Acute-on-chronic liver failure (ACLF) is a dynamic syndrome that should be assessed repeatedly. An algorithm for risk stratification in decompensated cirrhosis was recently proposed by the EASL-CLIF (European Association for the Study of the Liver-Chronic Liver Failure) Consortium.
AimTo validate the EASL-CLIF Consortium scores in patients with and without ACLF.
Materials and methodsRetrospective single-center cohort study including patients admitted for acute decompensation of cirrhosis between January 2014 and December 2015, and followed-up until December 2016. We separated patients with and without ACLF and compared the various EASL-CLIF Consortium scores to Child–Pugh and MELD for predicting 28-day (M28), 90-day and 12-month mortality. These scores were recalculated at different time points over 28 days.
Results106 patients were included (age 60.3±10.7 years; 87.7% male), 35.8% of whom met ACLF criteria on admission (50%) or during hospitalization. A CLIF-C AD Score ≥60 on admission was associated with a higher risk of developing ACLF. The onset of ACLF during hospitalization portended a poor prognosis. The prognostic performance of the CLIF-C ACLF Score (AUROC for M28: 0.856±0.071) was globally comparable to that of Child–Pugh and MELD. Overall, ACLF resolved in 54.1% patients, resulting in increased survival. Almost 40% of the patients reached their final ACLF grade after ≥8 days, with 13.9% of ACLF patients experiencing resolution by then.
DiscussionWe confirmed the accuracy and clinical value of the several proposed scores in our population. Prognosis was better defined by the early clinical course than by the initial evaluation, emphasizing the importance of repeated assessments.
La insuficiencia hepática crónica agudizada (IHCA) es un síndrome dinámico que se debe evaluar repetidamente. El Consorcio EASL-CLIF (Asociación Europea para el Estudio del Hígado-Insuficiencia Hepática Crónica) ha propuesto recientemente un algoritmo para la estratificación del riesgo en la cirrosis descompensada.
ObjetivoValidar las puntuaciones del Consorcio EASL-CLIF en pacientes con y sin IHCA.
Materiales y métodosestudio de cohorte unicéntrico retrospectivo que incluyó a pacientes ingresados por descompensación aguda de cirrosis entre enero de 2014 y diciembre de 2015, a los cuales se les hizo seguimiento hasta diciembre de 2016. Separamos a los pacientes con y sin IHCA, y comparamos las distintas puntuaciones del Consorcio EASL-CLIF con Child-Pugh y MELD en la predicción de mortalidad a los 28 días (M28), a los 90 días y a los 12 meses. Estas puntuaciones se recalcularon en diferentes momentos en el curso de los 28 días.
Resultadosse incluyó a 106 pacientes (edad: 60,3±10,7 años; 87,7% varones), el 35,8% de los cuales cumplieron con los criterios de IHCA, en el momento del ingreso (50%) o durante la hospitalización. Una puntuación de CLIF-C AD ≥60 en el momento del ingreso se asoció con mayor riesgo de desarrollar IHCA. El inicio de IHCA durante la hospitalización presagiaba un mal pronóstico. El rendimiento pronóstico de CLIF-C ACLF Score (AUROC de M28: 0,856±0,071) fue globalmente comparable al de Child-Pugh y MELD. En general, el IHCA se resolvió en el 54,1% de los pacientes, lo que produjo un aumento de la supervivencia. Casi el 40% de los pacientes alcanzaron su grado final de IHCA después de ≥8 días y el 13,9% de los pacientes con IHCA experimentaron su resolución para entonces.
DiscusiónConfirmamos la precisión y el valor clínico de las diversas puntuaciones propuestas en nuestra población. El pronóstico se definió mejor por el curso clínico temprano que por la evaluación inicial, lo que recalca la importancia de las evaluaciones repetidas.
Acute-on-chronic liver failure (ACLF) is a syndrome characterized by an acute decompensation of cirrhosis with organ failure(s) and high mortality.1 Although several diagnostic criteria have been proposed,2–5 the largest observational study designed to define ACLF was the CANONIC Study (EASL-CLIF ACLF in cirrhosis),6 a prospective study conducted by the EASL-CLIF (European Association for the Study of the Liver-Chronic Liver Failure) Consortium involving 1343 patients hospitalized for acute decompensation of liver cirrhosis. This study defined the criteria of ACLF based on the analysis of patients with organ failures according to the CLIF-SOFA (Sequential Organ Failure Assessment) Score and high 28-day mortality rate (>15%). The authors concluded that ACLF occurred in younger patients, was more frequent in alcoholic cirrhosis and was often associated with bacterial infections and active alcoholism, although no precipitating event could be identified in 40% of patients. Despite potentially developing at any stage of liver cirrhosis, ACLF was associated with higher mortality in patients with no prior decompensation of liver cirrhosis.
ACLF is believed to develop in the setting of an excessive inflammatory response1 either in response to pathogens or to endogenous molecules released by damaged cells, a process referred to as immunopathology.7 Other mechanisms include direct damage by pathogens and dysfunction of immune tolerance.1
The CLIF-C (CLIF-Consortium) ACLF Score is a prognostic score developed by the authors of the CANONIC Study that is calculated using the patient's age, white cell count and CLIF-C OF (Organ Failure Score) Score – an adapted version of the CLIF-SOFA Score.8 When compared to MELD (Model for End-Stage Liver Disease) and Child–Pugh Scores, CLIF-C ACLF Score was superior predicting 28-day, 90-day, 180-day and 265-day mortality. The authors also proposed a prognostic score for patients who did not fulfill ACLF criteria, the CLIF-C AD (Acute Decompensation) Score.9 These three scores (CLIF-C AD, CLIF-C OF and CLIF-C ACLF) were intended to integrate an algorithm to guide patient management. In patients without ACLF, a CLIF-C AD Score ≥60 was associated with higher 3-month mortality (>30%) and thus classified as high-risk. On the other hand, in patients with ACLF, this algorithm is aiming to help the clinician as to the need for intensive care unit admission or determination of futility.1 Indeed, after concluding that most patients reached their final ACLF grade 3–7 days after diagnosis, Gustot et al.10 proposed that intensive care should be discontinued at this point in patients who were not candidates to liver transplantation and had ≥4 organ failures or CLIF-C ACLF >64, owing to futility.
