients, which suggests that they were mostly moderate flares. The information about steroid-resistance or steroid-dependence is also limited in the mentioned studies: only the percentage of patients who are receiving steroid treatment is reported.There are two RCTs, however, that include exclusively steroid-resistant patients undergoing a severe flare.45,46 The results are contradictory, as infliximab is greatly superior to placebo45 in one and ineffective in the other,46 although this last study includes few patients and has a very high beta error risk. The Scandinavian study45 is the most complete and includes a mortality analysis (absent in both groups) and an analysis of the need for colectomy over the short and long term. A significant risk reduction is observed (RR 0.44; (95% CI: 0.22–0.87)) in the rate of colectomy at 13 months, which is relevant from the clinical standpoint. Recently, it has been shown that the effect of this single intervention in the acute phase persists at 36 months.47 The pooled analysis of the four RCTs on the efficacy shows the superiority of infliximab compared to placebo, both for inducing response (OR 3.60 (2.67, 4.85)) and remission (OR 4.56 [1.98–10.52]) (we have remission data just in two RCTs). The comparison of infliximab with steroids is analyzed in two open RCTs with small populations, without any statistically significant differences. Though serious adverse effects are uncommon (<5%), there is a greater incidence of infections, especially opportunistic, that may result in mortality (<1%). The risks seem to be greater if infliximab is associated to other immunomodulators.In summary, infliximab is superior to placebo in treatment of severe UC flare, with an acceptable risk of serious adverse effects, occasionally associated to mortality. Recently, several reports of European CYSIF study results were published in abstract form, in which infliximab is randomly compared to cyclosporine in the severe flare of steroid-resistant UC. There are no differences between both, and both drugs seem to be very effective with a rate of colectomy at 90 days of 20% and a good safety profile. There are no data for direct comparison with surgery. In spite of the important number of patients and studies, we have to qualify the quality of the evidence as moderate due to the heterogeneity, the lack of definition of the exact clinical scenario in many cases, and the relatively low number of severe flares analyzed, although there are more observational data available confirming what was described in the mentioned studies.48 | Moderate quality |
| Although data are very heterogeneous, infliximab is more effective than placebo in the induction of remission of severe UC flare, and specifically in severe steroid-resistant flares; and apparently it is as effective as cyclosporine. It increases the rate of clinical remission and reduces the rate of colectomies, persisting the statistical significance of this effect over the mid-term. Observational studies support these results. We do not have final data available showing its superiority in severe flares compared to steroids, or even less so, compared to cyclosporine. Side effects are possible, potentially severe, and their safety profile is well known, as it is analogous to the one described in Crohn's disease (CD), although burdened by the severity of this situation. | Moderate quality |
| After considering the results of Cysif study, the lack of differences between infliximab and cyclosporine mandates to decide a recommendation in terms of individual circumstances of each patient and of the site's local conditioning factors. Furthermore, the long-term follow-up of the patients of the Scandinavian study confirms a very important issue: a successful intervention in the acute phase means less probability of colectomy in the mid and long term47 |
- -
Is heparin (at anti-coagulant doses) effective in induction of remission in patients with a severe UC flare?
| There is a Cochrane review available49 based on two controlled trials, comparing low-molecular weight heparin (LMWH) to placebo in the UC flare. No differences were found with placebo in terms of induction of remission (OR 1.09; 95% CI: 0.26–4.23). There was also no evidence in other studies that adding LMWH to other treatments improved the response. Moreover, the study comparing non-fractioned heparin (intravenous sodium heparin) to steroids50 (as standard treatment of the severe UC flare, see “Steroids” section) showed a clear inferiority of heparin, with an OR of 0.02 (95% CI: 0–0.4, p=0.01) in terms of the point of analysis of clinical response, with more side effects (haemorrhage) in the heparin group. | Low quality |
| All of the above refer to heparin as a primary therapeutic agent, used at anti-coagulant doses. Even without controlled data available, a series of indirect tests strongly suggest that the use of low-molecular-weight heparin at antithrombotic doses is advisable in practically all the patients with severe UC flare. The basal risk of thromboembolic phenomena in this clinical scenario is very high and, in fact, they are one of the most important causes of mortality.51,52 |
- -
Are antibiotics effective in induction of remission in patients with a severe UC flare?
| We have a systematic review available53 which includes 10 RCTs comparing antibiotic treatment to placebo in 509 patients with active UC: 263 on antibiotics versus 267 on placebo plus the regular treatment; however, the severity of flares is not specified. The moment of assessment of the response is carried out early (1–14 days) in all the studies except in one of them in which it is evaluated at 180 days. The studies included use different types of antibiotics at different doses, with different methods of administration and even different associations: vancomycin 7%, metronidazole 7%, tobramycin 31%, ciprofloxacin 38.5%, rifaximine 5.5%, metronidazole plus tobramycin 7%, metronidazole plus amoxicillin plus tetracycline 4%. The RR found is 1.30 [1.14–1.49], which translates into a possible benefit of using antibiotics in these patients (always associated to steroids). If we analyze the early response, excluding the long-term study, the OR still remains at 2.02 [1.36–3.00]. Adverse effects are not evaluated. A very recent clinical trial54 with a combination of amoxicillin, tetracycline and metronidazole did not show any differences in the short-term remission, but did improve the response and the remission at 12 months. Due to the type of study, most patients included suffered a mild or moderate UC flare. | Very low quality |
| Even if no recommendations can be established as primary agents, in severe cases with a high risk of perforation, and especially in the cases of megacolon, practically all the experts recommend to use an antibiotic treatment aimed at Gram negative germs and anaerobes. Depending on the local rates of resistance, the following are recommended: cefotaxime plus metronidazole, imipenem, meropenem or piperacillin-tazobactam. These recommendations are not different from the ones issued in other clinical scenarios of severe intra-abdominal infection. |
- -
Is surgery effective in induction of remission in patients with a severe UC flare?
Colectomy was established as treatment for UC when there were no medical alternatives. Gradually, with technical advances in anaesthesia and in postoperative control, colectomy reached mortality rates that are clearly below the spontaneous course of the flare. The absence of controlled studies in this field is one of the clearest examples of situations that are self-evident, but impossible to demonstrate. There is no reasonable doubt that colectomy is a very effective treatment in severe UC flares, a scenario for which, in the absence of medical treatment, mortality rates of 22–77% were described. Even if we do not have controlled studies either, the results of the Oxford series in the 1960s and 1970s in studies conducted by Sidney Truelove in the steroid era55 are very convincing. The best results were obtained when a fixed day was set a priori in which the patient with no response to steroids was intervened (in the Oxford series, this was decided to be on the fifth day of treatment; in the Scandinavian series, it was decided to be a little later, but it yielded very similar results56). It is curious to see how, after introducing new medical alternatives such as cyclosporine or infliximab, most experts emphasize on making an early decision (third, fifth, seventh or tenth day, according to the authors). On some occasions, surgery is the best choice: perforation, severe haemorrhage that is not controlled with endoscopic treatment, toxic megacolon of more than 72h duration. In others, it is probably the preferred choice: e.g., severe colitis with a very prolonged clinical history and/or a previous diagnosis of dysplasia. In most cases, however, it is just a back-up option and it is only indicated if the previous medical treatment has failed. In any case, uncontrolled but extensive experience suggests that in the severe UC flare treatment, the collaboration of a surgical team from the moment the patient is admitted helps make more coherent decisions every time, taking into consideration several treatment options.Summary of the evidence
| We do suggest surgery as an option in severe flares of steroid-resistant UC, although in most cases, a rescue treatment with infliximab or cyclosporine must be tried previously. In some clinical scenarios, the indication is absolute, and so surgery is to be recommended: perforation, massive haemorrhage, and refractory toxic megacolon. | Weak for |
| Surgery is a therapeutic alternative for patients in severe UC flares; and in some clinical circumstances (free perforation, massive haemorrhage, and refractory toxic megacolon) it is the only option. The decisions in these clinical scenarios may be very difficult and must result from the fluent and open interaction between the medical team, the surgical team, the relatives or associated people and, mainly, a well-informed patient. Acknowledging that on occasions, the indication for treatment is practically absolute, the degree of the general recommendation has to be weak in favour due to the absence of comparative studies with the rest of treatment options. |
- -
Is parenteral nutrition effective in induction of remission in patients with a severe UC flare?
There are not enough studies to create a GRADE table in the case of parenteral nutrition either. However, we can state several basic principles, either due to the existence of controlled studies, or the existence of direct and/or experimental data.57
- •
In contrast with what was suggested in the first half of the XX century, absolute diet does not have any primary therapeutic effectiveness and hence, except in cases of absolute oral intolerance, intestinal obstruction or extreme severity, oral nutrition must not be discontinued.
- •
Parenteral nutrition has no primary efficacy in the treatment of UC, and generally, enteral nutrition must be preferred.58 Moreover, it is more expensive and it entails a higher risk of infection and thromboembolic phenomena when compared to enteral nutrition.
- •
In case a complete caloric and nutritional supply cannot be assured, enteral nutrition must be used, either as a supplement or even as the sole source of nutrition.
- •
In case of severe flares, a relatively extensive hospital stay should be foreseen. There are studies from the 1980s,59 corroborated in years as recent as 200860; showing that hospitalized patients are discharged (on average) in a worse nutritional condition than the one they had on admission. Moreover, numerous experimental and clinical tests confirm that an adequate nutrition is necessary for the correct functioning of the immune system. In patients with severe UC, the risk of nosocomial infections, either opportunistic or not, is very high due to several factors: hospitalization, use of tubes and catheters, an increased intestinal permeability (rupture of the mucous barrier), malnourishment and the use of immunomodulating drugs being the most important. The measures for the control of infections will help reduce the risk, but we can ensure a greater medical impact by assuring a proper nutrition. Also, we can decrease the negative effects of some drugs, keeping a good nutritional status (calcium and vitamin D to prevent osteoporosis; prevention of hypercholesterolemia and hypomagnesaemia in case cyclosporine is used).
| Undoubtedly, an adequate nutrition is essential in the treatment of severe UC flares. However, there is no evidence of a primary effect on the activity of the disease, and parenteral nutrition is burdened with costs and risks. Therefore, it is advisable to feed each patient as necessary, with a normal diet (the fewest), with enteral nutrition (the majority) or with total parenteral nutrition (a minority). At times, in clinical practice, a possible primary benefit of total parenteral nutrition is expected, which may unnecessarily delay making key decisions. |
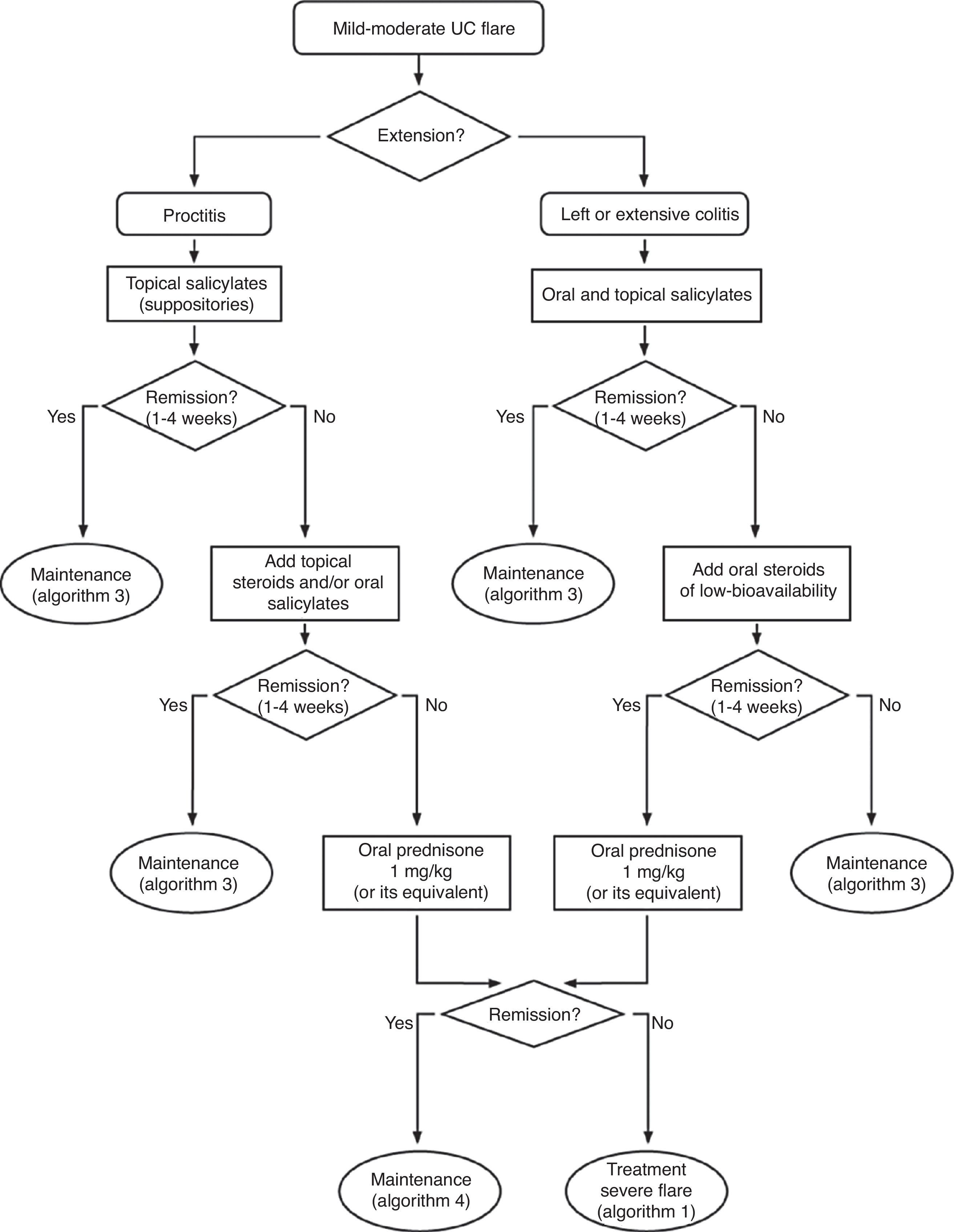
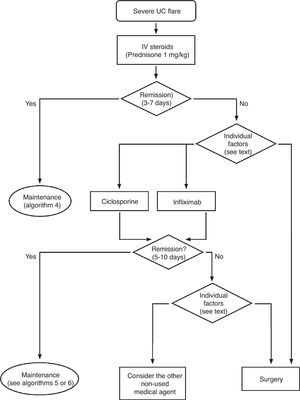
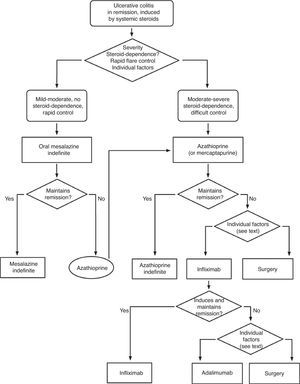
Severe flare: algorithm and explanatory text
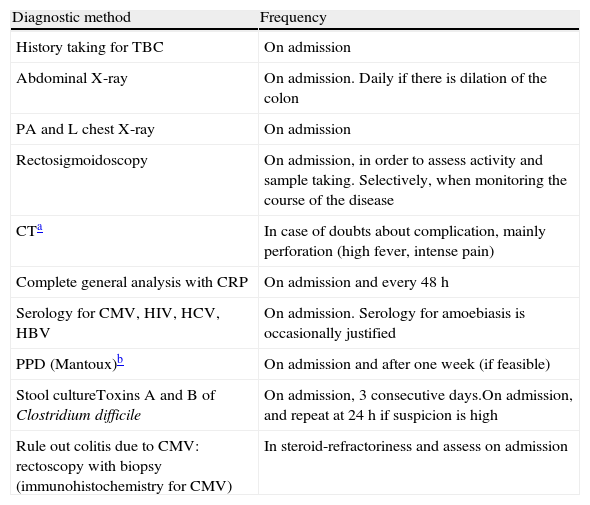
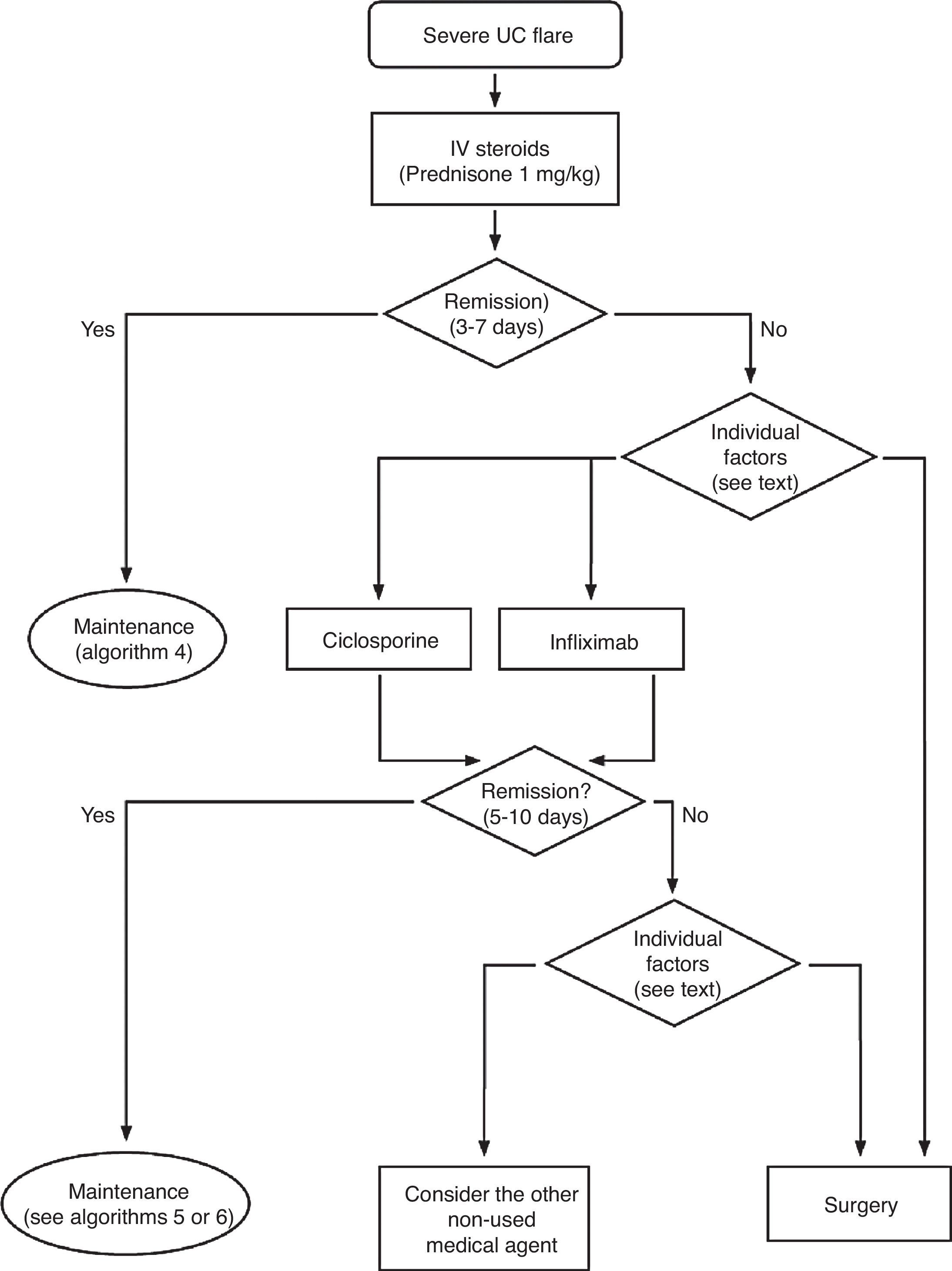
Treatment of severe UC flare is summarized in Algorithm 1. When the diagnosis of severe UC flare is established, in most cases hospitalization is a prudent strategy.8,13,17,61 From the first moment, a strategy must be proposed that includes immediate measures, the options in case these fail and also the possible treatment in the mid and long term. Therefore, all the diagnostic information must be reviewed (Table 10) by re-evaluating all the data that may be of interest when it comes to making decisions in each specific case.61
Review of the diagnostic information prior to the treatment of the severe flare.
