Helicobacter pylori infection is very common in the Spanish population and represents the main cause of chronic gastritis, peptic ulcer, and gastric cancer. The last iteration of Spanish consensus guidelines on H. pylori infection was conducted in 2016. Recent changes in therapeutic schemes along with increasing supporting evidence were key for developing the V Spanish Consensus Conference (May 2021). Fourteen experts performed a systematic review of the scientific evidence and developed a series of recommendations that were subjected to an anonymous Delphi process of iterative voting. Scientific evidence and the strength of the recommendation were classified using GRADE guidelines. An eradication therapy, when prescribed empirically, is considered acceptable when it reliably achieves, or preferably surpass, 90% cure rates. Currently, only quadruple therapies (with or without bismuth) and generally lasting 14 days, accomplish this goal in first- and second-line therapies. A non-bismuth quadruple concomitant regimen (proton pump inhibitor, clarithromycin, amoxicillin, and metronidazole) or a quadruple bismuth-based combination (proton pump inhibitor, bismuth, tetracycline, and metronidazole), are recommended as first-line regimens. Rescue therapies after eradication failure and management of H. pylori infection in peptic ulcer disease were also reviewed.
La infección por Helicobacter pylori es muy frecuente entre la población española y representa la causa fundamental de gastritis crónica, úlcera péptica y cáncer gástrico. Previamente se han llevado a cabo cuatro reuniones de Consenso sobre el manejo de la infección por H. pylori en España, la última de ellas en 2016. Los cambios en los esquemas de tratamiento y la creciente evidencia disponible al respecto han justificado la organización de esta V Conferencia Española de Consenso en mayo de 2021, centrada en el tratamiento de esta infección. Participaron 14 expertos sobre el tema, que realizaron una búsqueda sistemática de la evidencia científica y elaboraron una serie de recomendaciones que fueron sometidas a un proceso de interacción de votaciones anónimas seriadas mediante metodología Delphi. Para clasificar la evidencia científica y la fuerza de las recomendaciones, se utilizó el sistema GRADE. Este consenso establece, como punto de partida, un aumento de la exigencia en la eficacia de los tratamientos recomendados, que deben alcanzar, o preferiblemente superar, el 90% de curación al ser administrados empíricamente. De este modo, tanto en primera como en segunda línea se recomiendan tratamientos cuádruples con o sin bismuto, generalmente prescritos durante 14 días. Como tratamiento de primera línea se recomienda una pauta cuádruple concomitante sin bismuto (inhibidor de la bomba de protones, claritromicina, amoxicilina y metronidazol) o una combinación cuádruple con bismuto (inhibidor de la bomba de protones, bismuto, tetraciclina y metronidazol). En el presente consenso se revisan también con detalle otras alternativas de tratamiento de rescate.
Helicobacter pylori (H. pylori) infection affects around 50% of the world population and plays a fundamental role in the development of various digestive diseases such as chronic gastritis, peptic ulcer and gastric cancer. Therefore, its proper diagnosis and effective treatment are crucial in clinical practice. In Spain, four consensus meetings on H. pylori infection have been organised to date: in 1999,1,2 2004,3,4 2012,5 and the last in 2016.6 The notable changes that have occurred in treatment regimens and the growing evidence available on the subject (especially in our setting) have justified the organisation of this V Spanish Consensus Conference in May 2021. Since there have been no significant advances in aspects related to indications for treatment or diagnostic techniques for the infection, this consensus conference will focus exclusively on updating the recommendations on the treatment of H. pylori infection.
MethodologyParticipants in the consensus: Spanish researchers were invited to participate who had published at least five articles during the last five years on the treatment of H. pylori infection, identified by the search strategy "Helicobacter pylori AND Spain" in PubMed. In all, 14 experts, including gastroenterologists, primary care physicians, and experts in scientific methodology and evidence-based medicine, were invited and all of them accepted. A gastroenterologist (JPG) acted as coordinator. The recommendations on the eradication treatment of H. pylori of this consensus group are primarily directed and applied to the adult population.
Bibliographic searches: priority was given to identifying systematic reviews and other documents of critical synthesis of the scientific literature. The following electronic databases were consulted: TRIP Database, NHS National Library of Guidelines, National Guideline Clearinghouse, Cochrane Database of Systematic Reviews (The Cochrane Library), Database of Abstracts of Reviews of Effects (DARE) and MEDLINE (access through PubMed). In a second phase, a search of individual studies, randomised clinical trials and observational studies was carried out, as well as a review of the bibliographic references of the documents included.
Classification of scientific evidence and strength of recommendations: to classify the scientific evidence and the strength of the recommendations, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system was used (http://www.gradeworkinggroup.org/). The GRADE system is a structured and explicit classification that has been widely adopted internationally, which has the advantage of overcoming the limitations of previous systems and standardising the system for formulating recommendations.7,8
Evaluation of the recommendations by the consensus group: the recommendations were initially drawn up by the coordinator. Subsequently, they were subjected to a process of interaction via a series of votes, using Delphi methodology.9 Two rounds of electronic voting were carried out.
For each recommendation, the participants rated their degree of agreement using a 6-point Likert scale (1: totally disagree; 2: strongly disagree; 3: somewhat disagree; 4: somewhat agree; 5: strongly agree; 6: totally agree). Any rating lower than 6 required the evaluators to make suggestions for improvement. After each vote, the coordinator reviewed the recommendations according to the comments and votes received, integrating the suggestions to maximise agreement. A recommendation was approved if more than 75% of the participants agreed (score 4–6 on the Likert scale).
Ethical aspects: the consensus was adjusted to the established ethical recommendations.10 Participants made conflict of interest declarations before and after the Delphi voting process (see the section Conflicts of interest).
Sponsorships, endorsements and funding: the conference was scientifically sponsored by the Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD) [Biomedical Research Networking Centre for Liver and Digestive Diseases]. This consensus document has been endorsed by the Asociación Española de Gastroenterología [Spanish Association of Gastroenterology] and by the Sociedad Española de Patología Digestiva [Spanish Society of Digestive Pathology], which have adhered to and support the consensus recommendations. There has been no funding from the pharmaceutical industry.
RecommendationsEach recommendation is accompanied by the result of the vote (percentage of agreement), the grade of recommendation (GR; strong or weak), the quality of the evidence (QE; high, moderate, low or very low) and the discussion of the corresponding evidence.