Our aim was to characterize a population of patients admitted for acute decompensation of liver cirrhosis regarding the presence of ACLF defined by the EASL-CLIF criteria and to validate the several scores proposed by the EASL-CLIF Consortium (CLIF-C AD in patients without ACLF; CLIF-C OF and CLIF-C ACLF in patients with ACLF). Additionally, in the subgroup of patients with ACLF, we sought to determine if an evaluation by 3–7 days indeed correlates best with prognosis.
Materials and methodsPopulation and data collectionWe conducted a unicentric retrospective cohort study of patients admitted in our unit for acute decompensation of cirrhosis between January 2014 and December 2015.
The diagnosis of liver cirrhosis was based on a combination of clinical signs, laboratory findings, imaging and endoscopy, corroborated by liver histology in selected cases. Acute decompensation was defined as the onset of ascites, hepatic encephalopathy, hypertensive gastrointestinal bleeding or bacterial infection. Patients were included only if they were admitted for at least 48h. Exclusion criteria included hepatocellular carcinoma outside Milan criteria and severe chronic extra-hepatic disease.
A single hospitalization was considered in each patient. In patients with more than one admission fulfilling ACLF criteria during the study period, the first ACLF episode was analyzed.
All patients were admitted in the gastroenterology department, which comprises a general ward and intermediate care unit. The majority of patients were initially assessed in the emergency department for stabilization, with few patients being admitted through the outpatient clinic. Whenever advanced organ support was needed, patients were transferred to an intensive care unit upon medical decision.
For each patient, analyzed data include physical examination, laboratory work-up and clinical history, covering past or active alcoholism (in the last 3-months) and previous admissions for decompensation. Acute kidney injury was defined using the AKIN (acute kidney injury network) criteria.11 Data were collected retrospectively from patients’ electronic medical records at the end of December 2016, ensuring a follow-up of at least 12 months from hospital admission.
All patients were characterized on admission using the Child–Pugh Score and the MELD (Model for End-Stage Liver Disease) Score, not combined with serum sodium. Mortality analysis was performed at 28 days (M28), 90 days (M90) and 12 months (M12m).
This study was approved by the hospital's Ethical Committee on the 9th February 2018 and conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Acute-on-chronic liver failureACLF was defined using the criteria proposed by the CANONIC study. Accordingly, ACLF was present if acute decompensation of liver cirrhosis was associated with kidney failure (serum creatinine ≥2mg/dL, renal replacement therapy or use of vasopressors indicating hepatorenal syndrome), two other organ failures (liver: serum bilirubin ≥12mg/dL; brain: grade III–IV hepatic encephalopathy based on West Haven criteria; coagulation: international normalized ratio [INR] ≥2.5; circulation: mean arterial pressure <70mmHg or use of vasopressors; lungs: PaO2/FiO2 <200 or SpO2/FiO2 <214) or one organ failure if associated with kidney dysfunction (serum creatinine 1.5–1.9mg/dL) or cerebral dysfunction (grade I–II hepatic encephalopathy). ACLF grade was defined by the number of organ failures: ACLF grade 1 (ACLF1) corresponding to one organ failure, ACFL grade 2 (ACLF2) to two organ failures and ACLF grade 3 (ACLF3) to three or more organ failures.
CLIF-C OF Score, ACLF grade and CLIF-C ACLF Score were calculated using the online tool of the CLIF Research platform.12 In patients who did not fulfill ACLF criteria, CLIF-C AD Score was also calculated in this platform.
Patients with ACLF, either on admission or during the course of hospitalization, where compared to those without ACLF, regarding age, gender, etiology of cirrhosis, history of previous decompensations and both clinical and laboratory findings during hospitalization.
The accuracy of CLIF-C AD and CLIF-C ACLF Scores predicting mortality was compared to that of Child–Pugh and MELD scores.
Similarly to Gustot et al.,10 in the ACLF group, these scores were assessed at ACLF onset, at 48h, 3–7 days and at the last available assessment in the first 28 days. Only patients with a complete assessment at 48h were included. Final and initial ACLF grades were compared to define ACLF resolution, improvement, fluctuating course (change of ACLF grade during the 28-day period, but with the same final and initial grades) or worsening.
Statistical analysisGroups with and without ACLF were compared using Student t, chi-square or Fisher's exact tests when appropriate. The AUROCs (area under receiver operating characteristic curves) for predicting mortality were compared using DeLong method. Kaplan–Meier's method was used for survival analysis, with log-rank test for comparisons. Statistical analysis was performed in SPSS 21.0® and MedCalc 17.5.3®. A p-value <0.05 was considered significant.
ResultsWe included 106 patients (age 60.3±10.7 years; 87.7% male), of which 38 (35.8%) fulfilled ACLF criteria, either at admission (50.0%) or during hospitalization (50.0%). Total number of hospital admissions was 203, corresponding to 1.92 admissions per patient, of which 61 with ACLF. Only one episode per patient was analyzed, with an average duration of 16.8±13.8 days. Mean follow-up was 353±300 days.