| Diagnostic method | Frequency |
| History taking for TBC | On admission |
| Abdominal X-ray | On admission. Daily if there is dilation of the colon |
| PA and L chest X-ray | On admission |
| Rectosigmoidoscopy | On admission, in order to assess activity and sample taking. Selectively, when monitoring the course of the disease |
| CTa | In case of doubts about complication, mainly perforation (high fever, intense pain) |
| Complete general analysis with CRP | On admission and every 48h |
| Serology for CMV, HIV, HCV, HBV | On admission. Serology for amoebiasis is occasionally justified |
| PPD (Mantoux)b | On admission and after one week (if feasible) |
| Stool cultureToxins A and B of Clostridium difficile | On admission, 3 consecutive days.On admission, and repeat at 24h if suspicion is high |
| Rule out colitis due to CMV: rectoscopy with biopsy (immunohistochemistry for CMV) | In steroid-refractoriness and assess on admission |
In most cases, the initial treatment (except if there is an absolute contraindication due to previous severe toxicity (for instance, steroid-associated psychosis) or a situation of surgical urgency) will be based on the administration of intravenous steroids, with a dose equivalent to 1mg/kg of prednisone per day. It can be administered in a single or fragmented dose, or even in continuous infusion. Furthermore, the treatment with other measures must be completed in order to prevent thromboembolism, obtain an adequate nutrition and, in selected cases, administer antibiotics (Table 11). The clinical follow-up must be, at minimum, daily, and preferably jointly managed with the surgeon. It is practical to include in the diagnostic evaluation those tests that inform us about the risks of the side effects of infliximab, cyclosporine or azathioprine, as they may allow to start the treatments with a greater safety, and if found to be necessary, instituting the necessary preventive measures.
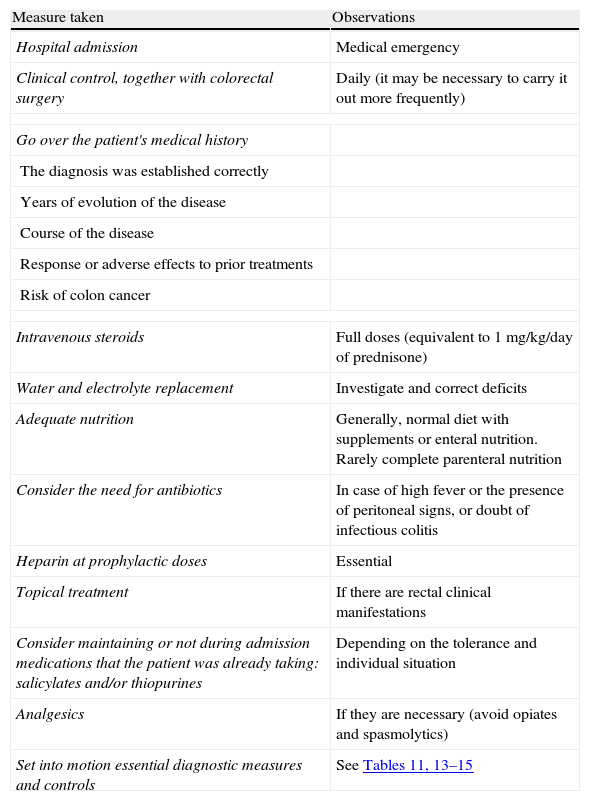
Summary of the general treatment of the severe flare.
| Measure taken | Observations |
| Hospital admission | Medical emergency |
| Clinical control, together with colorectal surgery | Daily (it may be necessary to carry it out more frequently) |
| Go over the patient's medical history | |
| The diagnosis was established correctly | |
| Years of evolution of the disease | |
| Course of the disease | |
| Response or adverse effects to prior treatments | |
| Risk of colon cancer | |
| Intravenous steroids | Full doses (equivalent to 1mg/kg/day of prednisone) |
| Water and electrolyte replacement | Investigate and correct deficits |
| Adequate nutrition | Generally, normal diet with supplements or enteral nutrition. Rarely complete parenteral nutrition |
| Consider the need for antibiotics | In case of high fever or the presence of peritoneal signs, or doubt of infectious colitis |
| Heparin at prophylactic doses | Essential |
| Topical treatment | If there are rectal clinical manifestations |
| Consider maintaining or not during admission medications that the patient was already taking: salicylates and/or thiopurines | Depending on the tolerance and individual situation |
| Analgesics | If they are necessary (avoid opiates and spasmolytics) |
| Set into motion essential diagnostic measures and controls | See Tables 11, 13–15 |
It is advisable to set, from the first day, a time point for evaluating the response, and it is most adequate to do it between the third and fifth day after starting the treatment. In fact, currently most authors tend to recommend an initial evaluation at 72h.61 At that moment, the patient may have reached remission (in which case, he/she will progressively move from the treatment phase to the maintenance phase) or not. If remission has not been obtained, there are several indices (for example, Ho's index,62Table 12) that allow us to predict the probability of response if the steroid treatment were to be extended. A recent simple model, evaluated prospectively in a hospital, suggests that with four parameters (rectal bleeding, CRP, platelet count and the number of bowel movements) evaluated by the third day, it may be decided with great precision if it is necessary to switch to another treatment or not. If this were to be confirmed in other sites, this model may allow us to make decisions with data available at any other site with great ease.63 On occasions, the situation is very clear, and a new medical treatment (infliximab or cyclosporine) is initiated between the third and fifth days. In other patients, the situation is not that clear, and it is preferred to carry out a re-evaluation between the fifth and seventh days after starting on steroids. If remission has not been reached by the seventh day it is best to start the alternative treatment.
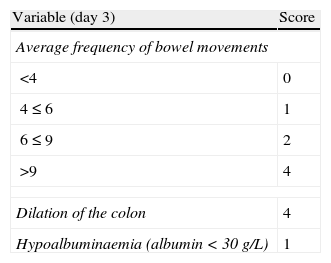
Probability of response to steroids (Ho index).
| Variable (day 3) | Score |
| Average frequency of bowel movements | |
| <4 | 0 |
| 4≤6 | 1 |
| 6≤9 | 2 |
| >9 | 4 |
| Dilation of the colon | 4 |
| Hypoalbuminaemia (albumin<30g/L) | 1 |
Assessment of the index (estimated probability of failure with the medical treatment):
Score index: probability of no response to the medical treatment.
0–1: 11%.
2–3: 43%.
≥4: 85%.
Source: Ho et al.62
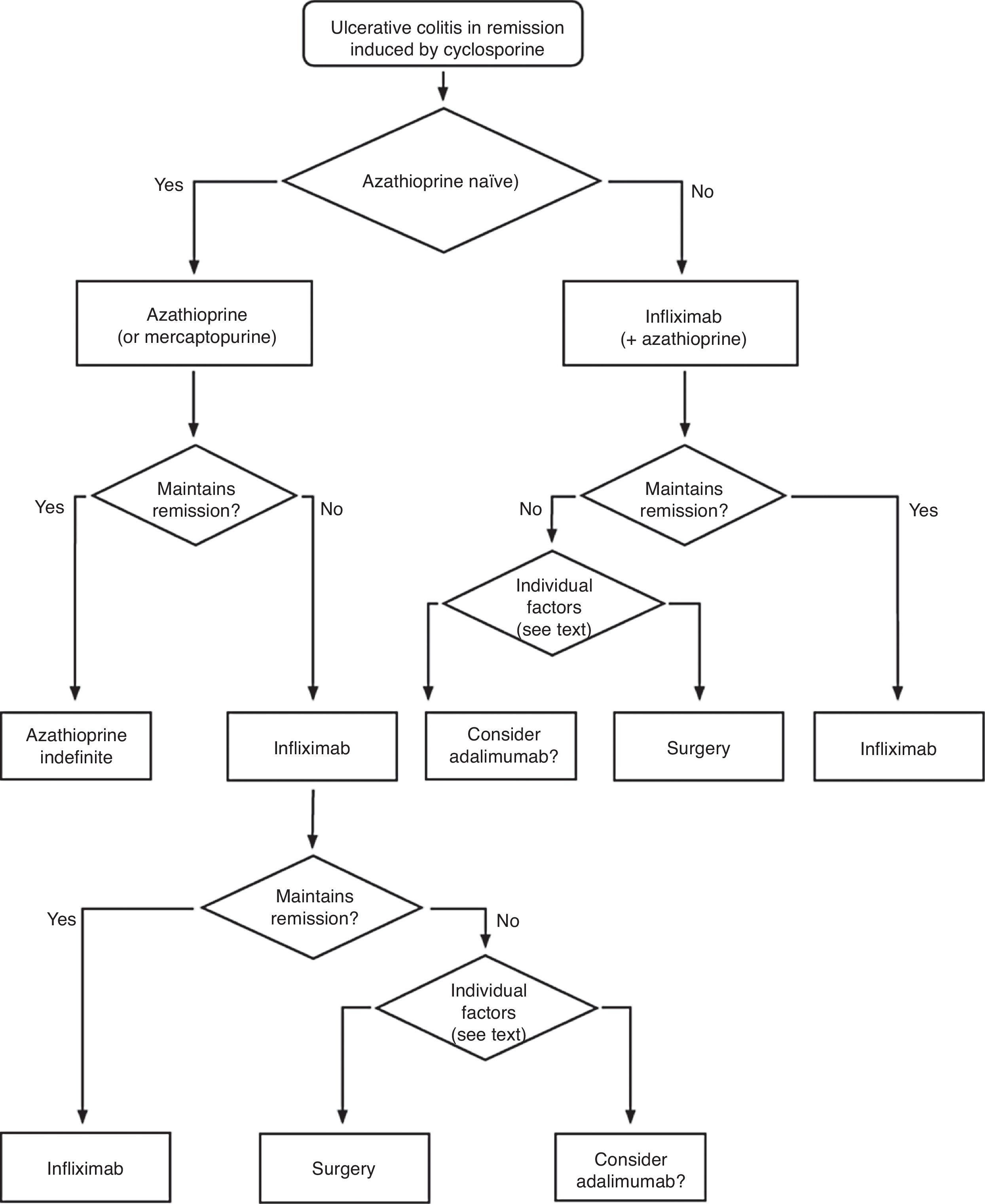
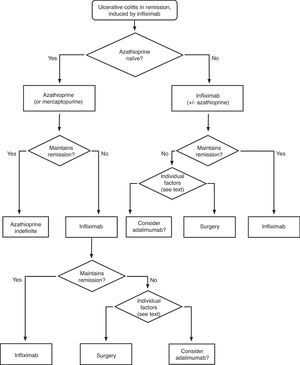
When steroids fail, there are mainly three options: cyclosporine, infliximab and surgery. The lack of comparative studies hinders us from being specific. In this decision node, the opinion of the well-informed patient is very important, with the collaboration of relatives and associated people, and it is absolutely necessary to rule out other causes of refractoriness, especially a colon infection by CMV64 and by Clostridium difficile (by means of the study of signs of infection by CMV in a rectal biopsy, and by looking for specific toxins in faeces, respectively). The following favour surgery: a prolonged history of the disease and/or the presence of dysplasia in previous controls, as well as two clinical situations: toxic megacolon and massive haemorrhage. However, in almost all cases, a medical option can be tried. If the patient has suffered a severe flare while already on treatment with thiopurines (drugs that would be necessary in the post-cyclosporine maintenance treatment), it would, probably, be most indicated to use infliximab. In the rest of the cases, the choice may be between cyclosporine37 or infliximab,43 a decision in which the team's experience and other local factors (the possibility of determining blood levels of cyclosporine, for example) are determinant. The general recommendations for the use of infliximab65 (Table 13) or cyclosporine37 must be followed (Tables 14–16).