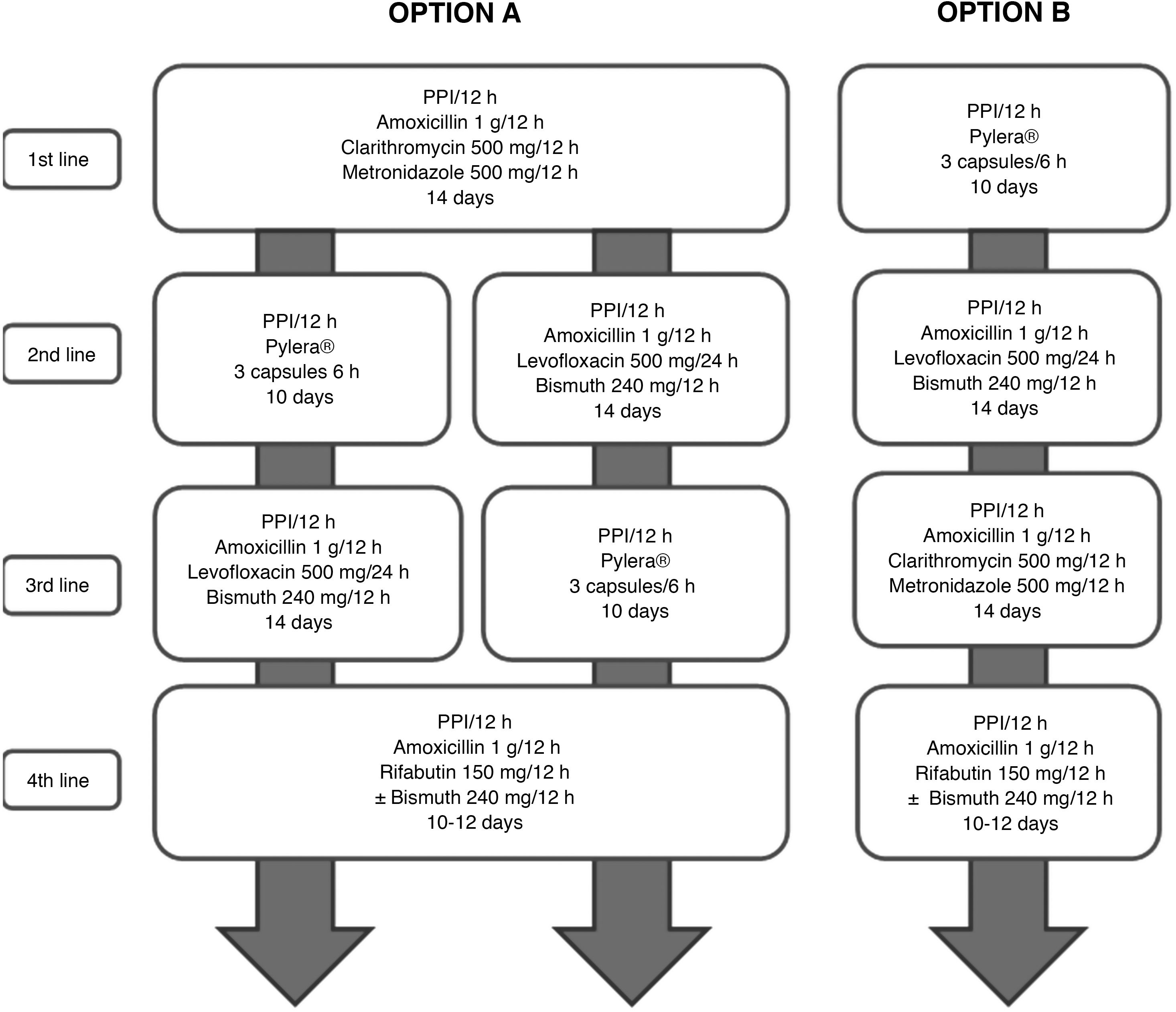
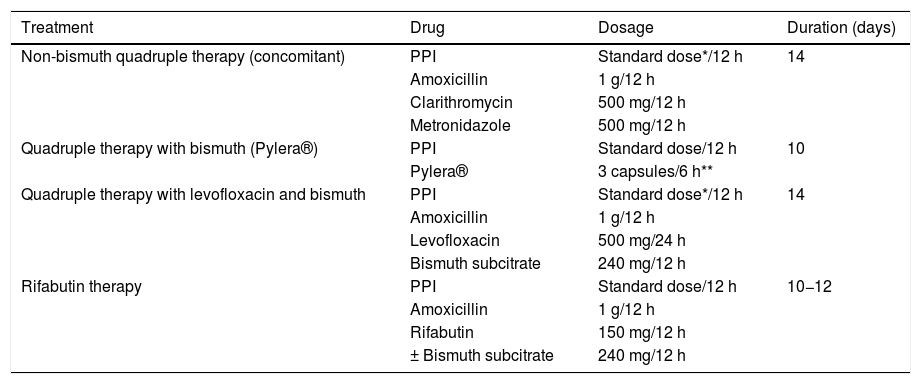
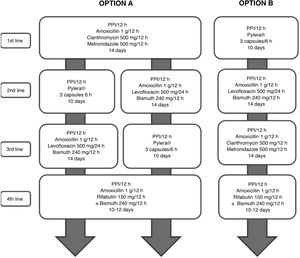
All the recommendations approved in this Spanish consensus on the treatment of H. pylori infection are included in Table 1. The drugs, duration and dose of each of the components of the recommended guidelines are detailed in Table 2. Finally, the algorithm for the initial and rescue treatment for the infection is shown in Fig. 1.
Recommendations for the treatment of H. pylori infection.
| Recommendation 1. Currently, it is recommended that an eradication therapy be considered effective when it is capable of curing H. pylori infection in close to, or preferably more than, 90% of patients. |
| Recommendation 2. As first-line treatment for H. pylori infection, a concomitant non-bismuth quadruple regimen (PPI, clarithromycin, amoxicillin and metronidazole) or a quadruple combination with bismuth (PPI, bismuth, tetracycline and metronidazole) is recommended. |
| Recommendation 3. It is recommended that the duration of concomitant non-bismuth quadruple therapy (PPI, clarithromycin, amoxicillin and metronidazole) be 14 days. |
| Recommendation 4. It is recommended that the duration of quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) be at least 10 days. |
| Recommendation 5. In patients allergic to penicillin, a quadruple regimen with bismuth (PPI, bismuth, tetracycline and metronidazole) is recommended as first-line treatment. |
| Recommendation 6. It is not recommended to combine probiotics with eradication therapy. |
| Recommendation 7. After the failure of a first treatment that includes clarithromycin (triple or quadruple), a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) is recommended. Another option is a quadruple regimen with levofloxacin (PPI, amoxicillin, levofloxacin and bismuth). |
| Recommendation 8. After the failure of a first treatment with a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole), a quadruple regimen with levofloxacin (PPI, amoxicillin, levofloxacin and bismuth) is recommended. |
| Recommendation 9. rescue treatment in patients allergic to penicillin: |
| a) After the failure of a first triple treatment (PPI, clarithromycin and metronidazole), it is suggested to use a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole). |
| b) After the failure of a first quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole), a quadruple therapy with PPI, levofloxacin, clarithromycin and bismuth is suggested. |
| Recommendation 10. After the failure of a first treatment with clarithromycin and a second treatment with levofloxacin, a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) is recommended. |
| Recommendation 11. After the failure of a first treatment with clarithromycin and a second-line quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole), a quadruple therapy with levofloxacin (PPI, amoxicillin, levofloxacin and bismuth) is recommended. |
| Recommendation 12. After the failure of a first quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) and a second-line treatment with levofloxacin, a concomitant quadruple therapy (PPI, amoxicillin, clarithromycin and metronidazole) is suggested. |
| Recommendation 13. After the failure of a third treatment, it is suggested to carefully reevaluate the need to eradicate the infection and, where appropriate, to indicate a fourth-line treatment with rifabutin. |
| Recommendation 14. In patients with uncomplicated duodenal ulcer who do not require non-steroidal anti-inflammatory drugs/aspirin, it is not recommended to continue antisecretory therapy after completing the H. pylori eradication therapy. |
| Recommendation 15. In patients with gastric ulcers who do not require NSAIDs/aspirin, it is recommended to maintain the antisecretory therapy for 4−8 weeks after the end of the H. pylori eradication therapy. |
| Recommendation 16. In patients with gastrointestinal bleeding from peptic ulcer, the eradication of H. pylori eliminates practically all relapses. Therefore, once eradication is confirmed and if the patient is not taking non-steroidal anti-inflammatory drugs/aspirin, it is recommended not to administer maintenance treatment with antisecretory drugs. |
Drugs, dose and duration of recommended H. pylori eradication treatments.
| Treatment | Drug | Dosage | Duration (days) |
|---|---|---|---|
| Non-bismuth quadruple therapy (concomitant) | PPI | Standard dose*/12 h | 14 |
| Amoxicillin | 1 g/12 h | ||
| Clarithromycin | 500 mg/12 h | ||
| Metronidazole | 500 mg/12 h | ||
| Quadruple therapy with bismuth (Pylera®) | PPI | Standard dose/12 h | 10 |
| Pylera® | 3 capsules/6 h** | ||
| Quadruple therapy with levofloxacin and bismuth | PPI | Standard dose*/12 h | 14 |
| Amoxicillin | 1 g/12 h | ||
| Levofloxacin | 500 mg/24 h | ||
| Bismuth subcitrate | 240 mg/12 h | ||
| Rifabutin therapy | PPI | Standard dose/12 h | 10−12 |
| Amoxicillin | 1 g/12 h | ||
| Rifabutin | 150 mg/12 h | ||
| ± Bismuth subcitrate | 240 mg/12 h |
PPI: proton pump inhibitor.