The most frequent etiologies of cirrhosis were alcohol (84.9%) and chronic hepatitis C (24.5%), occasionally overlapping, with chronic hepatitis B (2.8%) and auto-immune liver diseases (1.9%) being less frequent.
In 29.1% patients, the present episode was the first admission for acute decompensation of cirrhosis, whereas 26.2% had experienced a decompensation in the previous 3 months, 15.1% 3–12 months before and 29.1% more than 12 months before.
Clinical manifestations on admission included ascites (58.5%), hepatic encephalopathy (46.2%), gastrointestinal bleeding (39.6%), acute kidney injury (38.7%), bacterial infection (36.8%) – comprising spontaneous bacterial peritonitis (11.3%) –, hepatic hydrothorax (7.5%) and alcoholic hepatitis (6.6%), confirmed by histology only in selected cases. Of the 7 (6.6%) patients diagnosed with alcoholic hepatitis, 3 received corticosteroids (among the 4 patients who did not, 1 had Maddrey's Discriminant Function <32 and 3 had evidence of infection), which had no significant impact on mortality (M28 and M90 were both 66.7% vs 50.0%, p=1.000).
In the large majority of cases (86.8%) a precipitating event was found, most commonly active alcoholism in the previous 3 months (55.8%), followed by gastrointestinal bleeding (39.6%), bacterial infection (36.8%) or transjugular intrahepatic portosystemic shunting (TIPS) in the previous 3 months (1.9%).
The most frequent organ failure was renal failure (24.5%), followed by cerebral (16.0%), hepatic (6.6%), pulmonary (4.7%), coagulation (3.8%) and circulatory failure (2.8%). Most of the patients with organ failures fulfilled ACLF criteria (96.2%). The global intensive care unit admission rate was 20.8%, including 47.4% of patients with ALCF and 5.9% without ACLF.
A total of 7 patients (6.6%) underwent liver transplantation during follow-up, including one ACLF patient who was transplanted 5 months after the initial admission.
Comparison of patients with and without ACLFIn the group of 38 patients with ACLF, 17 (63.2%) were classified as ACLF1, 9 (23.7%) as ACLF2 and 5 (13.2%) as ACLF3.
When comparing patients with and without ACLF (Table 1), no significant difference was found regarding age (62.0±10.0 vs 59.4±11.0 years, p=0.248), gender (89.5% vs 86.8% male, p=0.767) or etiology of cirrhosis, namely alcoholic (89.5% vs 82.4%, p=0.326) or chronic hepatitis C (23.7% vs 25.0%, p=0.880). There were also no differences concerning active alcoholism (50.0% vs 59.0%, p=0.369) or presence of a precipitating event for decompensation (86.8% vs 86.8%, p=0.991). The onset of ACLF was equally as frequent in previously compensated patients as in patients with history of decompensation (33.3% vs 36.8%, p=0.734).
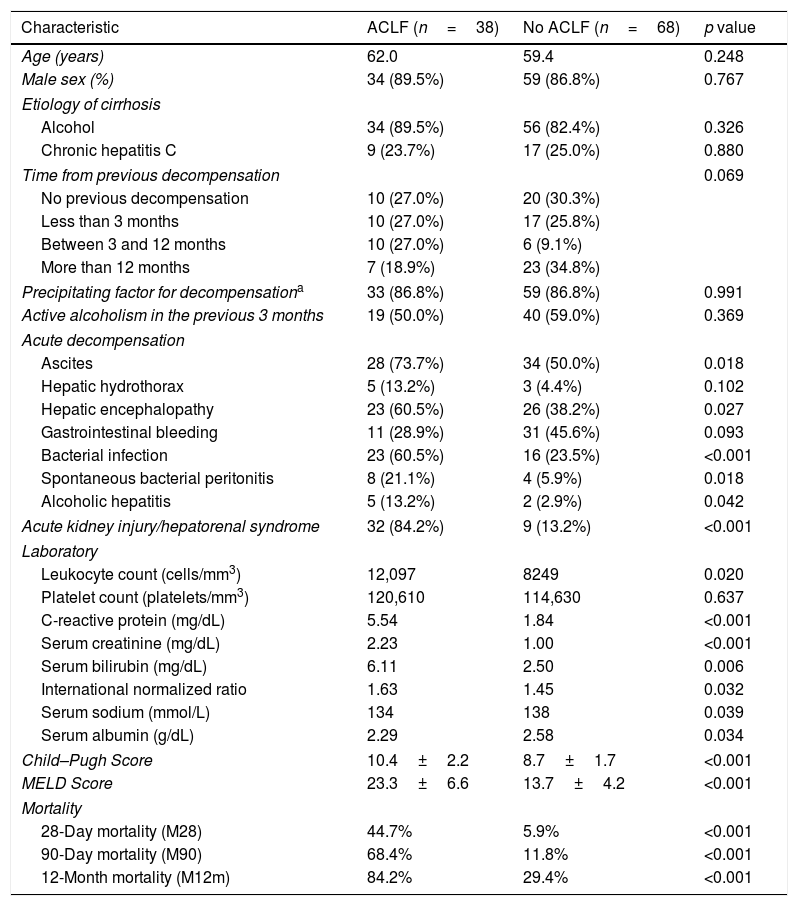
Characterization and outcomes of patients with and without ACLF.