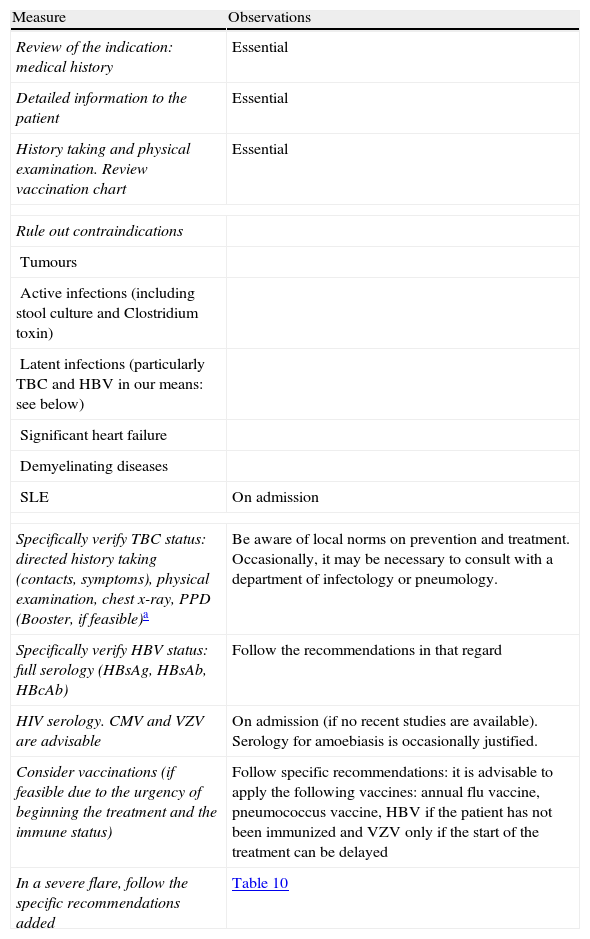
Measures prior to starting the treatment with infliximab.
| Measure | Observations |
| Review of the indication: medical history | Essential |
| Detailed information to the patient | Essential |
| History taking and physical examination. Review vaccination chart | Essential |
| Rule out contraindications | |
| Tumours | |
| Active infections (including stool culture and Clostridium toxin) | |
| Latent infections (particularly TBC and HBV in our means: see below) | |
| Significant heart failure | |
| Demyelinating diseases | |
| SLE | On admission |
| Specifically verify TBC status: directed history taking (contacts, symptoms), physical examination, chest x-ray, PPD (Booster, if feasible)a | Be aware of local norms on prevention and treatment. Occasionally, it may be necessary to consult with a department of infectology or pneumology. |
| Specifically verify HBV status: full serology (HBsAg, HBsAb, HBcAb) | Follow the recommendations in that regard |
| HIV serology. CMV and VZV are advisable | On admission (if no recent studies are available). Serology for amoebiasis is occasionally justified. |
| Consider vaccinations (if feasible due to the urgency of beginning the treatment and the immune status) | Follow specific recommendations: it is advisable to apply the following vaccines: annual flu vaccine, pneumococcus vaccine, HBV if the patient has not been immunized and VZV only if the start of the treatment can be delayed |
| In a severe flare, follow the specific recommendations added | Table 10 |
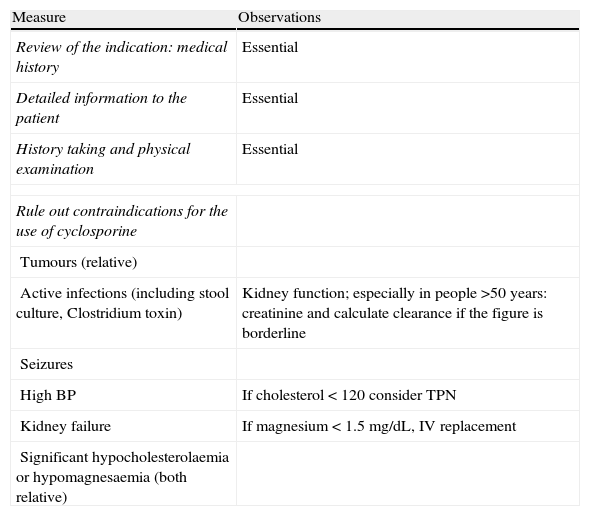
Measures prior to starting the treatment with cyclosporine.
| Measure | Observations |
| Review of the indication: medical history | Essential |
| Detailed information to the patient | Essential |
| History taking and physical examination | Essential |
| Rule out contraindications for the use of cyclosporine | |
| Tumours (relative) | |
| Active infections (including stool culture, Clostridium toxin) | Kidney function; especially in people >50 years: creatinine and calculate clearance if the figure is borderline |
| Seizures | |
| High BP | If cholesterol<120 consider TPN |
| Kidney failure | If magnesium<1.5mg/dL, IV replacement |
| Significant hypocholesterolaemia or hypomagnesaemia (both relative) | |
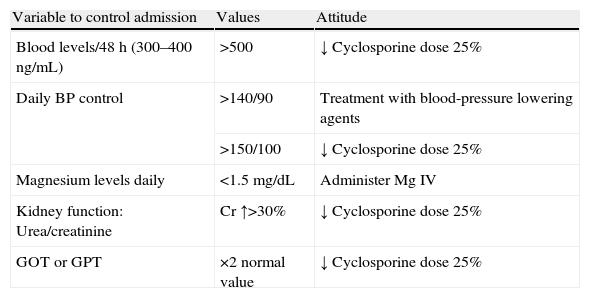
Follow-up measures for the treatment with cyclosporine.
| Variable to control admission | Values | Attitude |
| Blood levels/48h (300–400ng/mL) | >500 | ↓ Cyclosporine dose 25% |
| Daily BP control | >140/90 | Treatment with blood-pressure lowering agents |
| >150/100 | ↓ Cyclosporine dose 25% | |
| Magnesium levels daily | <1.5mg/dL | Administer Mg IV |
| Kidney function: Urea/creatinine | Cr ↑>30% | ↓ Cyclosporine dose 25% |
| GOT or GPT | ×2 normal value | ↓ Cyclosporine dose 25% |
| Variables to control on an outpatient basis | Attitude |
| Triple immunosuppression | Associate trimetoprim-sulfamethoxazole |
| Full blood chemistry, weekly during the first month | ↓ Progressive steroid dose and begin Azathioprine/mercaptopurine |
| Complete blood chemistry, every 15 days, 2nd month | |
| Full blood chemistry, subsequently on a monthly basis |
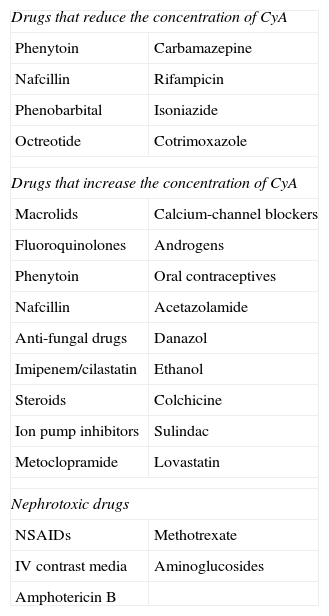
Pharmacological interactions of cyclosporine.
| Drugs that reduce the concentration of CyA | |
| Phenytoin | Carbamazepine |
| Nafcillin | Rifampicin |
| Phenobarbital | Isoniazide |
| Octreotide | Cotrimoxazole |
| Drugs that increase the concentration of CyA | |
| Macrolids | Calcium-channel blockers |
| Fluoroquinolones | Androgens |
| Phenytoin | Oral contraceptives |
| Nafcillin | Acetazolamide |
| Anti-fungal drugs | Danazol |
| Imipenem/cilastatin | Ethanol |
| Steroids | Colchicine |
| Ion pump inhibitors | Sulindac |
| Metoclopramide | Lovastatin |
| Nephrotoxic drugs | |
| NSAIDs | Methotrexate |
| IV contrast media | Aminoglucosides |
| Amphotericin B | |
In any of the medical options chosen, a new moment of evaluation must be planned, and probably, it would be most reasonable to carry out an evaluation after seven days of having started the new treatment. At this point, there are no set norms. If there is a worsening or an absolute lack of improvement, in most cases, the most reasonable course of action is surgery; if there is remission, we may move on to the maintenance regimen. Nonetheless, it is very common for intermediate situations to take place, in which all the possibilities must be evaluated. Thus, it has been described that in spite of the failure with cyclosporine, some patients may avoid surgery by using infliximab,66 and also, less frequently, the opposite path has been taken.
In all the decision nodes, the opinion of the patient, or that of his/her relatives and/or friends, the opinion of the surgeon and the opinion of the gastroenterologist must be considered carefully. We cannot emphasize enough on how relevant it is to share all the information with the patient from the start.
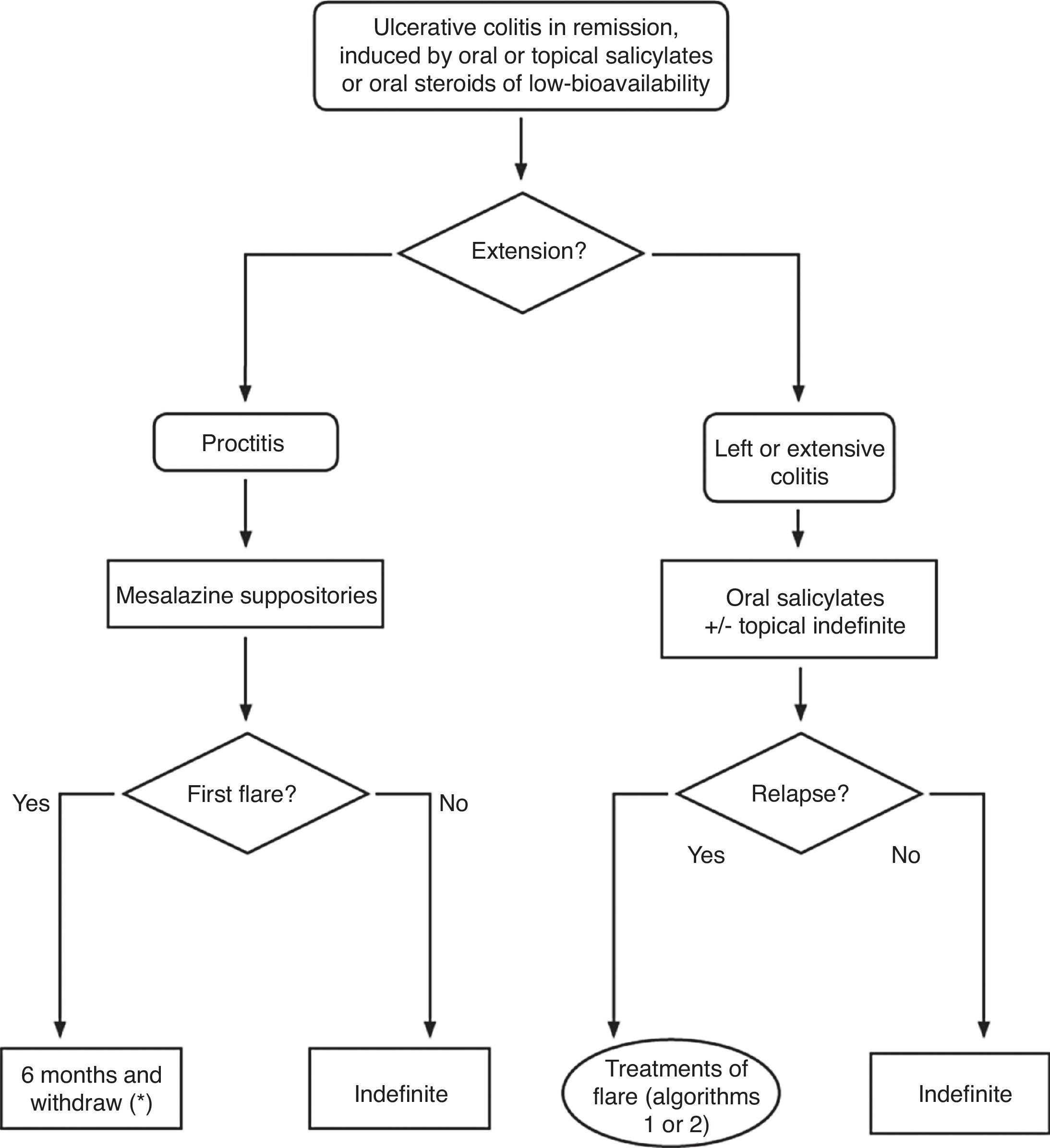
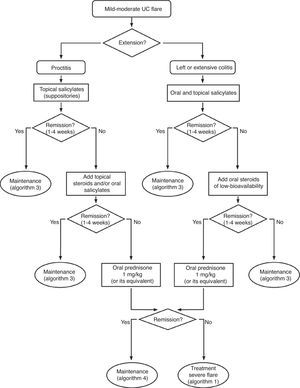
6.2Mild and moderate UC flare (Algorithm 2)6.2.1Salicylates- -
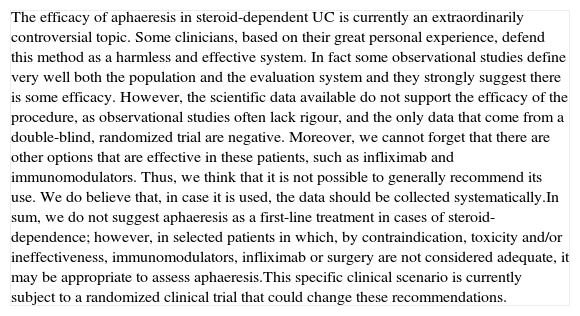
Is the treatment with oral salicylates effective in induction of remission in patients with a mild–moderate UC flare?
| In oral salicylates, the objective to assess is clinical remission after four weeks of treatment. Neither the response, nor the remission can be evaluated at two weeks’ time, as there are not enough studies available for these data. In literature, we have identified 11 high-quality RCTs that compare mesalazine to placebo in the treatment of the mild–moderate UC flare: 9 included in a Cochrane review67 and 2 subsequent ones with the new MMX formulation.68,69 Furthermore, there is a recent meta-analysis in which 20 RCTs are included, comparing the efficacy and safety of sulfasalazine against other salicylates.70In the Cochrane review,67 9 RCTs are included (1495 patients) comparing the induction of remission with mesalazine–placebo and 12 RCTs comparing mesalazine–sulfasalazine that assess the remission obtained between the fourth and twelfth week of treatment. Moreover, a study with mesalazine MMX69 compared 2.4g/day against 4.8g/day and placebo, both doses being more effective than placebo, without being any differences between them. Globally, the calculated global remission RR was of 1.92 [1.50–2.47], indicating a benefit from the use of mesalazine in these patients. Sulfasalazine is equally effective as the different salicylates used in the induction of remission, though there are a greater percentage of side effects that prompt the withdrawal from RCTs that compare sulfasalazine to balsalazide.70When the rate of remission is analyzed in terms of the dose, there is a tendency for the greater the dose, the greater the rate of remission (p=0.002); and when the analyzed variable is endoscopic remission, the doses of 3g/day or higher are more effective. It is interesting that in all the studies in which a single dose of mesalazine is used in 24h, it works at least as effectively as distributed doses,71,72 an attitude that is supported by data on bioavailability coming from pharmacokinetic studies.In all the studies available, which are numerous, no significant differences were seen in the side effects between placebo group and patients treated with different doses of mesalazine. | High quality |
| Although in a recent review73 comparative studies of different doses of mesalazine are evaluated, and there seem to be no differences in induction of clinical remission from 2.4g, we believe that these results must be interpreted cautiously because: (a) the numbers are smaller when the groups are reduced to compare the doses and, therefore, the conclusions are weaker; (b) doses of 3g/day or more are not significantly more toxic; (c) in some studies, higher doses obtain remission earlier, which, if confirmed, would be of great importance for the patients; (d) the comparisons made with a pharmaceutical formula cannot necessarily be completely extrapolated to the rest. This opinion is supported in a recent study of high methodological quality (ASCEND III)74 (with 772 patients in whom one dose of 4.8g/day is superior to 2.4g/day). Therefore, it is the opinion of the elaborating group that the standard dose of remission must be 3g daily (or more). What seems very clear, nowadays, is that a single daily dose is at least as effective as distributed doses, and because of its easier compliance, it would probably be even more effective.75 |
- -
Is the combined treatment with oral and topical salicylates more effective than oral treatment alone in induction of remission in patients with a mild–moderate UC flare?