The benefit of administering a latest generation PPI (rabeprazole or esomeprazole) and the use of double PPI doses is not clearly established, unlike what occurs with standard triple treatment. However, it is possible that these optimisations also add eradicating benefits to these therapeutic regimens.68
Although this is the dose included in the SmPC, a recent study based on data from the European Registry on Helicobacter pylori management (Hp-EuReg) suggests that the dose of 4 capsules/8 h could have a similar effectiveness and tolerance.264
Since the advantage of guiding eradication therapy by studying the antimicrobial susceptibility of H. pylori compared to the empirical administration of treatment has not been sufficiently confirmed,11–14 and as this study is not generally accessible,15,16 the comments included in this consensus document have been based on the assumption that the individual susceptibility (of the particular patient) is unknown. Nevertheless, it seems reasonable to recommend routine susceptibility testing (culture or PCR), even before prescribing a first-line eradication therapy, in specialised centres interested in the management of H. pylori infection. In any case, the scope of application of this consensus is limited to Spanish territory and to other geographic areas that present a similar frequency of antibiotic resistance to the recommended drugs, especially clarithromycin, metronidazole and levofloxacin.
Recommendation 1. Currently, it is recommended that an eradication therapy be considered effective when it is capable of curing H. pylori infection in close to, or preferably more than, 90% of patients.
100% agreement; votes: totally agree (85.7%); strongly agree (14.3%). GR: strong. QE: very low.
The goal of treatment aimed at eliminating any microorganism should be to achieve 100% success, and H. pylori infection should not be an exception.17 However, in all the initial European18–21 and Spanish2,4,5 consensus guidelines, it was established that a cure rate equal to or greater than 80% could be considered sufficient. Given that we currently have antibiotic therapies with cure rates close to or even higher than 90% for most bacterial infections, it is considered that this efficacy threshold – albeit arbitrary – should also be that required for a treatment, both first-line and rescue, to be considered effective against H. pylori infection (although it is true that in second-line and successive treatments this threshold may be more difficult to reach). These eradication figures (≥90%) refer to the intention-to-treat (ITT) analysis, that is, considering the worst scenario (penalised by the possible incorrect compliance with the treatment by the patient) and not only taking into account clinical trials but also, and fundamentally, clinical practice studies. To achieve this goal, all treatments must be optimised in terms of duration, dose and interval of administration of proton pump inhibitors (PPIs) and antibiotics22,23 (Fig. 1).
Recommendation 2. As first-line treatment for H. pylori infection, a concomitant non-bismuth quadruple regimen (PPI, clarithromycin, amoxicillin and metronidazole) or a quadruple combination with bismuth (PPI, bismuth, tetracycline and metronidazole) is recommended.
100% agreement; votes: totally agree (100%). GR: strong. QE: moderate.
The choice of first-line treatment for H. pylori infection will depend primarily on the rate of resistance of this bacterium to the prescribed antibiotics.24 The classic triple therapy (PPI, clarithromycin and amoxicillin) is not recommended when the resistance rate to clarithromycin is greater than 15%,25 since the eradication figures are unacceptably low above this threshold.22 Other factors that influence the efficacy of eradication therapy are the patient's adherence and their previous history of antibiotic use, which could determine the choice of the first therapeutic option.22,26,27 The available evidence regarding potential first-line treatment guidelines is reviewed below.
Triple therapy (PPI, clarithromycin and amoxicillin)The mean efficacy of triple therapy in Spain was 80% and 70% in two systematic reviews published in 2011 and 2013.28,29 The efficacy of such treatment in the studies published later in Spain has usually been less than 75%.30–34 The European Registry on H. pylori Management (Hp-EuReg) provides us with information of great interest in this regard. More than 300 researchers from 30 countries participate in this registry, which evaluates how H. pylori infection is managed by European gastroenterologists.35 When analysing the Spanish data from this registry, which included approximately 3000 patients from 2013 to 2020, it was found that the eradication rate achieved with triple treatment with clarithromycin was only 80%, in the "modified" ITT analysis36 (this analysis aims to obtain a result as close as possible to that of clinical practice, including all patients who complete follow-up and in whom a confirmatory test of eradication success is performed, regardless of treatment compliance; henceforth, when the Hp-EuReg results are mentioned, we will always be referring to this analysis). Despite these poor results, this treatment continues to be used with some frequency in Spain (and in Europe37), especially in the primary care context.38 The use of vonoprazan (a competitive H+/K+ ATPase pump inhibitor), instead of a PPI, is associated with a marked increase in the efficacy of triple therapy (and even dual therapy with clarithromycin alone),39–44 but studies are still scarce and there has been no experience with this drug in our setting, where it is not yet marketed.
The mean rate of resistance to clarithromycin in Spain was 14% in 200945 and 17–18% in more updated reviews.27,29 A recent Spanish study has shown a clarithromycin resistance rate of 34% in children,46 while in adults the figures published in recent studies have been approximately 20%.47–50 Cumulatively, all this evidence points to continuing with the recommendation not to prescribe triple therapy as first-line treatment in Spain, especially when there are currently therapeutic alternatives capable of obtaining significantly better cure rates.51 These include quadruple therapies with or without bismuth, which will be reviewed below.
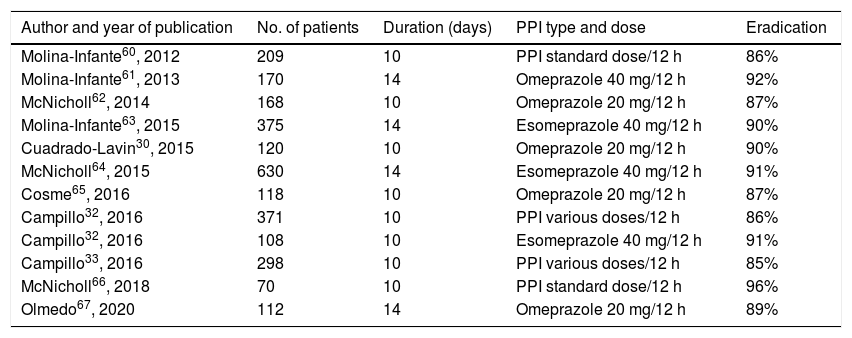
Non-bismuth quadruple therapies (PPI, amoxicillin, clarithromycin and metronidazole)The efficacy of non-bismuth quadruple therapies will depend fundamentally on the rate of dual resistance (at the same time) to clarithromycin and metronidazole.22 So-called "concomitant" therapy is the most effective non-bismuth quadruple therapy in high-resistance situations. It has been estimated that sequential, hybrid, and concomitant therapy will achieve cure rates >90% when this double resistance rate is below 5%, 9%, and 15%, respectively. In our setting, the dual resistance rate is still below 15%. Various studies carried out with concomitant therapy in many different countries have shown an efficacy close to or greater than 90% in the ITT analysis.52–54 A recent systematic review and meta-analysis that included 107 studies (55 of them randomised) and almost 30,000 patients, calculated a mean efficacy of concomitant therapy of approximately 90% (with good results even in the presence of resistance to clarithromycin or metronidazole), with results superior to standard triple treatment and sequential treatment.55 However, in certain regions of Europe and Asia, with much higher rates of resistance to clarithromycin and metronidazole, the efficacy of concomitant therapy has been suboptimal.56–59 The results obtained with this concomitant quadruple therapy in various studies carried out in Spain are summarised in Table 3, with a mean eradication efficacy of approximately 90%.30,32,33,60–67 When considering the Spanish studies that prescribe this treatment for 14 days, the eradication rates range between 89% and 92%, which is significantly higher than those obtained by classic triple therapy optimised and prolonged up to two weeks (81%).63 These favourable results have been described not only when the prescription has been made by gastroenterologists but also in the primary care context.67 Finally, the Hp-EuReg data have confirmed the excellent results of concomitant therapy, both in Europe in general68,69 and in Spain in particular,36 with eradication rates (by ITT) higher than 90% in both cases (if the treatment was prescribed for 14 days, which, as is specified below, is considered the appropriate duration).