| Characteristic | ACLF (n=38) | No ACLF (n=68) | p value |
|---|---|---|---|
| Age (years) | 62.0 | 59.4 | 0.248 |
| Male sex (%) | 34 (89.5%) | 59 (86.8%) | 0.767 |
| Etiology of cirrhosis | |||
| Alcohol | 34 (89.5%) | 56 (82.4%) | 0.326 |
| Chronic hepatitis C | 9 (23.7%) | 17 (25.0%) | 0.880 |
| Time from previous decompensation | 0.069 | ||
| No previous decompensation | 10 (27.0%) | 20 (30.3%) | |
| Less than 3 months | 10 (27.0%) | 17 (25.8%) | |
| Between 3 and 12 months | 10 (27.0%) | 6 (9.1%) | |
| More than 12 months | 7 (18.9%) | 23 (34.8%) | |
| Precipitating factor for decompensationa | 33 (86.8%) | 59 (86.8%) | 0.991 |
| Active alcoholism in the previous 3 months | 19 (50.0%) | 40 (59.0%) | 0.369 |
| Acute decompensation | |||
| Ascites | 28 (73.7%) | 34 (50.0%) | 0.018 |
| Hepatic hydrothorax | 5 (13.2%) | 3 (4.4%) | 0.102 |
| Hepatic encephalopathy | 23 (60.5%) | 26 (38.2%) | 0.027 |
| Gastrointestinal bleeding | 11 (28.9%) | 31 (45.6%) | 0.093 |
| Bacterial infection | 23 (60.5%) | 16 (23.5%) | <0.001 |
| Spontaneous bacterial peritonitis | 8 (21.1%) | 4 (5.9%) | 0.018 |
| Alcoholic hepatitis | 5 (13.2%) | 2 (2.9%) | 0.042 |
| Acute kidney injury/hepatorenal syndrome | 32 (84.2%) | 9 (13.2%) | <0.001 |
| Laboratory | |||
| Leukocyte count (cells/mm3) | 12,097 | 8249 | 0.020 |
| Platelet count (platelets/mm3) | 120,610 | 114,630 | 0.637 |
| C-reactive protein (mg/dL) | 5.54 | 1.84 | <0.001 |
| Serum creatinine (mg/dL) | 2.23 | 1.00 | <0.001 |
| Serum bilirubin (mg/dL) | 6.11 | 2.50 | 0.006 |
| International normalized ratio | 1.63 | 1.45 | 0.032 |
| Serum sodium (mmol/L) | 134 | 138 | 0.039 |
| Serum albumin (g/dL) | 2.29 | 2.58 | 0.034 |
| Child–Pugh Score | 10.4±2.2 | 8.7±1.7 | <0.001 |
| MELD Score | 23.3±6.6 | 13.7±4.2 | <0.001 |
| Mortality | |||
| 28-Day mortality (M28) | 44.7% | 5.9% | <0.001 |
| 90-Day mortality (M90) | 68.4% | 11.8% | <0.001 |
| 12-Month mortality (M12m) | 84.2% | 29.4% | <0.001 |
Data are expressed in absolute number and percentage.
Patients with ACLF had a higher prevalence of acute kidney injury (84.2% vs 13.2%, p<0.001), ascites (73.7% vs 50.0%, p=0.018), hepatic encephalopathy (60.5% vs 38.2%, p=0.027), bacterial infection (60.5% vs 23.5%, p<0.001), spontaneous bacterial peritonitis (21.1% vs 5.9%, p=0.018) and alcoholic hepatitis (13.2% vs 2.9%, p=0.042). No significant differences were found in gastrointestinal bleeding (28.9% vs 45.6%, p=0.093) and hepatic hydrothorax (13.2% vs 4.4%, p=0.102).
These patients also had higher leukocyte count (12,097 vs 8249/mm3, p=0.020) and higher levels of C-reactive protein (5.54 vs 1.84mg/dL, p<0.001), serum creatinine (2.23 vs 1.00mg/dL, p<0.001), total bilirubin (6.11 vs 2.50mg/dL, p=0.006) and international normalized ratio (1.63 vs 1.45, p=0.032), as well as lower serum sodium (134 vs 138mmol/L, p=0.039) and albumin levels (2.29 vs 2.58g/dL, p=0.034). Platelet count did not differ between groups (120,610 vs 114,630/mm3, p=0.637).
Mortality analysisGlobal mortality rate was 19.8% at 28 days (M28), 32.1% at 90 days (M90) and 49.1% at 12 months (M12m).
Considering the whole cohort, the presence of infection (p<0.001), ascites (p=0.039) or hepatic encephalopathy (p<0.001) had a significant negative impact on survival analysis (log-rank test), whereas gastrointestinal bleeding did not (p=0.170). Previously compensated patients had the same prognosis as the others, both in the whole cohort (M28 16.7% vs 21.1%, p=0.610; M90 30.0% vs 32.9%, p=0.774; M12m 40.0% vs 52.6%, p=0.241) and specifically in the ACLF group (M28 50.0% vs 42.9%, p=0.727; M90 70.0% vs 67.9%, p=1.000; M12m 80.0% vs 85.7%, p=0.644).