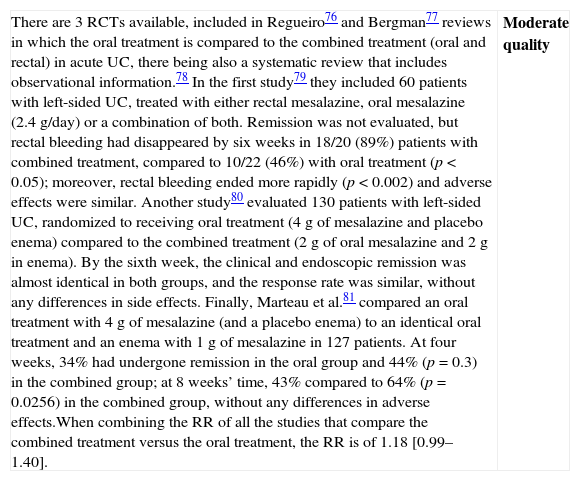
| There are 3 RCTs available, included in Regueiro76 and Bergman77 reviews in which the oral treatment is compared to the combined treatment (oral and rectal) in acute UC, there being also a systematic review that includes observational information.78 In the first study79 they included 60 patients with left-sided UC, treated with either rectal mesalazine, oral mesalazine (2.4g/day) or a combination of both. Remission was not evaluated, but rectal bleeding had disappeared by six weeks in 18/20 (89%) patients with combined treatment, compared to 10/22 (46%) with oral treatment (p<0.05); moreover, rectal bleeding ended more rapidly (p<0.002) and adverse effects were similar. Another study80 evaluated 130 patients with left-sided UC, randomized to receiving oral treatment (4g of mesalazine and placebo enema) compared to the combined treatment (2g of oral mesalazine and 2g in enema). By the sixth week, the clinical and endoscopic remission was almost identical in both groups, and the response rate was similar, without any differences in side effects. Finally, Marteau et al.81 compared an oral treatment with 4g of mesalazine (and a placebo enema) to an identical oral treatment and an enema with 1g of mesalazine in 127 patients. At four weeks, 34% had undergone remission in the oral group and 44% (p=0.3) in the combined group; at 8 weeks’ time, 43% compared to 64% (p=0.0256) in the combined group, without any differences in adverse effects.When combining the RR of all the studies that compare the combined treatment versus the oral treatment, the RR is of 1.18 [0.99–1.40]. | Moderate quality |
| The differences do not reach statistical significance, but this may be due to the study design. The results are greatly conditioned by Vecchi's study, in which the groups are not homogenous: the group treated recently receives an oral dose of mesalazine that is lower than that of the other group. Marteau's study, which has the best methodology, shows that rectal therapy accelerates the response. Taking these data into account, we believe that the combined treatment must be offered to the patient, especially if rectal symptoms are intense. It is important to point out that adverse effects are not at all important. However, given the special discomfort of the treatment route, and the scarce evidence, the opinion of each patient will be decisive. |
- -
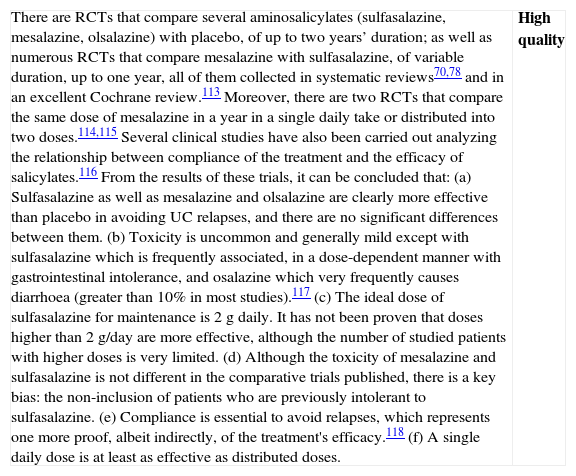
Is the treatment with topical salicylates alone effective in induction of remission in patients with a mild–moderate flare of left-sided UC?
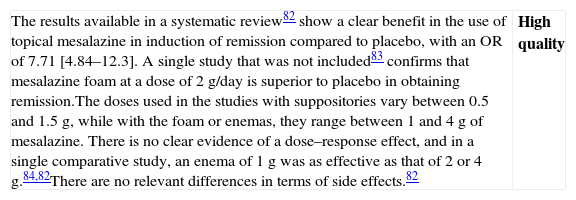
| The results available in a systematic review82 show a clear benefit in the use of topical mesalazine in induction of remission compared to placebo, with an OR of 7.71 [4.84–12.3]. A single study that was not included83 confirms that mesalazine foam at a dose of 2g/day is superior to placebo in obtaining remission.The doses used in the studies with suppositories vary between 0.5 and 1.5g, while with the foam or enemas, they range between 1 and 4g of mesalazine. There is no clear evidence of a dose–response effect, and in a single comparative study, an enema of 1g was as effective as that of 2 or 4g.84,82There are no relevant differences in terms of side effects.82 | High quality |
Formulation of the recommendation
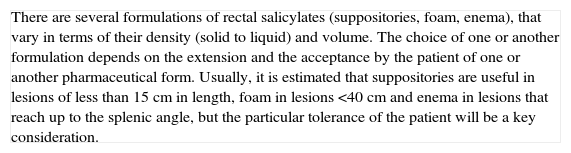
| There are several formulations of rectal salicylates (suppositories, foam, enema), that vary in terms of their density (solid to liquid) and volume. The choice of one or another formulation depends on the extension and the acceptance by the patient of one or another pharmaceutical form. Usually, it is estimated that suppositories are useful in lesions of less than 15cm in length, foam in lesions <40cm and enema in lesions that reach up to the splenic angle, but the particular tolerance of the patient will be a key consideration. |
- -
Is treatment with topical salicylates more effective than oral treatment in induction of remission in patients with left-sided UC flare?
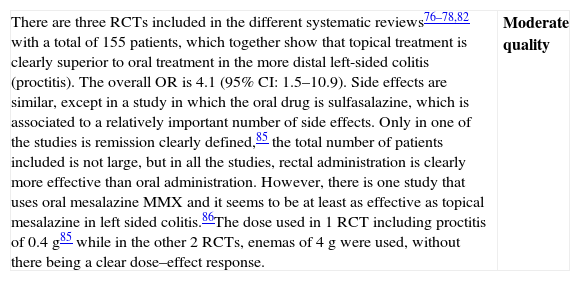
| There are three RCTs included in the different systematic reviews76–78,82 with a total of 155 patients, which together show that topical treatment is clearly superior to oral treatment in the more distal left-sided colitis (proctitis). The overall OR is 4.1 (95% CI: 1.5–10.9). Side effects are similar, except in a study in which the oral drug is sulfasalazine, which is associated to a relatively important number of side effects. Only in one of the studies is remission clearly defined,85 the total number of patients included is not large, but in all the studies, rectal administration is clearly more effective than oral administration. However, there is one study that uses oral mesalazine MMX and it seems to be at least as effective as topical mesalazine in left sided colitis.86The dose used in 1 RCT including proctitis of 0.4g85 while in the other 2 RCTs, enemas of 4g were used, without there being a clear dose–effect response. | Moderate quality |
| Both the observational studies as well as the clinical experience strongly suggest that rectal salicylates are more effective than oral salicylates in left-sided UC. Furthermore, in basic studies, it has been proven that the mucous concentration of the drug that is reached is much higher when applied topically. For this reason, and although it is not always possible, the doctor must try to overcome the reluctance many patients have to a rectal treatment. It is not at all easy to recommend a specific dose, because the studies are scarce, and what is most important is, most likely, to achieve good compliance by the patient. There are suppositories (of 500mg and of 1g), enemas (of 1g and 4g) and foam (of 1g), and at the moment, it is not possible to issue general recommendations.Some authors have suggested that the new pharmaceutical form (MMX) could obtain better results in left sided UC, as has been reported also with mesalazine in microgranules, but both claims are based in only one study each and need independent confirmation before being useful to guide clinical decisions. |
- -
Are systemic oral steroids effective in induction of remission in patients with mild–moderate UC flares?
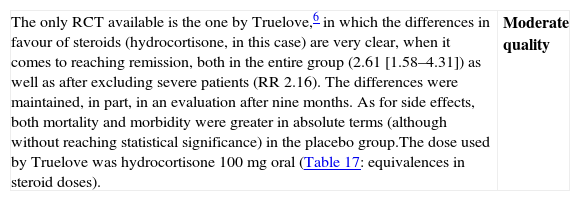
| The only RCT available is the one by Truelove,6 in which the differences in favour of steroids (hydrocortisone, in this case) are very clear, when it comes to reaching remission, both in the entire group (2.61 [1.58–4.31]) as well as after excluding severe patients (RR 2.16). The differences were maintained, in part, in an evaluation after nine months. As for side effects, both mortality and morbidity were greater in absolute terms (although without reaching statistical significance) in the placebo group.The dose used by Truelove was hydrocortisone 100mg oral (Table 17: equivalences in steroid doses). | Moderate quality |
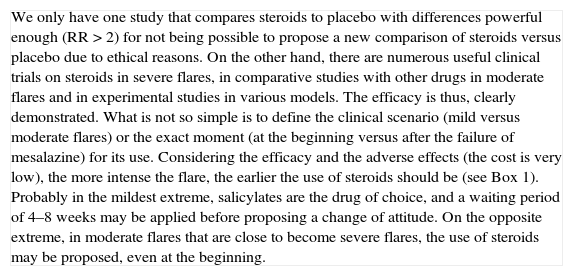
| We only have one study that compares steroids to placebo with differences powerful enough (RR>2) for not being possible to propose a new comparison of steroids versus placebo due to ethical reasons. On the other hand, there are numerous useful clinical trials on steroids in severe flares, in comparative studies with other drugs in moderate flares and in experimental studies in various models. The efficacy is thus, clearly demonstrated. What is not so simple is to define the clinical scenario (mild versus moderate flares) or the exact moment (at the beginning versus after the failure of mesalazine) for its use. Considering the efficacy and the adverse effects (the cost is very low), the more intense the flare, the earlier the use of steroids should be (see Box 1). Probably in the mildest extreme, salicylates are the drug of choice, and a waiting period of 4–8 weeks may be applied before proposing a change of attitude. On the opposite extreme, in moderate flares that are close to become severe flares, the use of steroids may be proposed, even at the beginning. |
- -
Are the new oral steroids of low bioavailability effective in induction of remission in patients with mild–moderate UC flares?
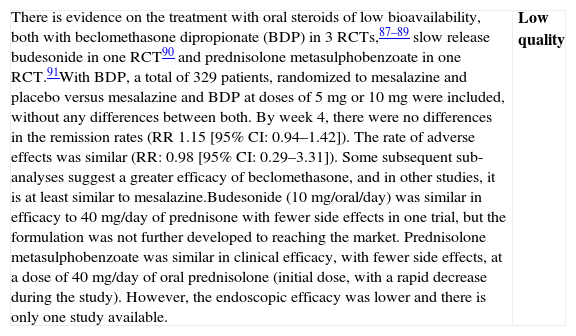
| There is evidence on the treatment with oral steroids of low bioavailability, both with beclomethasone dipropionate (BDP) in 3 RCTs,87–89 slow release budesonide in one RCT90 and prednisolone metasulphobenzoate in one RCT.91With BDP, a total of 329 patients, randomized to mesalazine and placebo versus mesalazine and BDP at doses of 5mg or 10mg were included, without any differences between both. By week 4, there were no differences in the remission rates (RR 1.15 [95% CI: 0.94–1.42]). The rate of adverse effects was similar (RR: 0.98 [95% CI: 0.29–3.31]). Some subsequent sub-analyses suggest a greater efficacy of beclomethasone, and in other studies, it is at least similar to mesalazine.Budesonide (10mg/oral/day) was similar in efficacy to 40mg/day of prednisone with fewer side effects in one trial, but the formulation was not further developed to reaching the market. Prednisolone metasulphobenzoate was similar in clinical efficacy, with fewer side effects, at a dose of 40mg/day of oral prednisolone (initial dose, with a rapid decrease during the study). However, the endoscopic efficacy was lower and there is only one study available. | Low quality |
| The evidence available suggests that adding BDP to mesalazine could improve the clinical response in mild–moderate UC flares. However, the data are limited and no differences have been shown in the course of endoscopic or histological lesions. A large part of the studies are not double-blind. Budesonide and prednisolone metasulphobenzoate could be an alternative to prednisolone, but the published data are too limited. | Low quality |
| The efficacy of rectal steroids, the experience in Crohn's disease, and the data of the study available, very strongly suggests that low bioavailability oral steroids of (the only one accessible in the market is beclomethasone) may be, in the future, an alternative in the treatment of mild UC flares. Specifically, they would be the first option in mesalazine failures, before moving on to systemic steroids, specifically in mild flares. However, the trials are limited in terms of methodological quality, and in the number of patients evaluated. Furthermore, an external validation by other groups not directly involved in the development of the drug is missing. Taking into account all the data available to date (that include an extensive observational experience by GETECCU),92 we believe that its use can be especially recommended in patients with mild flares that do not reach a complete response to mesalazine. In any case, we recommend an early evaluation of response (two to four weeks) and in case of failure, to consider the introduction of systemic steroids. On the other hand, the most used dose is 5mg/day, but some (not all) of the studies suggest that 10mg/day could be a more effective alternative and with a similar toxicity. |
- -
Is the treatment with rectal steroids effective in induction of remission in patients with a mild–moderate flare of left-sided UC?
| We divided rectal steroids into two groups:-High bioavailability steroids: hydrocortisone, prednisolone, methylprednisolone and betamethasone-Low bioavailability steroids: budesonide, beclomethasone and prednisolone metasulphobenzoateAlthough we only have two studies that are now old,93,94 steroids of high bioavailability are clearly more effective than placebo (OR of 0.21 [0.07–0.71]). With steroids of low bioavailability we evaluated a study in which it was superior to placebo,95 and another in which significant differences were not noted in all the doses, but it was confirmed that the 2mg enema is clearly superior to placebo.96 | Moderate quality |
| Although there are few comparative studies with placebo available, the information coming from comparative studies with mesalazine is much more important (see below), and it confirms the effectiveness of steroids in this clinical context, aside from the indirect evidence provided by studies of oral steroids. Being mesalazine at least as effective and probably less toxic and cheaper, rectal steroids are a second choice, alone or associated to mesalazine. |
- -
Are low bioavailability rectal steroids more effective than those of high bioavailability in the induction of remission in patients with mild–moderate UC flares?