Studies evaluating the efficacy of first-line concomitant non-bismuth quadruple therapy in Spain.
| Author and year of publication | No. of patients | Duration (days) | PPI type and dose | Eradication |
|---|---|---|---|---|
| Molina-Infante60, 2012 | 209 | 10 | PPI standard dose/12 h | 86% |
| Molina-Infante61, 2013 | 170 | 14 | Omeprazole 40 mg/12 h | 92% |
| McNicholl62, 2014 | 168 | 10 | Omeprazole 20 mg/12 h | 87% |
| Molina-Infante63, 2015 | 375 | 14 | Esomeprazole 40 mg/12 h | 90% |
| Cuadrado-Lavin30, 2015 | 120 | 10 | Omeprazole 20 mg/12 h | 90% |
| McNicholl64, 2015 | 630 | 14 | Esomeprazole 40 mg/12 h | 91% |
| Cosme65, 2016 | 118 | 10 | Omeprazole 20 mg/12 h | 87% |
| Campillo32, 2016 | 371 | 10 | PPI various doses/12 h | 86% |
| Campillo32, 2016 | 108 | 10 | Esomeprazole 40 mg/12 h | 91% |
| Campillo33, 2016 | 298 | 10 | PPI various doses/12 h | 85% |
| McNicholl66, 2018 | 70 | 10 | PPI standard dose/12 h | 96% |
| Olmedo67, 2020 | 112 | 14 | Omeprazole 20 mg/12 h | 89% |
“Intention-to-treat” eradication rates.
PPI: proton pump inhibitor.
Regarding sequential therapy (PPI together with amoxicillin during the first 5–7 days, followed by PPI together with clarithromycin and metronidazole during the last 5–7 days), various studies and meta-analyses have shown that it is not superior to 14-day triple therapy.70–74 Meanwhile, concomitant therapy is significantly superior to sequential therapy when both are prescribed for a similar duration.54 Furthermore, suboptimal results have been published with sequential therapy in our setting.62,75,76 Therefore, the use of sequential therapy is currently discouraged.
In summary, as a first-line treatment for H. pylori infection in Spain, a concomitant a non-bismuth quadruple regimen is recommended as one of the options. The duration and dose of its components (i.e., PPI, clarithromycin, amoxicillin, and metronidazole) are summarised in Table 2. For more details about the duration of concomitant therapy, see recommendation 3.
Quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole)Quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) represents a valid alternative to non-bismuth quadruple therapy, since it consists of drugs, such as bismuth and tetracycline, against which H. pylori is never or only exceptionally resistant.77 Meanwhile, resistance to metronidazole can be partially compensated by prolonged use of this antibiotic, at short intervals and with high doses.77
Three meta-analyses carried out in the last decade have coincided in emphasising that quadruple therapy with bismuth (mean efficacy: 81%, 78%, 77%) does not offer any advantage over triple therapy.78–80 However, quadruple therapy with bismuth was prescribed for only 7 days in the studies on which these meta-analyses are based, and these were also published a decade or more ago, when clarithromycin resistance rates (which reduce the efficacy of triple therapy) were considerably lower. In fact, a multicentre clinical trial, carried out between 2013 and 2016, in a geographic area with high resistance to clarithromycin, concluded that quadruple therapy with bismuth administered for 10 days was superior to the classic 14-day triple therapy (efficacy of 90% vs. 84%).81
The experience in Spain and in other countries with conventional bismuth quadruple therapy has been limited by the lack of availability of tetracycline hydrochloride, and by the fact that doxycycline (which is marketed here) is associated with worse results.82 The recent marketing of Pylera® (a capsule containing bismuth, tetracycline and metronidazole), has once again permitted the prescription of this therapeutic combination. A European multicentre trial initially demonstrated good results with Pylera® administered for 10 days, reaching an efficacy by ITT analysis (based on the result of a breath test) of 90%, significantly higher than that of triple therapy.83 Subsequently, numerous studies have been published, which have been summarised in a recent meta-analysis, showing that treatment with first-line Pylera® (21 studies included) achieved an eradication efficacy (by ITT) of 90%.84 These results were obtained regardless of the type and dose of PPI, and even in patients infected with strains of H. pylori resistant to clarithromycin or metronidazole.84 In fact, the high efficacy of this first-line treatment regimen (close to or greater than 90%) has been demonstrated in two recent studies carried out in China and Thailand,85,86 countries with a very high rate of resistance to clarithromycin (approximately 50%). The tolerance of this treatment is acceptable, although the incidence of adverse effects is relatively high (as is the case with all eradication therapies87). Most are mild, and only exceptionally do they oblige the suspension of treatment.36,84,88
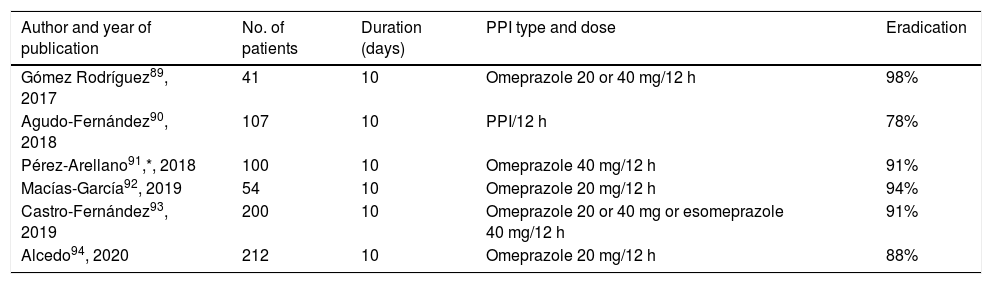
The results obtained with Pylera® in various studies carried out in Spain are summarised in Table 4, showing a mean efficacy of approximately 90 %.89–94 The Hp-EuReg data have confirmed these excellent results, both in Europe in general88 and in Spain in particular36 (currently with more than 2500 patients treated with Pylera®), obtaining eradication rates above 90%. A very recent update of this registry, including in this case more than 5000 patients, has once again confirmed these findings (94% eradication by ITT, as first-line treatment).95
Studies evaluating the efficacy of first-line therapy with Pylera® in Spain.
| Author and year of publication | No. of patients | Duration (days) | PPI type and dose | Eradication |
|---|---|---|---|---|
| Gómez Rodríguez89, 2017 | 41 | 10 | Omeprazole 20 or 40 mg/12 h | 98% |
| Agudo-Fernández90, 2018 | 107 | 10 | PPI/12 h | 78% |
| Pérez-Arellano91,*, 2018 | 100 | 10 | Omeprazole 40 mg/12 h | 91% |
| Macías-García92, 2019 | 54 | 10 | Omeprazole 20 mg/12 h | 94% |
| Castro-Fernández93, 2019 | 200 | 10 | Omeprazole 20 or 40 mg or esomeprazole 40 mg/12 h | 91% |
| Alcedo94, 2020 | 212 | 10 | Omeprazole 20 mg/12 h | 88% |
“Intention-to-treat” eradication rates.
PPI: proton pump inhibitor.
Finally, a very recent meta-analysis has summarised the clinical trials that compared bismuth versus concomitant quadruple therapy as first-line therapy (10 studies, although not all used the standard guidelines of these regimens) and has shown similar efficacy and safety with both therapies.96 In particular, a Spanish prospective (although not randomised) study compared these two regimens (Pylera® for 10 days versus concomitant for 14 days) and has shown similar eradication rates (94% and 98%, respectively), with similar tolerance.92 Another more recent Spanish study, also prospective (although not randomised), has again confirmed that both therapies (Pylera® and concomitant) are equivalent (eradication rates of 88% and 86%, respectively).94
A variant of quadruple therapy with bismuth consists of adding this drug to the classic triple therapy (that is, the combination of a PPI, clarithromycin, amoxicillin and bismuth), with which good results have been obtained in some studies.23 However, experience with this treatment in our setting is very limited (just one study has been published in Spain97) and the results in other countries have not always been very encouraging.23
In summary, quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole), and in particular using the Pylera® formulation, can now be considered a valid first-line alternative, together with concomitant therapy. The duration and dose of its components are summarised in Table 2. For more details about the duration of treatment, see recommendation 4.