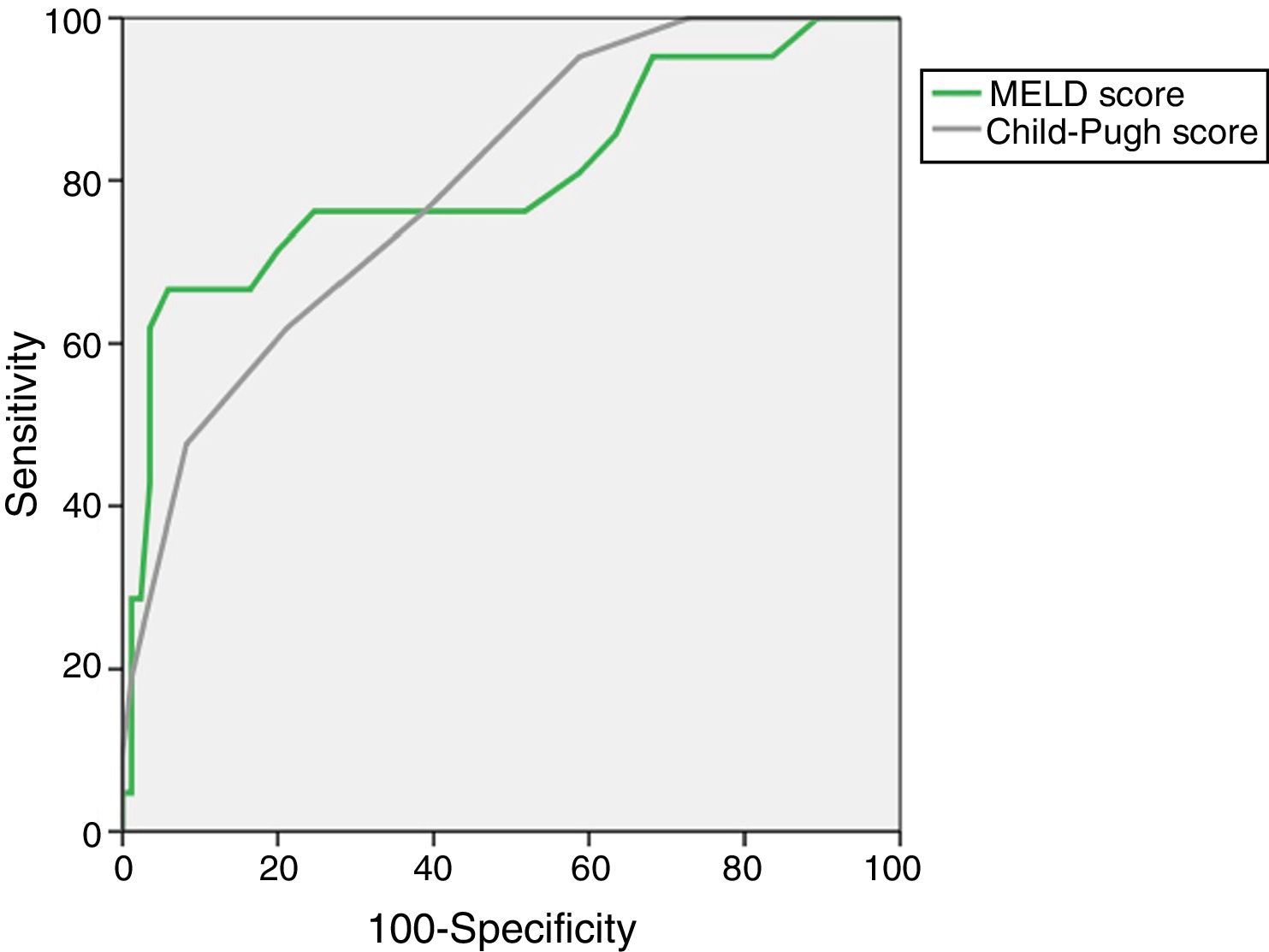
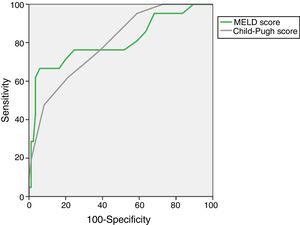
The AUROC of MELD (0.805±0.063) and Child–Pugh (0.798±0.052) predicting M28 in the entire cohort are depicted in Fig. 1.