| In a systematic review,97 5 RCTs were included in which high bioavailability rectal steroids are compared to low bioavailability steroids: 3 RCTs compare budesonide enemas at doses of 2–2.5mg to prednisolone enemas, 1 RCT compares budesonide enemas to methylprednisolone, and 1 RCT compares budesonide enemas to hydrocortisone enemas. The OR for clinical remission of these 5 RCTs was of 0.85 [95% CI: 0.44–1.63], without differences between the two groups. Data for endoscopic remission (1.14 [95% CI: 0.69–1.88]) or histological remission (0.68 [95% CI: 0.28–1.67]) were not significant either. The suppression of the adrenal axis (the most relevant adverse effect, in comparison) was lower when low bioavailability steroids are used. Other subsequent trials,98–100 confirm these results using beclomethasone, prednisolone phosphate, budesonide and hydrocortisone. | Moderate quality |
| An evaluation using a formal GRADE table has not been possible due to the enormous heterogeneity of studies, with very different drugs and doses. Even if the differences between the different low bioavailability steroids are probably very subtle, budesonide enemas are the only ones that have been compared to placebo, to high bioavailability steroids, and to others of low bioavailability (aside from mesalazine), and it is the drug with most scientific evidence of the agents analyzed, with several studies of a high quality published, which is why, at this moment, it may be considered the rectal steroid of reference. |
- -
Are topical salicylates more effective than rectal steroids in the induction of remission in patients with a mild–moderate UC flare?
| Mesalazine versus rectal high bioavailability steroidsWith a total of 9 RCTs available included in a systematic review97 comparing rectal steroids (100–356mg of hydrocortisone) to rectal mesalazine (1–4g), the mesalazine preparations are more effective in obtaining clinical remission with an OR of 2.42 [95% CI: 1.72–3.41]. The same was observed in endoscopic and histological remissions. There are two RCTs comparing with para-aminosalicylic acid (4-ASA) with similar results. | High quality |
| Mesalazine versus rectal steroids of low bioavailabilityIn two RCTs of high quality included in Marshall's review97 comparing budesonide with mesalazine, there are no differences (OR 0.95 [95% CI: 0.43–2.10]).In a review101 of clinical trials with mesalazine versus beclomethasone, of a low methodologic quality, no significant differences were seen, although mesalazine reached superiority (RR: 0.89 [95% CI: 0.78–1.01]).There is a RCT of good quality, but with a very limited number of patients, which suggests that the combination of steroids (beclomethasone) and mesalazine could have synergic effects.102 | Low quality |
| Topical mesalazine is at least as effective as rectal steroids, it has a lower toxicity potential, it is cheaper, and it exists in several pharmaceutical formulations that allow for its adaptation to almost all clinical scenarios. However, it is not tolerated by every individual patient, nor does it have an absolute efficacy. In these cases, rectal steroids alone or combined with mesalazine are the adequate alternative. If steroids are used, those of low bioavailability are clearly preferable, and among them, the one that has the most scientific evidence in its favour is budesonide. |
- -
Are thiopurine immunomodulators effective in induction of remission in patients with a moderate flare of steroid-dependent and/or steroid-resistant UC?
| In a recent meta-analysis103 that includes 4 RCTs in which the efficacy of AZA/MP is compared against placebo and/or salicylates, no significant differences are reached and also, the different studies are heterogeneous.However, there are numerous observational studies that obtain remission rates of 65% [95% CI: 55–75%] which suggest a possible efficacy in this indication. | Low quality |
| Numerous studies show that thiopurines are drugs for which their efficacy in inflammatory bowel diseases requires a minimum time of administration to obtain efficacy104 (at least one month, probably in most cases more than two months).Without being able to rule out a beta-type error (the number of patients included in these studies is very scarce), the short duration of the observation could explain the inefficacy. In any case, such a long latency is unacceptable when the patient presents with acute symptoms, which is why thiopurines are not drugs to be used in the induction of response. |
- -
Is methotrexate effective in induction of remission in patients with a moderate flare of steroid-dependent UC?
| We found several studies in literature trying to analyze the efficacy of methotrexate for induction of remission in patients with UC, and that have been described in a recent Cochrane review.105 Of the 17 localized studies identified, only 3 were randomized; these 3 only include the study carried out by Oren,106 being the rest excluded due to severe methodological deficiencies. In this trial, 67 patients with an active UC (Mayo>7), of which 70% received steroids at variable doses were randomized to oral methotrexate 12.5mg/week (n=30) or placebo (n=37) for 9 months. There were no differences, either in the rate of remission (OR 0.92; 95% CI: 0.35–2.42), or in the time to remission, nor in the rate of serious adverse effects. In the excluded studies, a relevant difference was not observed either. | Low quality |
| Currently, the trials available with methotrexate in UC are of a minimum quality. The subgroups of patients who are heterogeneous in terms of steroid-dependence are not well defined, and the methotrexate dose used is (if extrapolated from what is known in Crohn's disease) is insufficient. Therefore, we can only conclude that it is unlikely that low-dose oral methotrexate is effective in the treatment of acute UC. An effect at higher doses, similar to the ones used in Crohn's disease, cannot be ruled out. As it is a cheap drug with an important cumulative experience in other diseases, it is evident that it should be a preferred field of study. |
- -
Is infliximab effective in induction of remission in patients with a moderate flare of steroid-dependent and/or steroid-resistant UC?
| See “Biological therapies” section regarding severe flares. Gisbert's systematic review43 is also the fundamental base of this summary. We will analyze separately the two most common and problematic situations in clinical practice: steroid-resistance and steroid-dependence.Steroid-resistance: there are several data in this clinical scenario showing that infliximab is effective in inducing remission and clinical response. Thus, in the quoted review43 52% of 877 patients were classified as steroid-resistant with a lack of response of 70% of infliximab versus 68% in non steroid-resistant patients. Specifically, in ACT studies in which a specific definition of steroid resistance (lack of clinical response after 2 weeks of oral prednisone 40mg/day; or the same dose intravenously for one week) response to infliximab was observed at 8 weeks in 73% of refractory patients versus 63% of non-refractory patients (NS).Steroid-dependence: although with some limitations as well (essentially derived from the difficulty of strictly applying the different definition), in ACT studies, a clear steroid-saving effect is shown. When evaluating patients in week 30 after starting the treatment, it was achieved to withdraw steroids in 22% of the patients treated with infliximab versus 7.1% of the patients treated with placebo (p<0.05).44 Another RCT107, although with much fewer patients, also reports similar results. | High quality |
| We have solid evidence that shows that infliximab is effective in inducing remission and response in moderate colitis flares, both in steroid-resistance or steroid-dependence. It obtains clinical remission and reduces the rate of colectomies. However, the rate of remission reached in controlled studies does not exceed 50%. The safety of the drug can be superimposed to the one known for Crohn's disease. | High quality |
| There are three considerations that can make this recommendation controversial. First, the remission rate is limited and below 50% in most studies. However, in many cases the only realistic alternative is surgery and a trial of infliximab seems warranted before colectomy. Second, there remain some concerns with the long-term risk of side-effects. However, most recent data both from gastroenterologic and reumathologic experiences are reassuring. Third, the cost of the drug is very high, and is an obvious limitation. However, the costs of surgery, hospitalizations, and short and long-term complications of surgery are in the other side of the coin. While awaiting formal cost-effectiveness analysis, a really difficult task when indirect costs are the most relevant, cost cannot be the main consideration. Considering all these facts it is not easy to decide between a recommendation or a suggestion.No specific GRADE tables were drafted due to a lack of specific, differentiated data in this specific scenario. |
- -
Is adalimumab effective in induction of remission in patients with a moderate flare of steroid-dependent and/or steroid-resistant UC?
| The available data are derived from a single controlled clinical trial108 that compares adalimumab to placebo in patients with moderate to severe UC without any response to concomitant steroids or immunomodulators, or with prior intolerance to these drugs. It included >500 patients, on an outpatient basis, with moderate to severe flares, defined as a Mayo index ≥ 6 and an endoscopic subindex ≥ 2. Patients who had previously received infliximab or another biological agent, anti-calcineuric agents within the previous 30 days, or intravenous steroids within the previous 14 days, were specifically included. It is not possible to analyze the data specifically in steroid-dependent or steroid-resistant patients, although 67% of the patients received steroids at the beginning of the study. Two different regimens of adalimumab were used for induction (160/80 and 80/40) that were compared with placebo. The primary objective of the trial was clinical remission at week 8 (Mayo index≤2 with no subindex>1). At that moment, the remission rate was 18.5% in the group treated with adalimumab 160/80 (p 0.031 versus placebo), 10.0% in the adalimumab group 80/40 (p 0.833 versus placebo) and 9.2% in the placebo group. There were no deaths in any of the groups. The rate of colectomies is not reflected in the study. Adalimumab was well tolerated, with a profile of adverse effects that is similar to that of previous clinical trials. In sum, these results show a significant efficacy of adalimumab, although it is only discretely higher than placebo and exclusive for the dose of 160/80. In any case, the high response obtained with placebo is notable, and it even reached a 40% of mucosal healing.Aside from these data derived from the single available clinical trial, there are several observational studies the results of which are consistent and support the efficacy of adalimumab in UC, even if most of them focus on patients controlled with infliximab who have lost the response or have experienced infusion reactions. | Moderate quality |
| We only have one clinical trial published on adalimumab in UC,108 with several observational studies with consistent results. Even if the results available are still short-term, and the differences obtained compared to placebo are small, adalimumab proved to be more effective in obtaining clinical remission in moderate to severe flares without any response to steroids or immunomodulators | Moderate quality |
| New controlled data on adalimumab in UC are necessary. Furthermore, results at 52 weeks of the published and commented trial will allow us to analyze them with a greater perspective. The increased dose of adalimumab could optimize its efficacy, as the sub-analyses of the results of the existing controlled trial suggest and they must be contemplated. |
- -
Is the treatment with aphaeresis effective in induction of remission in patients with a moderate flare of steroid-dependent UC?
| There are numerous studies with aphaeresis, but most of them are of low quality, with very heterogeneous response measures, open-labelled assignment to treatment groups, or concomitant treatments that make assessing the study very difficult. The data from the single randomized, open-label trial109 do not confirm the efficacy of aphaeresis. However, other open-label, randomized, observational studies,110,111 suggest that in steroid-dependent patients, adding aphaeresis may be followed by clinical response. The coherence of the results of several independent studies in the response percentage (around 2/3 of the patients), and at the moment of the response (almost all the studies show there is a response after the third session) suggests that there may be a real therapeutic effect of the procedure.112 However, blinded comparative data are still lacking. It is a high-cost technique, but the side effects are scarce. No common serious adverse events have been described in the available studies. | Low quality |
| The efficacy of aphaeresis in steroid-dependent UC is currently an extraordinarily controversial topic. Some clinicians, based on their great personal experience, defend this method as a harmless and effective system. In fact some observational studies define very well both the population and the evaluation system and they strongly suggest there is some efficacy. However, the scientific data available do not support the efficacy of the procedure, as observational studies often lack rigour, and the only data that come from a double-blind, randomized trial are negative. Moreover, we cannot forget that there are other options that are effective in these patients, such as infliximab and immunomodulators. Thus, we think that it is not possible to generally recommend its use. We do believe that, in case it is used, the data should be collected systematically.In sum, we do not suggest aphaeresis as a first-line treatment in cases of steroid-dependence; however, in selected patients in which, by contraindication, toxicity and/or ineffectiveness, immunomodulators, infliximab or surgery are not considered adequate, it may be appropriate to assess aphaeresis.This specific clinical scenario is currently subject to a randomized clinical trial that could change these recommendations. |
Mild to moderate flare: algorithm and explanatory text
We had to group patients this way, as clinical trials use this classification system. Of course, within the same global definition, an almost infinite number of possible clinical scenarios are included (Box 1). The clinician will recommend very different actions with very mild or almost severe situations: all of them fit the definition. Aside from severity, the clinician needs information about the extension of the disease and (fundamentally) about the response and tolerance to treatments of the specific patient in previous flares. Hence, there are patients who tolerate topical therapy very poorly, or patients in whom mesalazine causes a paradoxical reaction with increased diarrhoea. Once more, we are forced to emphasize on the extraordinary practical importance of a complete medical history. Being the flare mild or moderate, the attitude will be completely different in proctitis, in which the local treatment will be basic, and the rest of colitis in which a combined local and oral treatment will be the norm. Of course, just as between the mildest flare and the most intense moderate flare there is a whole continuum; the limits of the treatment in terms of extension are not absolutely rigid: it may be acceptable to treat a case of proctosigmoiditis exclusively with topical treatment, and it may also be acceptable to treat an extensive colitis exclusively with oral medication.
Just as we have reviewed in detail, the clinician has a variety of treatment measures that, generally, he/she uses in an escalated manner: more simple measures and with less risks in the milder flares that broadens if no response is obtained. A strategy of using the less aggressivea options first is only acceptable if a strategy in the mid and long term that involves knowing the patient's response to the treatment in a reasonable time frame has been planned from the beginning. Actually, it is impossible to define a uniform timeframe exactly, due to the enormous inter-individual variability. Generally speaking, the milder the clinical case, the greater the interval may be before knowing the patient's response, but in no case must it be over one month, in order to be able to choose other treatment systems if the one we have recommended fails. Nowadays we have to bear in mind some factors that may make follow-up easier. First, the communication with the patient does not always have to be in person, as the telephone and the Internet can perfectly work for maintaining a contact that is much more fluent, rapid and simple. Second, within the Units of Inflammatory Disease, the nursing staff has an increasingly more important role, and among their missions it may be included to more closely monitor the patients’ situation. In fact, software tools have been developed that have proven to be helpful in gaining more control in controlled trials. In the entire process, the patient's participation is fundamental, and therefore, all efforts must be made to transmit the information and to listen to the patient's point of view.
The therapeutic tools that are managed in most of the mild to moderate active flares are salicylates, low bioavailability steroids and systemic steroids. Furthermore, one can choose between rectal and oral routes, and a combination of both. The algorithm suggests a reasonable path, especially if we start from a mild flare, by progressively adopting more aggressive measures, sequentially. However, depending on the patient's medical history and the severity of the flare, the clinician may recommend to directly starting the treatment regimen in a much more advanced point. Thus, for example, if a patient who is receiving 3g daily of maintenance oral mesalazine, with an extensive colitis, and that presents with a UC flare since a week ago with five bloody bowel movements daily, fever and asthenia, after ruling out infection, it is reasonable to directly begin the treatment with oral steroids, completing the treatment with topical salicylates in the case of intense rectal symptoms being present. Moreover, of course, in this case, follow-up will be carried out in only one week's time, aside from leaving open communication channels with the patient in case there was a previous worsening. That is, depending on the clinical scenario, the history and the specific experiences of the patient, both the patient and the healthcare professionals must come to an agreement that modulates the recommendations of the general algorithm.
7Maintenance treatment of patients with ulcerative colitis in remissionColitis in remission is defined as the one in which the patient have a complete resolution of the symptoms accompanied by mucosal healing defined with clinical and endoscopic indices. In routine daily clinical practice, endoscopic assessment is usually not considered necessary, as the clinical assessment of the patients has a sensitivity of 86% and a specificity of 76% and would be defined as the presence of at least 3 bowel movements/day or maybe even better, the recovery of the patient's prior normal bowel rhythm, without rectal bleeding or defecation urgency.