Recommendation 3. It is recommended that the duration of concomitant non-bismuth quadruple therapy (PPI, clarithromycin, amoxicillin and metronidazole) be 14 days.
100% agreement; votes: totally agree (100%). GR: strong. QE: moderate.
Concomitant non-bismuth quadruple therapy, developed in the late 1990s, was initially designed to reduce the duration of eradication therapy.53 In fact, initial studies in Germany and Japan suggested that a duration of 3–5 days could be sufficient to achieve acceptable cure rates.98,99 However, more recently, several clinical trials that compared this therapy with different durations have shown higher cure rates with longer treatments: 3 days (81%) vs. 5 days (89%)100; 5 days (87%) vs. 7 days (90%)101; 5 days (89%) vs. 10 days (96%)102; 5 days (78%) vs. 14 days (86%)103; 10 days (80%) vs. 14 days (96%).104 In a recent non-randomised Spanish study, a 14-day concomitant therapy (together with a high PPI dose) was superior to a 10-day treatment (with a standard PPI dose; 87% vs. 91%, p < 0.01).64 Similarly, the first meta-analysis published on this therapy showed that the efficacy of concomitant therapy was dependent on its duration.52 In the Hp-EuReg, concomitant therapy was administered to 4164 patients, confirming a higher eradication rate with the 14-day versus 10-day regimens (92% vs. 88%).68 Finally, this same trend can be observed in the collective Spanish experience obtained in recent years, where 14-day treatments are the only ones that have consistently reached (or even exceeded) the 90% efficacy threshold (Table 3).
In summary, it is currently recommended that the duration of concomitant quadruple therapy be 14 days.
Recommendation 4. It is recommended that the duration of quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) be at least 10 days.
100% agreement; votes: totally agree (100%). GR: strong. QE: moderate.
It has been suggested that 10-day quadruple therapy with bismuth would be highly effective against strains sensitive to metronidazole, but it is possible that a 14-day is more effective than a 10-day therapy against microorganisms resistant to this antibiotic.85 However, various studies have suggested that a duration of 10 days might be sufficient, as reviewed below.
In a meta-analysis published in 2004 on different eradication therapies, it was observed that the efficacy of bismuth quadruple therapy administered for 1–3, 4, and 7 days was lower than with a duration of 10–14 days.105 Likewise, it was found that a duration equal to or greater than 10 days achieved cure rates higher than 85%, even in regions with a high prevalence of resistance to metronidazole.105 To date, there is only one study (including 417 patients) that directly compares 10-day and 14-day quadruple therapy with bismuth, and found no differences between the two regimens (92% vs. 93%).106
Meanwhile, in recent years excellent results have been obtained with bismuth quadruple therapy being presented in a single capsule (Pylera®), which is sold in packs/bottles that imply its prescription for 10 days. Thus, a recent meta-analysis, that included 30 studies and more than 6000 patients treated with Pylera® for 10 days, confirmed an efficacy (by ITT) of 90% as first-line treatment.84 In eight studies, the proportion of patients with resistance to clarithromycin or metronidazole was estimated, and Pylera® was highly effective despite the presence of such resistance.84
Finally, the Hp-EuReg has recently analysed the effectiveness of Pylera® (10 days) in clinical practice in various European countries (mainly Spain, Italy and Portugal), where 2100 patients were evaluated.88 Its efficacy (by ITT) was 95% as first-line, 89% as second-line, and 92% as third- to sixth-line rescue treatment.88 Although a culture was performed only to evaluate antibiotic resistance in 48 cases, Pylera® was also effective (>90%) in those patients with H. pylori strains resistant to clarithromycin or metronidazole, or to both.88 A very recent update of this European registry included more than 5000 patients treated with this 10-day bismuth quadruple therapy in a single capsule and confirmed the excellent eradication rates (by ITT): 94% as first-line treatment, 90% as second-line, and 86% in subsequent treatment lines.95
Unfortunately, there are no studies that directly compare the traditional bismuth quadruple therapy (with its components administered separately) and Pylera® (in a single capsule), nor between different durations (10 vs. 14 days) of the latter presentation. Therefore, comparative studies evaluating the efficacy, safety and cost of different regimens are necessary to clarify the ideal duration of bismuth quadruple therapy, especially depending on the pattern of resistance to metronidazole. In the meantime, it seems prudent to recommend that the duration of this treatment be at least 10 days.
Recommendation 5. In patients allergic to penicillin, a quadruple regimen with bismuth (PPI, bismuth, tetracycline and metronidazole) is recommended as first-line treatment.
100% agreement; votes: totally agree (100%). GR: strong. QE: moderate.
Amoxicillin is one of the most effective antimicrobial agents against H. pylori and therefore many of the eradication therapies include this antibiotic. Until now, experience with eradication therapy in patients allergic to penicillin has been limited, although in clinical practice this circumstance is relatively common. However, it must be taken into account that only a minority of patients who report a (theoretical) history of allergy to penicillin actually have evidence of immune-mediated hypersensitivity, and for this reason reliable confirmation of this is essential.107
In patients with allergy to beta-lactams, triple therapy with PPI, clarithromycin and metronidazole has traditionally been recommended.21 In a meta-analysis conducted more than 20 years ago, treatment with PPI, clarithromycin and nitroimidazole was considered relatively effective, with mean eradication rates above 80%.108 However, in a prospective Spanish study, this regimen was administered for 7 days to 12 patients allergic to penicillin and an eradication rate (by ITT) of only 58% was obtained.109 In another subsequent Spanish study, in this case a multicentre study, eradication figures as low as 55% were achieved when using this same treatment in 50 patients.110 The disappointing cure rates (<60%) in these Spanish studies109,110 could be due, at least in part, to the recent increase in resistance rates to both clarithromycin and metronidazole.29,45,111
A few years ago, two groups of researchers evaluated the efficacy of a regimen with PPI, tetracycline and metronidazole for 10 days in 5 and 17 patients, with penicillin allergy, obtaining eradication rates (by ITT) of 80–85%.112,113 These encouraging results suggested that this triple combination (or better yet, with the addition of bismuth, which would result in a quadruple regimen) could be a better alternative for first-line treatment in the presence of penicillin allergy (mainly in areas with high resistance to metronidazole or clarithromycin). This would probably be the case because the negative effect of resistance to metronidazole is overcome by the co-administration of bismuth114 and because the efficacy of this regimen is not influenced by resistance to clarithromycin.83
In this regard, the results of a prospective Spanish multicentre study have recently been updated, in which 267 patients allergic to penicillin were administered a first-line treatment of PPI, clarithromycin and metronidazole for 7 days or of PPI, bismuth, tetracycline and metronidazole for 10 days.115 The eradication rate (by ITT) with the triple therapy was only 57%, being clearly higher with the quadruple regimen (74%). Adherence to treatment was 94% and 98%, respectively. Adverse effects (all mild) were reported in 14% of patients with both regimens. It was therefore concluded that, despite the fact that in areas of low resistance to clarithromycin, perhaps a triple combination with PPI, clarithromycin and metronidazole could be prescribed in patients allergic to penicillin, quadruple therapy with bismuth should be preferred in areas, such as Spain, with high resistance to clarithromycin.
Along the same lines, the results have recently been published of a Hp-EuReg study specifically aimed at analysing the experience of patients (more than 1000) allergic to penicillin.116 In first-line treatment, the effectiveness of the combination of a PPI, clarithromycin and metronidazole was only 69%, while the bismuth quadruple therapy (either in its traditional format or with the single Pylera® capsule) reached 91%.
Finally, Liang et al. randomised 109 penicillin-allergic patients to receive a classic bismuth quadruple therapy (PPI, bismuth, tetracycline and metronidazole) or a modified bismuth quadruple regimen (PPI, bismuth, tetracycline and furazolidone).117 Eradication rates (by ITT) were 88% and 92%, respectively, supporting the effectiveness of bismuth-containing quadruple regimens in penicillin-allergic patients.