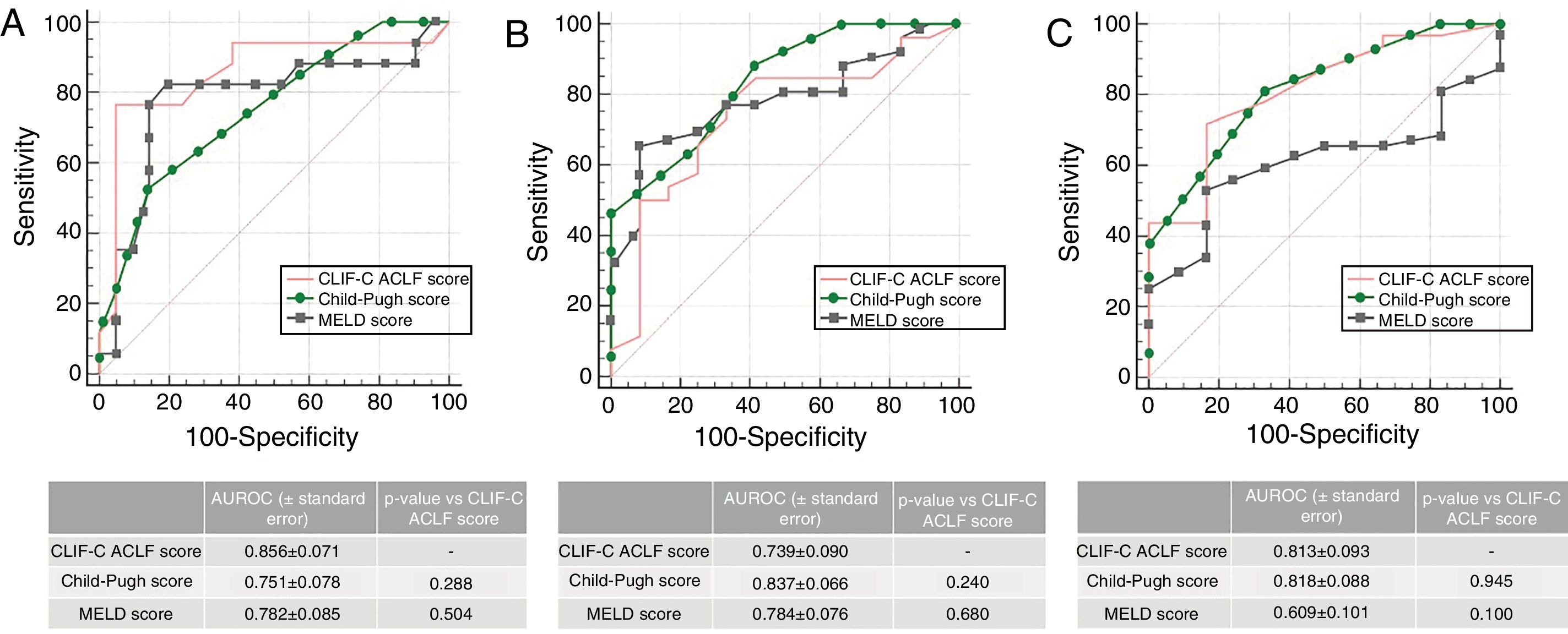
When analyzing patients with ACLF (n=38), the AUROC for CLIF-C ACLF Score for the prediction of M28 (0.856±0.071), M90 (0.739±0.090) and M12m (0.813±0.093) was not inferior to MELD of Child–Pugh, as depicted in Fig. 2. Patients with ACLF had significantly higher M28 (44.7% vs 5.9%, p<0.001), M90 (68.4% vs 11.8%, p<0.001) and M12m (84.2% vs 29.4%, p<0.001). Also, M28 was higher in ACLF2/3 when compared to ACLF1 (71.4% vs 29.2%, p=0.011), the same happening with M90 (92.9% vs 54.2%, p=0.027), but not reaching significance in M12m (100.0% vs 75.0%, p=0.067).
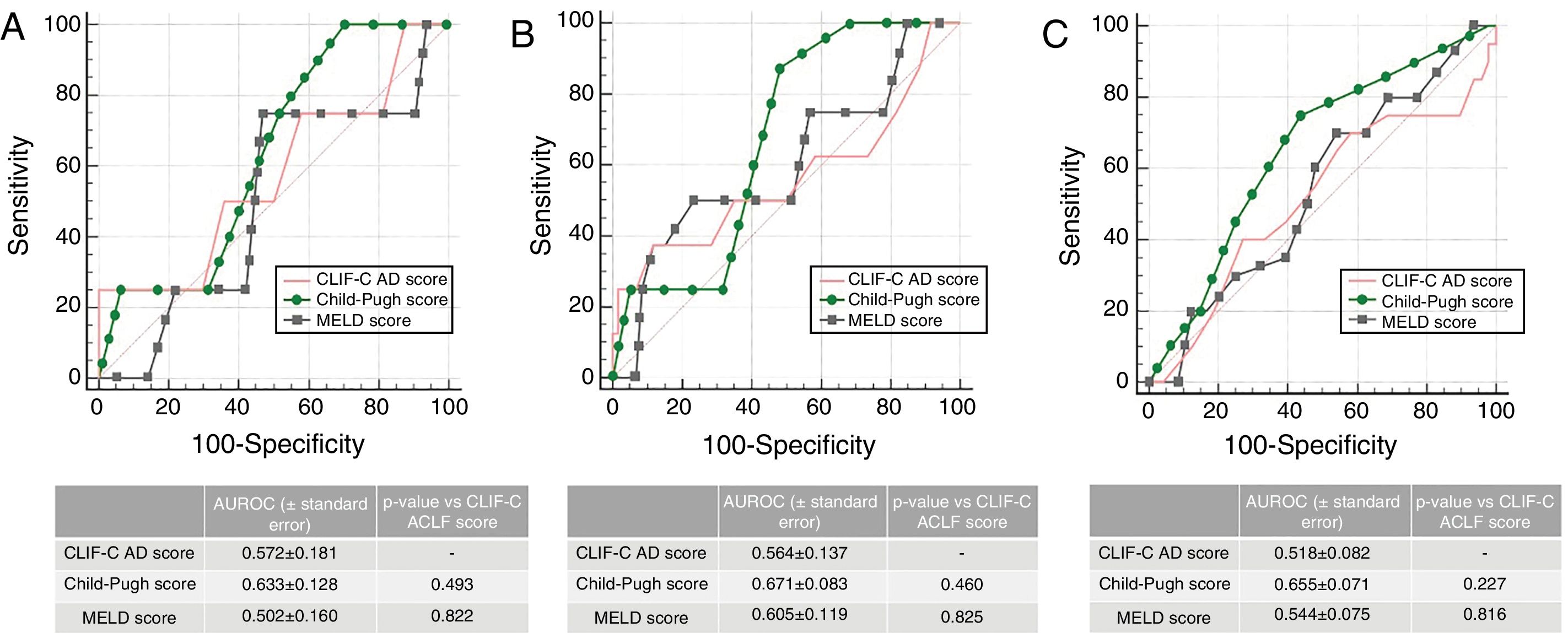
In patients without ACLF (n=68), the AUROC for CLIF-C AD Score for the prediction of M28 (0.572±0.0181), M90 (0.564±0.137) and M12m (0.518±0.082) was also not statistically inferior to MELD or Child–Pugh (Fig. 3), although all three scores presented a poor performance.