7.1Maintenance of remission obtained after a mild–moderate flare7.1.1UC not treated with steroids (Algorithm 3)- -
Is treatment with oral salicylates effective in maintenance of remission in patients after a mild–moderate UC flare treated with salicylates?
| There are RCTs that compare several aminosalicylates (sulfasalazine, mesalazine, olsalazine) with placebo, of up to two years’ duration; as well as numerous RCTs that compare mesalazine with sulfasalazine, of variable duration, up to one year, all of them collected in systematic reviews70,78 and in an excellent Cochrane review.113 Moreover, there are two RCTs that compare the same dose of mesalazine in a year in a single daily take or distributed into two doses.114,115 Several clinical studies have also been carried out analyzing the relationship between compliance of the treatment and the efficacy of salicylates.116 From the results of these trials, it can be concluded that:(a) Sulfasalazine as well as mesalazine and olsalazine are clearly more effective than placebo in avoiding UC relapses, and there are no significant differences between them.(b) Toxicity is uncommon and generally mild except with sulfasalazine which is frequently associated, in a dose-dependent manner with gastrointestinal intolerance, and osalazine which very frequently causes diarrhoea (greater than 10% in most studies).117(c) The ideal dose of sulfasalazine for maintenance is 2g daily. It has not been proven that doses higher than 2g/day are more effective, although the number of studied patients with higher doses is very limited.(d) Although the toxicity of mesalazine and sulfasalazine is not different in the comparative trials published, there is a key bias: the non-inclusion of patients who are previously intolerant to sulfasalazine.(e) Compliance is essential to avoid relapses, which represents one more proof, albeit indirectly, of the treatment's efficacy.118(f) A single daily dose is at least as effective as distributed doses. | High quality |
| Salicylates are effective in the treatment of long-term maintenance of UC in which remission has been obtained with salicylates. In patients who tolerate sulfasalazine, it is at least equivalent to mesalazine, although its effect on spermatogenesis could limit its indications. A single daily dose is as useful as distributed doses. | High quality |
| The evidence to use salicylates in the maintenance treatment of UC is incontrovertible. It may be added that it is possible (although it has been heavily discussed) that salicylates have a chemo-preventive effect, reducing the rate of colon cancer in these patients. Most experts recommend indefinite treatment, and the greatest controversy lies in the dose. If sulfasalazine is used, the total dose is 2g a day, and in order to avoid intolerances, doses must be divided at least into two. In the case of mesalazine, a minimum dose of 1.5g/day is recommended, although there are many data suggesting that a greater dose might be more effective, and there are actually no arguments for reducing the dose that has been effective in the acute treatment. In any case, a single daily dose should be administered. The efficacy has been shown in studies of up to two years, but an accumulation of observational data suggests that the best is an indefinite treatment. |
- -
Is the treatment with topical salicylates effective in the maintenance of remission in patients after a mild–moderate flare of left sided UC treated with salicylates?
| Several RCTs show that topical salicylates are more effective than placebo in the maintenance treatment of left sided colitis, especially in proctitis, summarized in various systematic reviews.76,78,119 In Marshall's systematic review82 that includes four RCTs, the overall OR is 5.6 (95% CI: 3–10.5) and in a more recent study120 a RR in favour of mesalazine is shown, being of 5.21 (95% CI: 1.99–13.6). The rate of adverse effects is, also, the same as with placebo. In dose-comparison studies, the results are less clear, with data of a similar efficacy between 500mg/day and 1g/day121 compared to others that suggest a superiority of the higher dose.122 | Moderate quality |
| The biggest problem of the topical treatment is the acceptance by the patient of a route of administration that is uncomfortable, especially in the long term. It is important to come to an agreement in each case with the patient, because both the formulation and the frequency of administration may be variable, and adapting to each patient is necessary to achieve good compliance. The effort of communication must be repeated at each visit. Initially, the daily dosage is advisable, but subsequently one may move on to a dose every other day, and even in some cases, to two or three doses per week, which have proven to be superior to placebo, and are clearly more acceptable for the majority of patients. |
Maintenance treatment after induction of remission with mesalazine and/or topical steroids: algorithm and explanatory text
In patients in whom it has not been necessary to use systemic steroids to control the disease, the maintenance treatment with salicylates must always be tried as the first option. There are many years of experience with sulfasalazine and it is a perfectly useful drug, as long as the patient is not a male who is trying to conceive. However, due to safety and convenience, most patients receive treatment with mesalazine. As it is a long-term treatment, the essential point is to achieve good compliance, and for this, it is probably very useful to use a single daily dose, regardless of the pharmaceutical formulation chosen. Fortunately, there are several formulations available nowadays in Spain: 400mg tablets, 500mg tablets, 1g sachets of microgranules, 1.2g tablets and 1.5g sachets of microgranules. The patient's preferences are very important in the selection of one or another form, in order to facilitate compliance. There are also differences in the cost that may be substantial and that could prompt the clinician to recommend one form or another.
In the distal forms, the proofs about the efficacy of the maintenance treatment are somewhat weaker, and it is not always easy to achieve good compliance. In fact, given the scarce evidence available in the long term, most authors recommend to maintain treatment only for 6 months if it is the first episode of proctitis, and if it has been mild; and to extend its duration in cases of refractoriness or frequent relapses. It should be assessed how hard it is for patients to permanently adhere to a topical treatment, which is always, at the very least, inconvenient. Adaptation to each circumstance is key in this case, and the information that is well transmitted to the patient is essential to come to an agreement on a treatment in each case.
7.1.2Steroid-dependent/steroid-resistant UC (Algorithm 4)- -
Is the treatment with thiopurine immunomodulators effective in the maintenance of remission in patients after a mild–moderate flare of steroid-dependent UC?
| There are two meta-analyses103,123 that analyze the efficacy of thiopurine drugs in the maintenance of remission in patients after a mild–moderate flare of steroid-dependent or steroid-resistant UC. In the meta-analysis by the Cochrane Institute,123 the superiority of azathioprine is shown compared to placebo or mesalazine in maintaining remission, with an OR of 1.56 (1.19–2.05). In any case, the studies were heterogeneous and of low quality. In the meta-analysis by Gisbert103 6 RCTs are included that compare AZA/MP to placebo or mesalazine, and an overall OR of 2.56 (95% CI: 1.51–5.3) is seen, confirming the findings of an accumulation of observational studies that show that azathioprine is a drug that is capable to maintain remission in patients with UC in the long term, after severe or moderate flares with a percentage of efficacy of 76%. The only high-quality clinical trial available,124 included in the last meta-analysis and carried out in patients treated with steroids shows that azathioprine is highly superior to mesalazine in maintaining remission in the first sixth months after the flare [OR 4.78 (1.57–14.5)]. Such a clear result was obtained in spite of the limited duration of the observation (6 months, a limited time taking into account that azathioprine often takes months in being effective) and the use of a fixed dose of 2mg/kg/day is very probably suboptimal in some patients. Moreover, this study points out the very scarce efficacy of mesalazine in this group of patients. Furthermore, new data suggest that, just as with CD, when azathioprine is withdrawn in patients who receive it chronically, the probability of a relapse is very high.125The counterpart lies in the relevant side effects, which are more frequent in the azathioprine treatment arm [OR 1.65 (1.22–2.23)], which makes the treatment not being applicable in all the patients. | Moderate Quality |
| It is clear that there are many more RCTs with thiopurines in Crohn's disease than in UC, which often makes many expert reviews indicate that the evidence for the use of thiopurines in UC is insufficient, and may dissuade its use. However, numerous observational good quality studies that include many patients followed during very long periods of time126 confirm that thiopurines are globally even more effective in UC and in CD. Curiously, thiopurines seem to be more effective in CD of the colon than in CD of the small bowel. Although this is applicable to all the patients with UC, the adverse effects of thiopurines and the efficacy and safety of mesalazine make it preferable in many patients. However, in the special subgroup of patients with steroid-dependence, the superiority of thiopurines is obvious, as it is shown in the study by Ardizzone et al.124; an example of a well designed independent clinical trial. Another advantage of thiopurines that leads us to such an evident recommendation is its low cost (150mg a day of azathioprine cost 60% of the cost of 1500mg of the cheapest mesalazine in Spain). If we add this data to the results of the Ardizzone study, in the steroid-dependent population, the superiority of thiopurines is quite clear. Taking into account that the results of the study can probably be optimized with an individualization of the dose, it seems that when facing a situation of steroid-dependence in UC, the clinician must always consider using thiopurines as the first option. However, their actual effectiveness will be limited by the side effects. These force one out of every five patients to withdraw from the treatment, and it is in these where the clinician must assess other methods, such as mesalazine, infliximab or surgery, depending on the severity, the history of the case, the duration of the disease and other individual factors. |
- -
Is methotrexate effective in the maintenance of remission in patients after a mild–moderate flare of steroid-dependent/resistant UC?
| In the review published by the Cochrane Institute,127 only 1 RCT106 is included that compares 12.5mg of oral methotrexate to placebo for the maintenance of remission in patients with UC, with a 9-month follow-up, without noting any differences. (OR 2.25; 95% CI: 0.54–9.45; p=0.27).Another RCT128 that is more complex, with 3 randomization arms and severe methodological deficiencies, did not find differences in the maintenance of UC either. | Low quality |
| Data available, both from controlled trials, as well as from observational studies, do not show a clear efficacy of methotrexate in this indication; however, in these studies, the quality of the methodology is very low and it is not possible to rule out that this drug is effective at greater ldoses. |
- -
Is the treatment with infliximab effective in the maintenance of remission in patients after a mild–moderate flare of steroid-dependent/resistant UC?
| Data available on infliximab in the maintenance treatment of UC are perfectly summarized in an aforementioned systematic review.44 Of all the patients included in the RCT, only the ACT provide data of an extended follow-up. These studies include patients with mild to moderate flares, in the ambulatory setting, many of which are steroid-dependent or steroid-resistant, who are treated with placebo or infliximab in the acute phase and who respond, and are in maintenance treatment. They offer data in the mid to long-term (results at 30 and 54 weeks). In them, infliximab is significantly greater for maintaining remission or response. Thus, in patients under maintenance with infliximab, the rate of remission is above 20% of those treated with placebo (OR 2.72; 95% CI: 1.92–3.86). The response rate is higher with infliximab by 30% compared to placebo (OR 3.4; 95% CI: 2.52–4.59). Data about the efficacy in preventing colectomy are not mentioned in the initial publication, but they are mentioned in a subsequent analysis.129 Thus, 17% of the patients treated with placebo and 10% of the patients treated with infliximab require colectomy (p=0.02, absolute risk reduction 7%). Data have also been published that suggest that infliximab reduces the use of hospital resources significantly and improve the quality of life.As for safety, the data are similar to those described in CD, and to what is mentioned in previous sections in these guidelines. In Gisbert's review,43 it is obvious that analyzing the adverse effects of all the series is not simple, because in some of the RCTs, only the total number of adverse effects is mentioned and not that of patients with adverse effects (the same patient may have more than one adverse effect). In this review, it is reflected that there are adverse effects in 83% (95% CI: 80–86%) of the patients treated with infliximab and in 75% (95% CI: 70–81) of those treated with placebo, with an OR of 1.52 and an NNT of 14 (95% CI: 5–25). Specifically in the ACTs (ACT 1 up to 54 weeks, ACT 2 up to 30 weeks) it was similar between placebo and infliximab in the doses used (proportion of patients with adverse effects in ACT 1: placebo 25.6%, 5mg/kg 21.5% and 10mg/kg 23.8%; ACT 2: 19%, 10.7% and 9.2%, respectively). However, some severe infections were recorded in patients treated with infliximab, as well as with early infusion reactions (8–12% of the patients) and late infusion reactions in some cases. Some subsequent observational studies and assessments in the longer term of some of the trials included in this review47,129 completely corroborate the conclusions, adding information. Furthermore, a study of 16 weeks of induction has been reported, which compares three arms: infliximab and azathioprine, infliximab and placebo, placebo and azathioprine; in which the efficacy of infliximab in the treatment of UC is confirmed. We do not have details of the study, which is why we have to be cautious about its interpretation. | High quality |
| Infliximab is more effective than placebo in the maintenance treatment of UC after a moderate steroid-resistant or steroid-dependent flare. Data available refer to patients already treated initially with infliximab to induce the response. Even if the differences with placebo are very clear, between 20 and 50% of the patients do not respond to the treatment with infliximab. | High quality |
| There are still many questions about its exact role, the need or not to combine it with immunomodulators, the duration of the treatment, and the necessary controls, something that many ongoing observational and controlled trials will clarify over the next few years.The studies clearly show that infliximab is superior to placebo globally. However, the heterogeneity of the patients included in the great controlled trials, the lack of comparative data with other drugs, the cost and the risk of infliximab have to be taken into account. Thus, before starting infliximab as maintenance treatment, we must consider the circumstances of each patient carefully, as well as the alternatives available. The experience of over one-year duration is limited. |
Maintenance treatment after induction of remission with systemic steroids: algorithm and explanatory text
If it is a first flare, and control has been obtained without an excessive difficulty (hospitalization has not been necessary, the response to steroids has been obtained quickly), it is worth to try a maintenance treatment based on the use of mesalazine (oral, and in some cases oral and rectal). However, if the flare has required hospitalization or if the control has been slow and laborious, or if the patient already meets criteria for steroid-dependence, the most reasonable option is to use thiopurines (azathioprine or mercaptopurine). Once again, it is necessary to create awareness for the patient about the importance of compliance. Moreover, regular controls must be instituted to monitor the appearance of a possible toxicity. In case thiopurines failing, either due to toxicity or to lack of efficacy, there are mainly two options. Up until relatively recently, the trend was to recommend the patient elective surgery. However, infliximab has been available for several years, and it obtains a significant response rate in this subgroup of patients. Choosing between both alternatives requires knowing each patient's circumstances, as the clinical scenarios are rather variable. Nowadays, in most cases, the treatment with infliximab is tried first, and only in some circumstances (for example, a long medical history with low-grade dysplasia in the last colonoscopy) is surgery preferred as the first line of treatment. The use of biological agents has represented a very clear advance in these patients, but there are still numerous uncertainties that the clinician must solve in each case: Should we administer immunomodulators simultaneously? If so, for how long? Is there any moment in which we must suppress the biological agent? Only over the next years will useful information be collected to respond to these questions.
In some of these patients, there is no response to immunomodulators or to biological agents, and both the clinician and the patient him/herself are reluctant to proceed with surgery because the presentation is not very severe. With the data currently available, this is probably the niche in which aphaeresis has a place. If used, it is recommended to do so within clinical trials, or at least in observational studies, so as to contribute to gathering information that will clarify the exact role that aphaeresis has in these patients. It would also be an option, in patients who respond initially to infliximab but who later lose the response, to try to use adalimumab. Observational studies suggest this.