In summary, in patients allergic to penicillin, it is recommended, in our setting, to use a quadruple regimen with bismuth (PPI, bismuth, tetracycline and metronidazole) as the first line of treatment.
Recommendation 6. It is not recommended to combine probiotics with eradication therapy.
100% agreement; votes: totally agree (100%). GR: strong. QE: low.
Probiotics are live microorganisms that, administered in adequate amounts, can confer beneficial health effects. Although there is some evidence in favour of the clinical usefulness of certain probiotics, the latest Clinical Practice Guide from the American Gastroenterological Association only recommends their use in the prevention of diarrhoea due to Clostridioides difficile and necrotising enterocolitis in preterm newborns.118 However, the heterogeneity in the methodology of the studies and the variability of the strains involved, probably grouped inadequately in many cases, could explain, at least in part, the inconsistent results obtained. The most commonly used microorganisms in probiotic formulations in clinical practice are Lactobacillus spp., Bifidobacterium and Saccharomyces, as well as Bacillus, Streptococcus and Escherichia coli. The potential beneficial effects include regulation of the intestinal microbiota, stimulation of the immune system response and inhibitory activity against H. pylori demonstrated in vitro and in vivo.119
Regarding eradication therapy against H. pylori, there is considerable scientific evidence, summarised in multiple meta-analyses, on the use of multiple probiotic formulations, which globally point to a reduction in adverse effects and, to a lesser extent, a possible improvement in eradication rates with eradication therapies.120–137 However, various negative results have been published with the use of probiotics in combination with, primarily, triple therapy.138–142 It is likely that these discordant results are related to the use of different strains and combinations of these, as well as different concentrations, doses and durations of treatment.143,144 On the other hand, the vast majority of published studies on probiotics have evaluated their impact on the classic triple therapy, a relatively well tolerated treatment, with insufficient efficacy and which is no longer recommended as a first-line treatment in our setting.23 In this context, a recent meta-analysis including 33 clinical trials and 4459 patients showed that the therapeutic benefit obtained with probiotics was greater the less effective the eradication therapy.125 In fact, probiotic supplementation did not provide any therapeutic benefit when the effectiveness of the eradication therapy was greater than 80%. Moreover, in a randomised clinical trial, the usefulness of probiotics in combination with concomitant quadruple therapy, which is one of the first-line treatments currently recommended in Spain, was nil.66 And neither were two recent randomised studies able to demonstrate the potential beneficial effect (in terms of eradication of H. pylori) of probiotics combined with bismuth quadruple therapy, which represents another of the first-line options in our setting.145,146
Finally, it should be noted that probiotics are not funded in Spain, which increases the cost of eradication therapy. In addition, it makes the quadruple therapy more complex, by adding a fifth compound. Lastly, no studies have been published that allow us to predict which patients have a higher risk of suffering side effects with antibiotic treatment, which would enable the administration of probiotics to be individualised. In any case, the use of probiotics could be considered in highly selected cases, such as in patients with poor tolerance or with side effects with previous antibiotic treatments.
In conclusion, more evidence is needed about the impact of probiotics on the effectiveness and safety of the new quadruple eradication therapies against H. pylori before they can be implemented in daily clinical practice. Therefore, at the moment it is not recommended to combine probiotics with eradication therapy.
Recommendation 7. After the failure of a first treatment that includes clarithromycin (triple or quadruple), a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) is recommended. Another option is a quadruple regimen with levofloxacin (PPI, amoxicillin, levofloxacin and bismuth).
100% agreement; votes: totally agree (100%). GR: strong. QE: moderate.
Following the failure of clarithromycin therapy, it is conceivable that H. pylori was already resistant to this antibiotic (primary resistance) or that it had developed resistance (secondary) to it after a failed eradication therapy. Therefore, using clarithromycin again should be avoided. In this regard, a combined analysis of eight studies found a very low eradication rate of 46% when a treatment containing this antibiotic was repeated.147
When standard triple therapy (PPI, clarithromycin and amoxicillin – a combination that is no longer recommended) has failed, the classic quadruple therapy (PPI, bismuth, tetracycline and metronidazole) has traditionally been recommended as a rescue treatment, with which, according to a meta-analysis published in 2013, an average eradication of 78% was achieved.147 Recently, a meta-analysis of nine studies using the same regimen has obtained similar results (76%).148 In recent years, experience has been steadily obtained in the use of Pylera® as a rescue treatment. Moreover, an updated meta-analysis concludes that this treatment has an efficacy (by ITT) of 89% as second-line treatment, with a good safety profile.84 In the same way, the experience of the Hp-EuReg is also encouraging, having confirmed the results of the aforementioned meta-analysis, reaching eradication rates of approximately 90% (by ITT), both in Europe and in Spain in particular, after the failure of a first attempt at eradication.36,88,95,149
Due to the complexity of the classic bismuth quadruple therapy and the lack of availability of tetracycline and bismuth salts in many countries, various studies have been carried out using levofloxacin as rescue treatment.150 The results have been similar to those obtained with bismuth quadruple therapy, with a mean eradication of 76–79%.147,148 Various meta-analyses have compared, as a second-line treatment, a triple regimen with levofloxacin versus a quadruple therapy with bismuth, and have shown similar efficacy with both regimens (or even somewhat higher with levofloxacin in some cases) and a lower incidence of adverse effects with quinolone-containing therapy.147,151–157
These promising results with levofloxacin were confirmed in a large Spanish multicentre study, in which 1000 patients, in whom a first eradication therapy with PPI, amoxicillin and clarithromycin had failed, received PPI, amoxicillin and levofloxacin for 10 days.158 Eradication was achieved in 74% of the patients and, although adverse effects were reported in one fifth of the cases, none of them was serious. In this study, it was also assessed whether the efficacy decreased over time, since the resistance to quinolones in Spain seems to be increasing fairly quickly.24,27 However, eradication rates remained stable over time (during the six years of the study).158
Non-bismuth quadruple therapies that include PPI, amoxicillin, clarithromycin and a nitroimidazole (mostly in a concomitant regimen) are widely used as first-line treatment today. Finding rescue therapies after the failure of these therapies, which use key antibiotics such as clarithromycin and nitroimidazoles, is challenging. A meta-analysis has recently been carried out to evaluate which second-line treatments have been investigated after a failed eradication attempt with these therapies.159 Most of the studies evaluated a rescue treatment with PPI, amoxicillin and levofloxacin, a combination with which an overall eradication rate (by ITT) of approximately 80% was obtained, after failure of a non-bismuth quadruple therapy.139,160–165 This triple therapy (PPI, amoxicillin and levofloxacin) was relatively effective both after failure of sequential therapy (81%)139,161–165 and concomitant therapy (77%).160,161,165
It is evident that the efficacy of triple therapy with levofloxacin can be improved (remember that currently our therapeutic objective must be to achieve an eradication efficacy equal to or greater than 90%, and we should not settle for less, regardless of whether it is an initial or rescue treatment). At the same time, as previously mentioned, the rate of resistance to quinolones seems to be increasing and this may negatively affect the efficacy of triple therapy with levofloxacin.166,167 It has been suggested that the addition of bismuth could reduce this negative effect, as this drug has a synergistic effect with certain antibiotics and largely overcomes resistance to clarithromycin and levofloxacin.168,169 In this regard, a recent study has shown that with the addition of bismuth (PPI, amoxicillin, levofloxacin and bismuth for 14 days) an eradication efficacy is obtained of 95% “by protocol” and 88% by ITT.169 These figures were higher than those obtained with a triple therapy − without bismuth − with PPI, amoxicillin and levofloxacin. These favourable results were obtained despite a high rate of resistance to quinolones (30%), higher than that described in our setting. This quadruple therapy (PPI, amoxicillin, levofloxacin and bismuth) achieved eradication in 98% of patients with quinolone-sensitive H. pylori strains and, more importantly, achieved eradication in a relatively high percentage (71%) of strains resistant to levofloxacin. However, when triple therapy with levofloxacin was used, the infection was only eradicated in 38% of patients with quinolone-resistant strains.