Accuracy of CLIF-C AD Score compared to that of Child–Pugh Score and MELD Score predicting 28-day (A), 90-day (B) and 1 year mortality (C) in patients with acute decompensation of cirrhosis without ACLF (n=68). AUROC (under receiver operating characteristic) curves were compared using DeLong method.
Considering the growing interest in the prognostic value of C-reactive protein,13 which is not included in EASL-CLIF Consortium Scores (CLIF-C ACLF and CLIF-C AD), this variable was tested in multivariate logistic analyses for M28 prediction in both groups (with CLIF-C ACLF and CLIF-C AD Scores, as appropriate). However, it was not found to predict M28 in either ACLF (p=0.346) or no ALCF (p=0.130) groups.
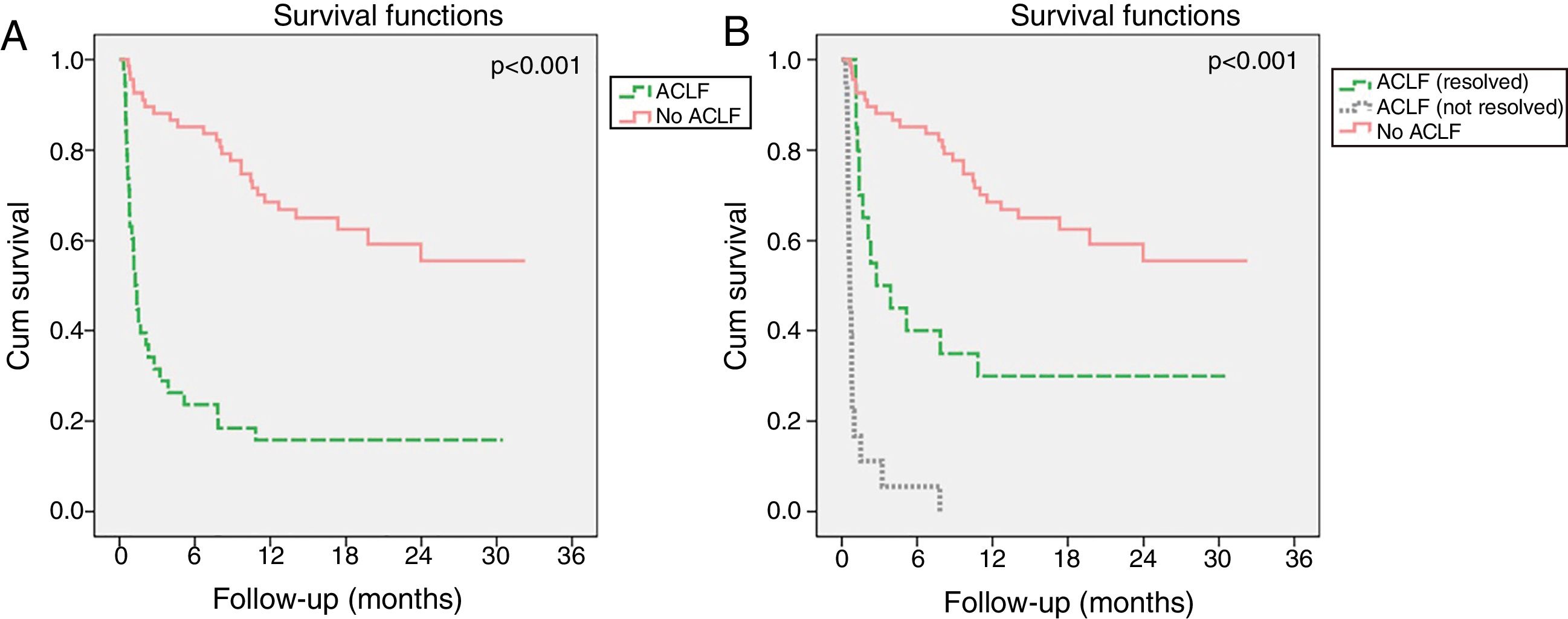
Evolution of ACLF and its impact in mortalityIn the ACLF group, the syndrome resolved in 54.1% of patients, improved in 8.1%, had a steady or fluctuating course in 13.5% and worsened in 24.3%. Resolution of ACLF was observed in 58.3% patients with ACLF1, 55.6% with ACLF2 and 25.0% with ACLF3. Although ACLF had a strong negative impact on long-term survival (Fig. 4A), its resolution resulted in significant improved survival relatively to patients in whom ACLF did not resolve (Fig. 4B). Indeed, patients with ACLF resolution had lower M28 (10.0% vs 82.4%, p<0.001), M90 (82.8% vs 50.0%, p=0.013) and M12m (100.0% vs 70%, p=0.022) than those in whom ACLF did not resolve.
Kaplan–Meier survival curves of patients with acute decompensation of cirrhosis (n=106) with and without ACLF (A). In B, patients with ACLF were further divided in two groups, according to whether ACLF resolved during the first 28 days of follow-up or not. Log-rank test was used for comparison of survival in different groups.
Final ACLF grade was reached during the first 48h in 38.9% patients, between days 3 and 7 in 22.2% and between days 8 and 28 in 38.9%.