7.2Maintenance of the remission obtained after a severe flare (Algorithms 5 and 6)- -
Is the maintenance treatment with thiopurine immunomodulators effective in patients with severe UC who have obtained remission with infliximab? (Algorithm 5)
| There are no clinical studies that specifically analyze the efficacy of immunomodulators after a severe flare controlled with infliximab (there are no data about its use alone or associated to infliximab in this clinical scenario). We also do not have any observational data. We do know that azathioprine maintains remission in a series of specific scenarios in which infliximab is used in UC (for example, steroid-dependence or in a moderate steroid-resistant flare). We also know that the use of thiopurine agents, together with the treatment with infliximab in CD, maintained during the first few months, seems to improve some aspects of the treatment. | Very low quality |
| Although we believe that, very likely, thiopurine immunomodulators will be effective in a significant group of patients in which remission has been induced with infliximab (especially if these are immunomodulator-naive patients), we cannot establish a general recommendation based on the tests. If we were allowed to extrapolate the evidence and experience available in UC when it has been treated with steroids and/or anti-calcineurinics (cyclosporine or tacrolimus) we could assume that thiopurines are a very reasonable option in these patients. |
- -
Is the maintenance treatment with infliximab effective in patients with severe UC who have obtained remission with infliximab? (Algorithm 5).
| In this clinical scenario, data are very limited (most patients included in the ACT suffered moderate flares, which is why the results do not have to be applicable to the severe flare).Thus, there are no controlled trials available that analyze the efficacy of infliximab as maintenance therapy after the severe flare controlled with this agent. Of the trials included in the systematic reviews,42,43 the studies about a severe flare do not offer any data about the course in the mid-term of the patients after the control of the flare, with the exception of the results published in the series by Järnerot.47 The rates of colectomy at 3 years of the severe flare were 50 and 76% in the groups of infliximab and placebo, respectively (12/24 compared to 16/21 patients, p=0.012). The follow-up of the ACT studies suggests that the rate of colectomy is clearly reduced when the treatment is maintained.129 There are also retrospective data, that suggest an efficacy of the maintenance treatment, although they are difficult to interpret in some points.48 | Low quality |
| As we pointed out in the case of thiopurines, we strictly have no information in the specific subgroup of patients with a severe flare. However, all the data that come from studies carried out in patients with a moderate flare indicate that very likely, it would be most adequate to use infliximab as the maintenance drug in these patients. In fact, this is the standard clinical practice of the large majority of experts. |
Maintenance treatment after induction of remission with infliximab: algorithm and explanatory text
When remission with infliximab has been obtained, we find ourselves with 2 different scenarios, according to whether the patient has taken thiopurines before or not. If the patient had not received them, the rational course of action would be to use the combination of infliximab and thiopurines for a period of time (perhaps six months to a year) and then try to withdraw infliximab. Not enough studies have been published, but some experts warn against this possibility, because when relapses appear, when trying to control them again by reintroducing infliximab, failure could be frequent. If the patient had already been treated with thiopurines, he/she must continue the treatment with infliximab as maintenance. Although it is not reflected in the algorithm, we may also distinguish two different situations in this scenario. The first would be when thiopurines had been withdrawn due to a prior severe toxicity. In this case, it is clear that maintenance should be made with infliximab as monotherapy. It could be discussed whether to combine mesalazine as, although there are no specific inconveniences for the association, no advantages have been shown either. The second situation would be those patients in whom thiopurines had failed. In this case, they also require maintenance with infliximab, but it seems reasonable to maintain the association with thiopurines at least for six months in order to reduce the immunogenicity of the anti-TNF agent. In the long term, in every case, the real (cost) or possible (risks) disadvantages of biological agents must be weighed against their advantages (perhaps a greater response rate). There are no data that allow us to establish a uniform recommendation in these patients. In any case, all the changes must be made with the patient in remission, and very likely at the moment of making decisions, in a subgroup of patients who require these complex medications for the control, remission must be defined clinically, through laboratory tests, endoscopically, and maybe even histologically.
If, in the course of the disease of these patients, there is failure with infliximab in spite of its “intensification” (either due to toxicity, or due to a loss of response), surgery must be proposed. The treatment with adalimumab is worth trying as compassionate use, because a series of observational studies suggest that this alternative may be effective in up to 2/3 of the patients.
- -
Is the maintenance treatment with azathioprine effective in patients with severe UC who have obtained remission with cyclosporine? (Algorithm 6)
| The evidence that analyzes the efficacy of thiopurines in the maintenance of remission induced by cyclosporine comes entirely from observational series. We have identified 7 series130–136 with variable follow-ups, which are at times very extensive (up to 7 years). In most cases, high rates of colectomy were reported in the mid-term. At one year, they were between 33 and 69%. In most series, thiopurines are used to maintain remission in the long term, and although there are differences, they generally suggest they are helpful. There are two very different strategies after remission with intravenous cyclosporine is obtained: (a) going from intravenous to oral cyclosporine for a few months and simultaneously starting azathioprine, to then maintain it continuously and (b) when the treatment with intravenous cyclosporine has finished, start only azathioprine, without oral cyclosporine. In both cases, the dose of steroids is progressively reduced. The results of these series generally show (one single exception) that azathioprine improves the course of the disease in this scenario. It even seems reasonable to avoid the use of associated oral cyclosporine, with equivalent results of both strategies and the greater risk of using triple immunomodulators.A recent series137 suggests that, if the patient was already being treated with azathioprine when he/she would experience the severe flare controlled with cyclosporine, the subsequent maintenance with azathioprine is of very little effectiveness in the mid-term. | Low quality |
| The quality of the published evidence is not very high, although this is greatly due to, actually, the frequency of severe flares requiring cyclosporine or tacrolimus is relatively low (4–10 cases yearly in large hospitals). Even if the published data are relatively few, in practically all the cases, all the clinicians experienced in the treatment of UC maintain the treatment with thiopurines if remission has been induced with cyclosporine and/or tacrolimus. However, the rate of colectomy observed is very high (at least 33% a year), which suggests that a more aggressive strategy may be proposed by using infliximab with or without thiopurines in this specific subgroup of patients, in order to reduce the rate of colectomies to a maximum. |
- -
Is the maintenance treatment with cyclosporine effective in patients with severe UC who have obtained remission with cyclosporine? (Algorithm 6).
| Due to its renal toxicity (which may be severe and irreversible), the use of cyclosporine in the long term does not seem advisable in a disease that is surgically treatable. There are no controlled studies available in this situation. In most series131,136,132 analyzing the course of the disease of patients after the flare has been controlled with cyclosporine, this route is maintained for a few months, while azathioprine is introduced simultaneously (see the previous section). Theoretically, this strategy aims at maintaining remission until azathioprine, an extended release drug, and starts having its effect. On the contrary, in other series, after the cyclosporine flare has been controlled, oral cyclosporine is not maintained and azathioprine is started directly. The response rates in this strategy do not seem different to the ones obtained in the other strategy. Finally, in some isolated series, cyclosporine is maintained as the single treatment for months, not adding azathioprine. One of the important factors in terms of the long-term response rate, after the control of a severe flare with cyclosporine, seems to be whether the patient was already taking azathioprine or not. | Very low quality |
| Data available do not allow us to establish a general recommendation, but knowing that UC is a disease that may be treated surgically, the option of using treatments chronically regarding the adverse effects of anticalcineurinics does not seem reasonable, except in exceptional cases (mainly those associated to a very high surgical risk). |
- -
Is the maintenance treatment with tacrolimus effective in patients with severe UC who have obtained remission with tacrolimus? (Algorithm 6).
| The data available, which are scarce and not all of them controlled, have been pooled into a systematic review40 with a subsequent important series41 and a Cochrane review42 which basically obtains the same conclusions.In the systematic review,40 it is summarized that it is not possible to adequately assess the efficacy of the long-term maintenance, given that in 40 patients (more than 60% of the patients); tacrolimus was used as “bridge” to the maintenance with other drugs; although apparently the safety was satisfactory with few relevant adverse effects.In the subsequent series,41 with a mean follow-up of 39±4.1 months (5–164 months), 40 patients with UC were evaluated (14 were steroid-dependent, 26 steroid-resistant), and remission was obtained in 27/40 (67.5%). The rate of colectomy was 22.5% (9/40), practiced between 1.6 and 41 months after beginning the treatment. At four years, 43.5% of the patients had been colectomized (failure after the treatment). It was achieved to withdraw or reduce steroids in 91% of the cases (33/36). The overall side effects in this series were infrequent and usually mild. | Very low quality |
| The recommendation may seem contradictory with the results of Baumgart's study. However, we must consider that it is a heterogeneous series of patients, some of whom receive other treatments, and they are evaluated when infliximab was not available (and hence, neither was the association between infliximab and thiopurines). Even if we cannot rule out that in very selective cases with a high surgical risk, the risks of a prolonged remission with tacrolimus can be assumed, the lack of comparative data with other strategies and the risks of the treatment prompt us to establish this recommendation. |
Maintenance treatment after induction of remission with cyclosporine (or tacrolimus): algorithm and explanatory text
Even though they have been used occasionally in other circumstances, in most cases, the use of cyclosporine or tacrolimus (from this moment onwards we will refer only to cyclosporine) is limited to severe flares that are refractory to steroids, and in some exceptional cases in which steroids are contraindicated. If remission is achieved, cyclosporine cannot be maintained indefinitely, as there is the risk of inducing irreversible renal toxicity. The alternative that is most used has been to maintain cyclosporine during a limited time (most groups recommend three months, although in Spain, several sites maintain it only for four weeks), associated to down-titrated steroids and to thiopurines. Together, the data available suggest that thiopurines reduce the need for colectomy in the mid and long term in this subgroup of patients with a reasonable toxicity. However, occasionally, the severe flare has been produced in a patient that was already being treated with thiopurines. In this specific case, clinicians are left with three options: to directly recommend elective surgery (an alternative that may have been assessed before, in the control of the severe refractory flare controlled with cyclosporine), start the maintenance treatment with infliximab, or try thiopurines once again. Of course, maintaining only thiopurines does not seem the most logical course of action, although it may be possible that in some specific circumstances, changing the patter of inflammation may recover its efficacy. In most cases, the patients and the healthcare professionals will have to choose between infliximab and surgery, and the decision will be made depending on the individual circumstances (duration of the disease, prior toxicity to drugs), of each patient's expectations (fear of surgery, accessibility to medical control) and of the local experience of the working group. When infliximab is chosen, which is a very reasonable decision in many cases, it is very reasonable to maintain thiopurines, at least during a minimum period of time that could be of 6–12 months.
8Other treatments8.1Possible treatments8.1.1ProbioticsThe literature about probiotics and UC is very difficult to interpret, as: (a) the type and formulation of the different probiotics used in the different studies vary greatly; (b) comparisons to placebo are scarce, and in many of the studies, the arm with probiotics also receives treatment with other drugs (particularly amino-salicylates) and (c) the published results are incredibly heterogeneous. Therefore, currently there is no solid evidence in favour or against the use of probiotics in UC, except for the case of pouchitis (an entity that is not the subject of these CPG). Some recent data suggest that VSL3 may be of use in UC138 in situations of a mild-moderate flare, both as isolated treatment and as complementary treatment to mesalazine, but these must be confirmed in spite of being a part of the basic treatment regimen.
8.2Complementary treatments8.2.1AnaemiaThe treatment of anaemia and iron deficiency without anaemia is an essential part in the management of UC. There is a Guideline of International Consensus,139 to which the reader is referred. It is never normal for patients to have anaemia or iron deficiency, and one of our goals must be to normalize haematological parameters by sensibly using transfusions, intravenous iron or oral iron according to the circumstances.
8.2.2OtherIn the treatment of UC, there may be some special circumstances that are not outlined in these CPG. In the vast majority of these scenarios, the evidence available is scarce or of low quality, although on occasions, there are more than enough data for establishing a recommendation. As it is not a subject of these Guidelines, we recommend the readers to refer to the ECCO Consensus, to their chapter about special situations in UC.140 It may also be useful to consult the ECCO consensus for the prevention of infectious complications.141 The use of biological therapies is considered when there are other recent reference documents.142 Moreover, the information about fertility, pregnancy and birth in patients with IBD has been critically examined in another very recent consensus document.143
9Future perspectivesCurrently, it seems unlikely that in a reasonable time frame (perhaps until 2013, at least) the practical clinician will have more pharmacological options available for the treatment of UC. This does not mean that improvements that are directly applicable to the patients cannot be produced. Our opinion is that they may be produced, prompted by:
- •
The application, in the daily practice, of the recommendations contained in Clinical Practice Guidelines, such as these ones.
- •
Acknowledging the importance of the maintenance treatment as a form of avoiding the appearance of flares and of substantially improving the patients’ quality of life.
- •
The study of barriers for a good treatment compliance, which is a problem that is at the root of many treatment failures, in order to be able to face this problem directly. This point is very important in the group of patients with UC, but it is even more so when topical treatments are necessary.
- •
The permanent education of patients to progressively acquire a shared responsibility in the long-term management of their disease. As this is a chronic entity, patients are better placed to control the problem, and only with knowledge of the disease and of the treatment possibilities will they be able to do so.
- •
New controlled and observational studies will provide critical information about the use of the therapeutic means that are already available. Specifically, at this moment at least 3 key studies are already advanced: a study about cyclosporine versus infliximab in severe UC flares, of which only its publication is pending (although it has already been reported in congresses, as we pointed out previously), another study about the efficacy of the combination of infliximab and thiopurines in the induction and maintenance of remission of UC, of which also only the publication is pending; and a study about the efficacy of granulocyte aphaeresis in the situation of steroid-dependence; this last one is still in the patient recruitment phase. Moreover, we expect the publication of a yet undetermined number of observational studies, especially regarding the cumulative experience with infliximab that would provide information about the duration of the response, possibilities of intensification and long-term toxicity. Jointly, all these data will make it necessary to review these guidelines in a maximum time frame of three years. Above all, we believe that these data will allow us to advance in the individualization of the treatment, by allowing to stratify patients, not just by the global clinical scenario, but also by other circumstances.
- •
Of course, the basic and clinical research will allow for an ever better definition of the different subgroups of patients, which would also make the individualized application of the treatments easier.
Box 1: Severity and extension, problematic definitions
Few of us would have difficulties in distinguishing a living being from a non living being, yet stating an exact definition of life is not at all an easy task.144 A similar paradox is produced practically every time we face a patient with ulcerative colitis (UC). For the patients, it is easy to know if they are doing well, not so well, poorly or very badly; and for the expert clinician it would not be very difficult to know – at least in appearance – if the patient is doing well, not so well, poorly or very badly. However, the problem arises when, in order to be able to compare two patients or two moments in the same patient, we have to use some sort of objective index. UC is a complex disease, with very variable intestinal and extraintestinal symptoms; and with repercussions in laboratory findings and/or endoscopically that are also very diverse and changing. Furthermore, it is frequent to find severe discrepancies between clinical data, data from laboratory tests, and/or endoscopic data. The first technical approximation to this issue was done by Sidney Truelove and Lloyd Witts in the classic clinical trial about hydrocortisone in colitis.145 From that moment on, numerous clinical, biological, endoscopic and histological indices have been used. Curiously, few have been validated formally, and the vast majority lack sufficient reproducibility. There is also an important limitation for the daily use of these classifications: the human brain is not fond of complex classifications.146 In order to create these CPG, we were forced to classify patients into two great situations of severity: mild to moderate versus severe. This is the only way of systematizing the information currently available in literature. The broadness of the mild-moderate concept is especially troublesome, as it spans from a patient who has 3 bowel movements per day and mild abdominal discomfort (if his/her normal situation is one bowel movement per day without any discomfort) to a patient who has 5 bowel movements with blood, that force him/her to get up at night, and who has an important asthenia linked to an anaemia with a haemoglobin of 11.2g/dL. In fact, it is possible that the former has mild symptoms and is not taking any treatment, while the latter has intense symptoms in spite of already receiving a combination of oral mesalazine, rectal mesalazine and oral steroids. For their inclusion in the clinical trial, both patients are in the same group; for the clinician it is obvious that the first patient would receive oral mesalazine, which would soothe him/her and the clinician would then follow-up one month later; however, for the second patient, the clinician may seriously consider using infliximab. It is not feasible to collect all of these possibilities into a few simple algorithms, nor do all circumstances fit in the GRADE tables, as complex as these may be. These GUIDELINES intend to make the work easier, but of course, they cannot replace the clinician.