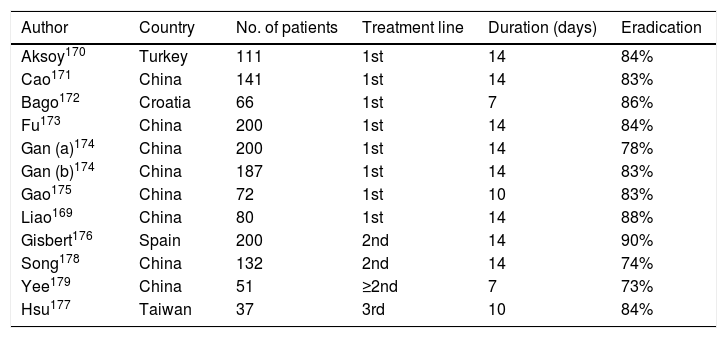
More recently, various studies have evaluated the aforementioned quadruple combination with levofloxacin (PPI, amoxicillin, levofloxacin and bismuth) as a second-line treatment,150 as shown in Table 5, achieving generally satisfactory eradication rates.169–179 Among them, a recent Spanish multicentre study administered a quadruple combination with PPI/12 h, amoxicillin (1 g/12 h), levofloxacin (500 mg/24 h) and bismuth (240 mg/12 h) for 14 days in 200 patients in whom triple therapy (PPI, clarithromycin and amoxicillin) or non-bismuth quadruple therapy had previously failed.176 In total, 96% of the patients took the medication correctly. Globally, ITT eradication rates were 90%. These figures were similar regardless of previous treatment: triple therapy (88.5%) vs. sequential therapy (93.8%) vs. concomitant therapy (91.9%). Therefore, quadruple therapy with bismuth and levofloxacin for 14 days constitutes an effective second-line therapy (≥90% cure), not only in patients after eradication failure with standard triple therapy but also in those with failure of quadruple therapy without bismuth.
Studies evaluating the efficacy of the combination of PPI, amoxicillin, levofloxacin and bismuth for the eradication of H. pylori.
| Author | Country | No. of patients | Treatment line | Duration (days) | Eradication |
|---|---|---|---|---|---|
| Aksoy170 | Turkey | 111 | 1st | 14 | 84% |
| Cao171 | China | 141 | 1st | 14 | 83% |
| Bago172 | Croatia | 66 | 1st | 7 | 86% |
| Fu173 | China | 200 | 1st | 14 | 84% |
| Gan (a)174 | China | 200 | 1st | 14 | 78% |
| Gan (b)174 | China | 187 | 1st | 14 | 83% |
| Gao175 | China | 72 | 1st | 10 | 83% |
| Liao169 | China | 80 | 1st | 14 | 88% |
| Gisbert176 | Spain | 200 | 2nd | 14 | 90% |
| Song178 | China | 132 | 2nd | 14 | 74% |
| Yee179 | China | 51 | ≥2nd | 7 | 73% |
| Hsu177 | Taiwan | 37 | 3rd | 10 | 84% |
“Intention-to-treat” eradication rates.
Gan (a): levofloxacin 500 mg/24 h; Gan (b): levofloxacin 200 mg/12 h; PPI: proton pump inhibitor.
In this regard, quadruple therapy with levofloxacin (PPI, levofloxacin, amoxicillin and bismuth) administered for at least 10 days proved to be the most effective treatment in a network meta-analysis that included 26 clinical trials on second-line eradication therapies,157 in accordance with the results of the previously cited meta-analysis (in which eradication reached 90% with this quadruple regimen).159 In the same vein, the most up-to-date data from the Hp-EuReg show how, after failure of a first-line treatment containing clarithromycin, optimal eradication (≥90%) is obtained with a quadruple therapy with bismuth, either the traditional one (with tetracycline and metronidazole) or with levofloxacin.95,149 In particular, the Spanish data from this registry confirm how eradication figures of approximately 90% can be achieved both with quadruple therapy with levofloxacin and with Pylera®.36 In the latter, quadruple therapy with PPI, bismuth, tetracycline and metronidazole has achieved promising results as rescue treatment after failure of a quadruple therapy without bismuth, but experience is still limited.180,181
It should be noted that it has been estimated that eradication therapy with levofloxacin will not achieve acceptable eradication rates (≥90%) if the proportion of H. pylori strains resistant to this antibiotic is >25%.182 In Spain, published data describe, in most cases, rates of resistance to levofloxacin that do not reach this threshold,47,49,50,183,184 but it is obvious that the increase in resistance to quinolones must be monitored locally.
Regarding the duration of treatment with levofloxacin, this should be at least 10 days, and probably better of 14 days.150 In this regard, three meta-analyses151–153 have shown, as have three recent randomised clinical trials,166,185,186 higher cure rates with regimens of 10–14 days than with treatments containing levofloxacin for only 7 days. Furthermore, two recent studies have compared the efficacy of triple therapy containing levofloxacin for 14, 10 and 7 days as rescue treatment, and have shown a higher eradication rate with the longer regimen.187
With regard to the dosage of levofloxacin, a dose of 500 mg per day is considered sufficient.150 Thus, levofloxacin 500 mg/day has been shown to be equally effective, but better tolerated, than higher doses (e.g. 1000 mg/day).166,188–190
Recently, the US Food and Drug Administration and the European Medicines Agency have published several alerts on serious adverse effects of fluoroquinolones. For this reason, both agencies advise against the use of fluoroquinolones for most mild and moderate infections or those in which there is a therapeutic alternative, restricting their use to infections in which the therapeutic benefit outweighs the risks.191 In this sense, taking into account the results obtained in clinical practice, we consider that, for the time being, the recommendations issued by the Conferencia Española de Consenso [Spanish Consensus Conference] on the treatment of previous H. pylori infection (IV)6 should not be substantially modified, as well as those by other international consensus groups,25,192 regarding the use of fluoroquinolones as rescue treatment after the failed eradication of H. pylori. That being said, it is necessary to insist on the need to make responsible use of all antibiotics – and of quinolones in particular, which in the case of H. pylori should be reserved for rescue therapies – to report all suspected adverse reactions and to remain attentive to possible communications from national and international health authorities on serious adverse effects of these drugs.191
Recommendation 8. After the failure of a first treatment with a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole), a quadruple regimen with levofloxacin (PPI, amoxicillin, levofloxacin and bismuth) is recommended.
100% agreement; votes: totally agree (92.8%); strongly agree (7.1%). GR: strong. QE: low.
After an eradication failure with bismuth quadruple therapy (PPI, bismuth, tetracycline and metronidazole), any treatment could theoretically be used, including the repetition of the same quadruple therapy but with higher doses and longer duration of metronidazole, given that the rate of acquired resistance after the use of amoxicillin, bismuth or tetracycline is negligible (<3%) and resistance to metronidazole could be overcome, at least partially, in this way.22 However, it seems logical not to repeat a treatment that has already failed.37 It also seems reasonable to assume that if quadruple therapy with bismuth has been used as the first option, it may be due to the existence of a high rate of resistance to clarithromycin (which reduces the efficacy of standard triple therapy) or a high rate of combined resistance to clarithromycin and metronidazole (which is associated with lower efficacy of non-bismuth quadruple therapies).22 In this sense, after a first eradication attempt with metronidazole has failed, there is a high probability that H. pylori is resistant to this antibiotic (either because it had previous primary resistance or because it developed secondary resistance after treatment). In any case, as is well known, the presence of dual resistance – to clarithromycin and to metronidazole – greater than 15% would significantly limit the efficacy of concomitant quadruple therapy. Thus, the use of a second-line treatment containing clarithromycin ± metronidazole after failure of a quadruple therapy with bismuth would probably not be the best option (although it could theoretically be considered to administer a quadruple combination with PPI, amoxicillin, clarithromycin and bismuth, although experience of this as a rescue treatment is very limited).23,97
On the contrary, it is well known that levofloxacin therapy is effective as a second-line treatment after failure of clarithromycin treatment150; therefore, this would seem more recommendable after the eradication failure of a quadruple regimen with bismuth. However, experience after failure of a quadruple therapy with bismuth in general, and with the administration of a rescue treatment with levofloxacin in particular, is remarkably limited. Studies that have evaluated the efficacy of a third-line treatment combining PPI, amoxicillin and levofloxacin for the eradication of H. pylori after failure of two treatments, the second line being the quadruple regimen with bismuth, are reviewed in recommendation 11 (obtaining an efficacy between 60% and 85%, with a mean of approximately 75%).