ACLF onset during hospitalization was associated with higher M28 (63.2% vs 26.3%, p=0.022) and M90 (84.2% vs 52.6%, p=0.036) than ACLF at admission, although this difference was not significant when considering M12m (89.5% vs 78.9%, p=0.660). Indeed, these patients developed a more severe form of ACLF (ACLF grades 2/3: 57.9% vs 15.8%, p=0.007). Infection was a common precipitant of ACLF in hospitalized patients (it was present in 73.7% of ACLF onset during hospitalization vs 47.4% in newly admitted ACLF patients, p=0.097), which possibly accounted for increased mortality.
On the other hand, from all patients without ACLF on admission (n=87), when comparing those who went on developing ACLF (n=19) to those who did not (n=68), we observed higher disease severity on admission, as reflected by higher MELD (17.7±3.3 vs 13.7±4.2, p<0.001), Child–Pugh (10.0±1.7 vs 8.7±1.7, p=0.006) and CLIF-C AD Scores (57.6±9.3 vs 52.0±7.8, p=0.024). Indeed, a CLIF-C AD Score ≥60 on admission, which is classified as high-risk by the EASL-CLIF Consortium, was associated with a higher probability of developing ACLF during the same hospitalization (42.1% vs 16.2%, p=0.026).
Despite not reaching statistical significance, we observed a trend for better performance of ACLF Grade at day 3–7 when compared to the initial grade in predicting M28 (AUROC 0.786±0.082 vs 0.655±0.090, p=0.241), but less so in M90 (0.654±0.093 vs 0.632±0.075, p=0.845).
A similar trend was present when comparing the CLIF OF (CLIF Organ Failure) Score at day 3–7 to the initial score in predicting M28 (0.841±0.068 vs 0.800±0.080, p=0.601) and M90 (0.853±0.047 vs 0.746±0.092, p=0.069). This comparison was not possible for CLIF-C ACLF Score due to a low number of patients in whom it can be calculated, since resolution of ACLF was observed in more than half of them (54.1%).
DiscussionIn this Portuguese cohort of 106 patients admitted for acute decompensation of liver cirrhosis, approximately one third (35.8%) fulfilled ACLF criteria, similarly to what is reported in the CANONIC Study.6 Patients with ACLF had a higher incidence of infection, as well as higher white blood cell count and C-reactive protein levels, as expected in a syndrome characterized by systemic inflammation. A precipitating event was identified in the majority of our patients (86.8%), the most common being active alcoholism in the previous 3 months (55.8%).
In the group of patients without ACLF, the performance of the CLIF-C AD Score predicting mortality was rather poor, although comparable to MELD and Child–Pugh. However, we found that this score was a useful tool for risk stratification on admission. In fact, a CLIF-C AD ≥60 was associated with a higher risk of developing ACLF during the same hospitalization, which is in agreement with the algorithm proposed by the EASL-CLIF Consortium 1whereby they are classified as high-risk, warranting a closer observation.
The presence and severity of ACLF had a high impact on both early (28-day) and long-term (12-months) mortality. Although the CLIF-C ACLF Score globally had a prognostic performance comparable to MELD and Child–Pugh, we emphasize its high accuracy predicting short-term mortality (M28), with an AUROC of 0.856±0.071.
The dynamic nature of this syndrome strongly influenced prognosis. Similarly to Gustot et al.,10 we found that ACLF resolved in around half of the patients, including one in four patients with ACLF3. Importantly, ACLF resolution during the first 28 days after onset led to a significant increase in 12-months survival, although not reaching that of patients without ACLF. Also, the absence of ACLF on admission was not reassuring, as ACLF development during hospitalization was associated with a higher mortality than ACLF on admission. Taken together, these observations further emphasize the need for frequent reevaluations of ACLF severity.
Regarding the proposed time point of 3–7 days after ACLF diagnosis for prognostic assessment,10 we indeed observed a non-significant increase in the predictive accuracy of both ACLF grade and CLIF-C OF when compared to the initial assessment, by ROC curve analysis. Lack of significance is probably related to the small size of our population. However, we underline that in our cohort only about 60% of patients had reached their final ACLF grade by day 3–7, as compared to 81% reported by those authors. In fact, 13.9% of ACLF patients experienced its resolution only after 8 or more days, including 3 patients with ACLF 2. These authors also suggested this time point for an evaluation of possible withdrawal of care in ACLF3 patients who were not candidates for a liver transplant and either had a CLIF-C ACLF Score >64 or had four or more organ failures, due to futility. In our cohort, only two patients fulfilled these criteria, both of whom died within 10 days of ACLF onset. We remind that, as a study based in a gastroenterology ward as opposed to an intensive care unit, it is not intended to accurately characterize patients with multiple organ failures.
We acknowledge that this is an observational retrospective study, with its inherent limitations. In particular, not every patients had complete assessments in all of the sequential time points evaluated, which probably limited this part of the comparative analysis.
In summary, in our cohort prognosis was better defined by the early course of ACLF than by the initial evaluation, illustrating the dynamic nature of this syndrome and the importance of repeated assessments. Indeed, resolution of ACLF was associated with a favorable prognosis. On the other hand, patients with a high CLIF-C AD Score on admission were at risk of developing ACLF during hospitalization, which was associated with high mortality. In the consecutive evaluation of ACLF severity, it was not clear whether there is a time point that correlates best with prognosis, as resolution of ACLF can occur even after 8 or more days in a considerable proportion of patients. Nevertheless, sequential determination of AD/ACLF scores allows for standardization of severity and may help to elucidate about futility or escalation of care.
Conflicts of interestThe authors state no conflicts of interest and no sources of funding for this work.