Box 2: Activity indices
The variable clinical course of inflammatory bowel diseases makes the concept of activity of the disease essential. However, there is no single definition of activity, as there are several different viewpoints (for example, the patient versus clinician versus investigator) and different consequences of the disease that are to be measured: biochemical changes, histological lesion, radiological lesion, endoscopic lesion, clinical symptoms, quality of life, financial repercussion, morbidity, mortality and others. The need to have a practical definition arose in the clinical trial of hydrocortisone compared to placebo, carried out by Sidney Truelove and Lloyd Witts between 1952 and 1955.6 To be able to evaluating the result of the treatment, they created a very simple classification that distinguished the extremes (mild versus severe), with an intermediate category, with parameters that were all clinical or in terms of laboratory tests. The simplicity of the classification, and the fact that it was the first, led it to be imposed both in clinical practice and in many studies. However, it has never been validated with a formal methodology (its internal reliability, reproducibility and external reliability are unknown). During the 56 years that have elapsed since then, numerous indices have appeared that are clinical, clinical–biological, endoscopic, histological, of quality of life, of work repercussion and of other types that have been used in very different studies. In fact, it is often very difficult to compare the results of several studies, because they use different measuring systems, and because even when they use the same, the cut-off points chosen for the definitions are not always the same. When the different options are analyzed with a methodological rigour, the results are discouraging: there is no correlation between laboratory values and endoscopy, between endoscopy and clinical presentation, between histology and laboratory tests, between the patient's and doctor's viewpoint, etc. Curiously, the last ones in arriving at the problem, the paediatric gastroenterologists expert in inflammatory bowel disease, have applied a more rigourous methodology from the beginning, and have developed the PUCAI (Paediatric Ulcerative Colitis Activity Index),147 probably the first index that has been validated correctly. For the purposes of these guidelines there is one more issue: even if we wanted to be rigourous with the indices, we could not, because the studies were made before the indices in many cases. Therefore, we decided to use the Montreal classification, which divides the possible situations of activity into four: remission, mild, moderate and severe. Data from most studies can be extrapolated to this type of classification. Of course, some confusion may arise, especially at the limits (Box 1). Many studies analyze not only clinical indices, but also endoscopic and at times, histological indices. Also, in this case, we have been forced to simplify sometimes, assuming a possible loss of quality of the data, compensated on many occasions by the great number of patients included. Although we admit that in the future, the concepts of remission (for example, recently it has been proposed to define remission as the absolute absence of clinical symptoms and mucosal healing demonstrated in colonoscopy148) will always include objective parameters (endoscopic, histological or biological), the definitions of response and remission are almost always clinical, because the studies analyzed, in many cases, do not allow for another approach. Finally, we have to point out that in the case of a severe flare, the variables chosen by the Committee are clearly definable: mortality or the need for colectomy; which makes the studies more easily comparable.
Box 3: Practical considerations about the doses of immunomodulating drugs
Steroids: Systemic steroids have been used by several routes (oral, intravenous, rectal) and in many different formulations and products. If we consider prednisone as the reference steroid (it is the one most commonly used), the standard dose for treatment start is 1mg/kg/day, orally. In order to adjust the doses of other steroids, we have to take into account the potency equivalences between them (Table 17), the route of administration that may vary the bioavailability significantly, and that there may be differences in the toxicity and individual effects of the different products. The IV steroid that is most used is methylprednisolone, which is more potent than prednisone (4mg of methylprednisolone are equal to 5mg of prednisone; not considering the obvious difference in bioavailability). The initial dose is usually maintained for two weeks, although depending on the response, it may be acceptable from one week up to four. When the dose is reduced, there are several guidelines for down-titrating the dose. The most usual practice is to remove 10mg from the daily dose of prednisone weekly until reaching 20mg/day, and from that point, remove 5mg from the daily dose weekly until discontinuing it. It is usually not justified to maintain the treatment for more than three months without having considered and tried an alternative treatment.
Equivalences in the doses of systemic steroids.
| Drug | Equivalent doses (mg) | Mineralosteroid activity (sodium retention) | Biological half life (h) |
| Short acting | |||
| Hydrocortisone | 20 | ++ | 8–12 |
| Cortisone | 25 | ++ | 8–12 |
| Prednisone | 5 | + | 12–36 |
| Prednisolone | 5 | + | 12–36 |
| Methylprednisolone | 4 | 0 | 12–36 |
| Triamcinolone | 4 | 0 | 12–36 |
| Deflazacort | 6–7.5 | 0 | 12–36 |
| Long acting | |||
| Betamethasone | 0.6 | 0 | 36–54 |
| Dexamethasone | 0.75 | 0 | 36–54 |
Note: For example, prednisone is 4 times more potent than hydrocortisone (i.e., 5mg of prednisone are equal to 20mg of hydrocortisone) and methylprednisolone is a little more powerful than prednisone (4mg of the former are equal to 5 of the latter).
Source: Gasull et al.149
Cyclosporine: Cyclosporine is used exclusively in the severe or moderate flare that is refractory to steroids, and its administration must not be extended for more than three to six months due to the risk of irreversible toxicity, particularly renal toxicity. Although defenders of the oral route are not lacking, the studies justifying its use have almost all been carried out initially using the intravenous route. Initially, a dose of 4mg/kg/day was chosen, although later an individual adjustment may be needed depending on the blood levels obtained and of toxicity. In a randomized study, it was shown that 2mg/kg/day were as effective as 4; although actually, in both groups, the mean final dose that was administered was very similar because adjustments by levels and toxicity were allowed. With the data available, it seems prudent to begin with 2mg/kg/day and up-titration if no toxicity is produced, and the levels considered therapeutic are not reached.
Tacrolimus: The doses used are of 0.01–0.02mg/kg when it is used intravenously, and of 0.1–0.2mg/kg when it is used orally; adjusting individually according to the blood levels, the toxicity and the response.
Azathioprine and mercaptopurine: The mean dose of azathioprine is of 2.5mg/kg/day and the mean dose of mercaptopurine is of 1–1.5mg/kg/day. However, there is great individual variability in the response and toxicity, which is why the doses may be adjusted in terms of the effectiveness and toxicity. If they are available, metabolite levels may be used as a guide, at least in selected patients. However, in most sites, adjustments have to be carried out considering the clinical presentation, the total leukocytes, transaminases and other analytical parameters. Their usefulness cannot be ruled out completely if they have been maintained for a period of less than 6 months.
11Conflicts of interest of the authors of the guidelineThis guide has been possible to some extent by an unrestricted grant of MSD. MSD was not involved in any part of the development or scientific aspects of the guide.
F. Gomollón has received grants for assistance to scientific meetings from Abbott and MSD. He has also received consultancy fees from FAES-FARMA, Abbott and MSD. He has also received research support from MSD.
S. García-López has received grants for assistance to scientific meetings from Abbott, MSD, FAES-FARMA, Ferring and Shire. He has also received consultancy fees from Abbott and MSD.
B. Sicilia has received grants for assistance to scientific meetings from Abbott and MSD.
Javier P. Gisbert has received grants for assistance to scientific meetings from Abbott and MSD. He has also received consultancy fees from FAES-FARMA, Abbott and MSD.
Joaquin Hinojosa has received grants for assistance to scientific meetings from Abbott and MSD. He has also received consultancy fees from FAES-FARMA, Abbott and MSD.
The rest of the authors of the guideline declare no conflict of interest.
President: Joaquín Hinojosa
Vice president: Fernando Gomollón
Secretary: Valle García
Treasurer: Francesc Casellas
Web coordinator: Pilar Nos Mateu
Vocals: José Luis Cabriada Nuño, Eugeni Doménech Morral, Fernando Muñoz, Daniel Ginard, Pilar Nos Mateu, Julián Panés, Javier P. Gisbert, Cristina Saro.
Joaquín Hinojosa
Fernando Gomollón
Javier P. Gisbert
Fernando Gomollón
Santiago García-López
Beatriz Sicilia
Javier P. Gisbert
Joaquín Hinojosa.
Montserrat Aceituno
Manuel Barreiro
Fernando Bermejo
Isabel Bernal
Xavier Calvet
Eugeni Domènech
María Esteve
Daniel Ginard
Esther García Planella
Joaquín Hinojosa
Antonio Lopez Sanromán
Ignacio Marín
Fernando Muñoz
Pilar Nos
Julián Panés
Elena Ricart
Malola Ruiz
Miquel Sans
Cristina Saro
Iván Sola.
Federico Argüelles (gastroenterologist)
Guillermo Bastida (gastroenterologist)
Belén Beltrán (gastroenterologist)
Eduard Cabré (gastroenterologist)
Ignacio Catalán (gastroenterologist)
María Chaparro (gastroenterologist)
Javier de Oca (surgeon)
Ana Echarri (surgeon)
Eloy Espín (surgeon)
Orlando García-Bosch (gastroenterologist)
Ana Gutiérrez (gastroenterologist)
Elena María Gutiérrez (patient)
Isabel Iborra (gastroenterologist)
Laureano López Rivas (gastroenterologist)
Olga Merino (gastroenterologist)
Amelia Nogueiras (nurse)
Lorena Oltra (nurse)
Maite Ortega (paciente)
Michel Papo (gastroenterologist)
Ildefonso Pérez (patient)
Sabino Riestra (gastroenterologist)
Toni Torrejón (nurse)
Concha Thomson (gastroenterologist)
Manuel VanDomselar (gastroenterologist)
José Antonio Velázquez (patient).
| Induction of remission in severe ulcerative colitis flare |
| Are steroids effective in induction of remission in patients with a severe UC flare? |
| Is cyclosporine effective in induction of remission in patients with a severe UC flare? And in steroid-resistance? |
| Is tacrolimus effective in induction of remission in patients with a severe UC flare? And in steroid-resistance? |
| Is infliximab effective in induction of remission in patients with a severe UC flare? And in steroid-resistance? |
| Is heparin (at anticoagulant dose) effective in induction of remission in patients with a severe UC flare? |
| Are antibiotics effective in induction of remission in patients with a severe UC flare? |
| Is surgery effective in induction of remission in patients with a severe UC flare? |
| Is parenteral nutrition effective in induction of remission in patients with a severe UC flare? |
| Induction of remission in mild to moderate ulcerative colitis flare |
| Are oral salycilates effective in induction of remission in patients with a mild-moderate UC flare? |
| Is combined treatment with oral and topical salycilates more effective than oral alone in induction of remission in patients with a mild-moderate UC flare? |
| Are topical salycilates alone effective in induction of remission in mild-moderate flares of left-sided colitis? |
| Are topical salycilates more effective than oral in induction of remission in patients with a moderate flare of left-sided UC? |
| Are systemic steroids effective in induction of remission in patients with mild-moderate UC flares? |
| Are oral steroids of low-bioavailability effective in induction of remission in mild-moderate UC flares? |
| Are rectal steroids effective in induction of remission in mild-moderate left-sided UC flares? |
| Are rectal steroids of low bioavailability more effective than those of high bioavailability in induction of remission in patients with mild-moderate left-sided UC flares? |
| Are topical salicylates more effective than rectal steroids in induction of remission in patients with a mild-moderate left-sided UC flare? |
| Are thiopurines effective in induction of remission in patients with a moderate flare of steroid-resistant or steroid-dependent UC? |
| Is methotrexate effective in induction of remission in patients with a moderate flare of steroid-dependent UC? |
| Is infliximab effective in induction of remission in patients with a moderate flare of steroid-dependent or steroid-resistant UC? |
| Is adalimumab effective in induction of remission in patients with a moderate flare of steroid-dependent or steroid-resistant UC? |
| Is treatment with aphaeresis effective in induction of remission in patients with a moderate flare of steroid-dependent UC? |
| Maintenance treatment of patients with ulcerative colitis in remission |
| Is treatment with oral salicylates effective in maintenance of remission in patients after a mild-moderate UC flare treated with salicylates? |
| Is treatment with topical salicylates effective in maintenance of remission in patients after a mild-moderate flare of left-sided UC treated with salicylates? |
| Is treatment with thiopurines effective in maintenance of remission in patients after a mild-moderate flare of steroid-dependent UC? |
| Is treatment with methotrexate effective in maintenance of remission in patients after a mild-moderate flare of steroid-dependent UC? |
| Is treatment with infliximab effective in maintenance of remission in patients after a mild-moderate flare of steroid-dependent or steroid-resistant UC? |
| Is maintenance treatment with thiopurines effective in patients with severe UC who have obtained remission with infliximab? |
| Is maintenance treatment with infliximab effective in patients with severe UC who have obtained remission with infliximab? |
| Is maintenance treatment with thiopurines effective in patients with severe UC who have obtained remission with cyclosporine? |
| Is maintenance treatment with cyclosporine effective in patients with severe UC who have obtained remission with cyclosporine? |
| Is maintenance treatment with tacrolimus effective in patients with severe UC who have obtained remission with tacrolimus? |
| Action (and clinical scenery) | Quality of evidence | Recommendation | For the clinician |
| Treatment of the flare | |||
| Severe flare | |||
| IV steroids | Moderate | Strong for (recommended) | DO IT |
| IV cyclosporine | Moderate | Strong for (recommended) | DO IT |
| Tacrolimus | Low | Weak for (suggested) | Probably DO IT |
| Infliximab | Moderate | Strong for (recommended) | DO IT |
| Heparin (anticoagulant) | Low | Strong against (not recommended) | DO NOT DO IT |
| Antibiotics | Very low | No recommendation | |
| Surgery | Low | Weak for (suggested) | Probably DO IT |
| Parenteral nutrition | Low | Weak against (not suggested) | Probably DO NOT DO IT |
| Mild-moderate | |||
| Oral salicylates | High | Strong for (recommended) | DO IT |
| Oral and topical | Moderate | Weak for (suggested) | Probably DO IT |
| Rectal salicylates (left-colitis) | High | Strong for (recommended) | DO IT |
| Rectal preferred (left-colitis) | Moderate | Strong for (recommended) | DO IT |
| Systemic steroids | Moderate | Strong for (recommended) | DO IT |
| Low availability oral steroids | Low | Weak for (suggested) | Probably DO IT |
| Topical steroids (left-colitis) | Moderate | Weak for (suggested) | Probably DO IT |
| Rectal versus systemic steroids (left-colitis) | Moderate | Strong for (recommended) | DO IT |
| Rectal salicylates versus rectal steroids (left-sided) | Moderate | Strong for (recommended) | DO IT |
| Thiopurines for steroid-failure | Low | Weak against (not suggested) | Probably DO NOT DO IT |
| Methotrexate for steroid-failure | Low | Weak against (not suggested) | Probably DO NOT DO IT |
| Infliximab for steroid-failure | High | Strong for (recommended) | DOIT |
| Adalimumab for steroid-failure | Moderate | Weak for (suggested) | Probably DO IT |
| Apheresis | Low | Weak against (not suggested) | Probably DO NOT DO IT |
| Maintenance treatment | |||
| Oral salicylates | High | Strong for (recommended) | DO IT |
| Topical salicylates (left-sided) | Moderate | Strong for (recommended) | DO IT |
| Thiopurines for steroid-failure | Moderate | Strong for (recommended) | DO IT |
| Methotrexate for steroid failure | Low | Weak against (not suggested) | Probably DO NOT DO IT |
| Infliximab for steroid failure | High | Strong for (recommended) | DO IT |
| Thiopurine (after remission with infliximab) | Very low | No recommendation | |
| Infliximab (after remission with infliximab) | Low | Weak for (suggested) | Probably DO IT |
| Thiopurine (after remission with cyclosporine) | Low | Weak for (suggested) | Probably DO IT |
| Cyclosporine (after remission with cyclosporine) | Very low | Strong against (not recommended) | DO NOT DO IT |
| Tacrolimus (after remission with tacrolimus) | Very low | Strong against (not recommended) | DO NOT DO IT |
GETECCU (Grupo Español de Trabajo de Enfermedad de Crohn y Colitis Ulcerosa or Spanish Group for Working on Crohn's Disease and Ulcerative Colitis).
The term aggressive is not the most adequate when referring to a treatment: what must predominate is the benefit-risk balance, and on many occasions, the most effective treatment, and even the most efficient treatment, may be the one that is apparently most aggressive.