As previously mentioned (see recommendation 7, devoted to rescue treatment after failure of clarithromycin therapies), the addition of bismuth to triple therapy with levofloxacin has achieved promising results, so it would therefore be the choice. In this regard, the results of the Hp-EuReg support this recommendation, by demonstrating that, after the failure of a quadruple therapy with bismuth, a second-line quadruple regimen with quinolones (i.e. PPI, levofloxacin, amoxicillin and bismuth), administered for 14 days, reaches optimal eradication figures (≈90%).149
Recommendation 9. Rescue treatment in patients allergic to penicillin:
- a)
After the failure of a first triple therapy (PPI, clarithromycin and metronidazole), it is suggested to use a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole). GR: weak. QE: very low.
- b)
After the failure of a first quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole), a quadruple therapy with PPI, levofloxacin, clarithromycin and bismuth is suggested.
100% agreement; votes: totally agree (92.8%); strongly agree (7.1%). GR: weak. QE: very low.
The eradication of H. pylori in penicillin-allergic patients is challenging, especially in those in whom a previous eradication attempt has already failed. In a Spanish pilot study, 15 penicillin-allergic patients in whom a first treatment with PPI, clarithromycin and metronidazole had failed received a second treatment with PPI, clarithromycin and levofloxacin for 10 days.110 Adherence to treatment was complete in all cases. Adverse effects, which were all mild, were reported in 20% of the patients. The eradication rate (by ITT) was 73%.
More recently, in a Spanish multicentre study, 267 penicillin-allergic patients received first-line treatment with PPI, clarithromycin and metronidazole or bismuth quadruple therapy (PPI, bismuth, tetracycline and metronidazole); and as rescue therapies, bismuth quadruple therapy or a regimen with PPI, clarithromycin and levofloxacin for 10 days.115 The eradication rate (by ITT) with PPI, clarithromycin and levofloxacin was 64%, both after failure of PPI, clarithromycin and metronidazole and of first-line quadruple therapy with bismuth, and adherence was 88–100%, with adverse effects (all mild) in 23–29% of patients. Therefore, the authors concluded that a triple therapy with PPI, clarithromycin and levofloxacin represents a second-line alternative in patients with a penicillin allergy.
In this regard, the results of the Hp-EuReg focusing specifically on patients allergic to penicillin have recently been published.116 In second-line treatment, after the failure of a combination of a PPI, clarithromycin and metronidazole, two rescue options showed similar efficacy: a quadruple therapy with PPI, bismuth, tetracycline and metronidazole (78%), and a triple combination with PPI, clarithromycin and levofloxacin (71%).116
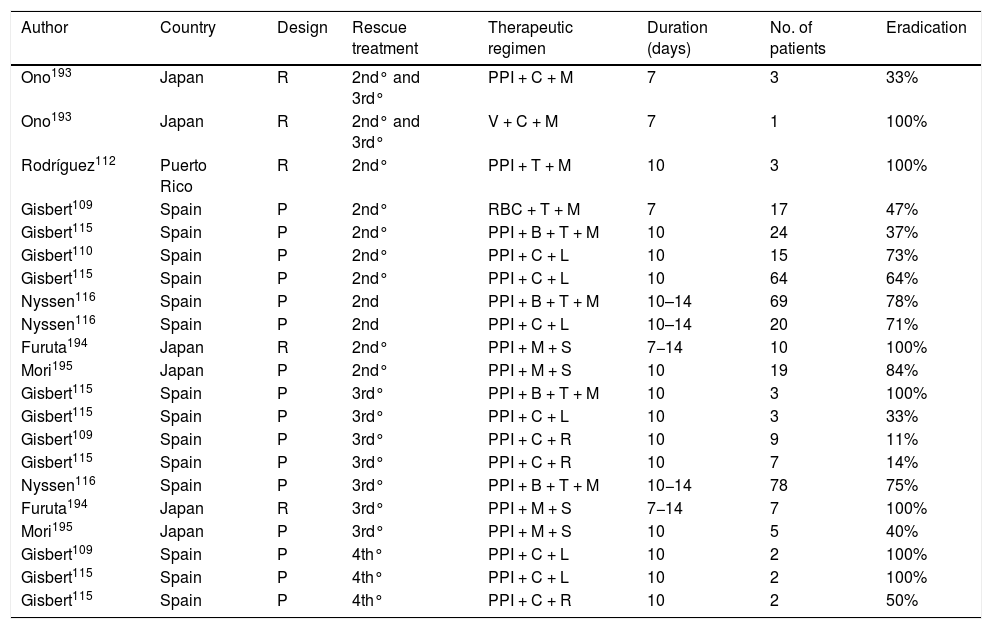
The few studies that have evaluated the various empirically prescribed rescue eradication therapies (without studying bacterial susceptibility) in patients with penicillin allergy are summarised in Table 6.109,110,112,115,116,193–195 From these it can be concluded that, in patients allergic to penicillin, after failure of a triple therapy (PPI, clarithromycin and metronidazole, a combination that is currently no longer recommended), it could be suggested to use a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole); whereas, after the failure of a first quadruple therapy with bismuth, a triple therapy with PPI, levofloxacin and clarithromycin could be considered. However, since, as previously discussed in other recommendations, the addition of bismuth to various triple therapies seems to increase their efficacy,23 it seems more reasonable to suggest, although there is no direct evidence to support it, the use of a quadruple therapy adding this last drug (PPI, levofloxacin, clarithromycin and bismuth).
Studies evaluating empirically prescribed H. pylori eradication rescue therapies (without bacterial susceptibility study) in patients with penicillin allergy.
| Author | Country | Design | Rescue treatment | Therapeutic regimen | Duration (days) | No. of patients | Eradication |
|---|---|---|---|---|---|---|---|
| Ono193 | Japan | R | 2nd° and 3rd° | PPI + C + M | 7 | 3 | 33% |
| Ono193 | Japan | R | 2nd° and 3rd° | V + C + M | 7 | 1 | 100% |
| Rodríguez112 | Puerto Rico | R | 2nd° | PPI + T + M | 10 | 3 | 100% |
| Gisbert109 | Spain | P | 2nd° | RBC + T + M | 7 | 17 | 47% |
| Gisbert115 | Spain | P | 2nd° | PPI + B + T + M | 10 | 24 | 37% |
| Gisbert110 | Spain | P | 2nd° | PPI + C + L | 10 | 15 | 73% |
| Gisbert115 | Spain | P | 2nd° | PPI + C + L | 10 | 64 | 64% |
| Nyssen116 | Spain | P | 2nd | PPI + B + T + M | 10–14 | 69 | 78% |
| Nyssen116 | Spain | P | 2nd | PPI + C + L | 10–14 | 20 | 71% |
| Furuta194 | Japan | R | 2nd° | PPI + M + S | 7−14 | 10 | 100% |
| Mori195 | Japan | P | 2nd° | PPI + M + S | 10 | 19 | 84% |
| Gisbert115 | Spain | P | 3rd° | PPI + B + T + M | 10 | 3 | 100% |
| Gisbert115 | Spain | P | 3rd° | PPI + C + L | 10 | 3 | 33% |
| Gisbert109 | Spain | P | 3rd° | PPI + C + R | 10 | 9 | 11% |
| Gisbert115 | Spain | P | 3rd° | PPI + C + R | 10 | 7 | 14% |
| Nyssen116 | Spain | P | 3rd° | PPI + B + T + M | 10−14 | 78 | 75% |
| Furuta194 | Japan | R | 3rd° | PPI + M + S | 7−14 | 7 | 100% |
| Mori195 | Japan | P | 3rd° | PPI + M + S | 10 | 5 | 40% |
| Gisbert109 | Spain | P | 4th° | PPI + C + L | 10 | 2 | 100% |
| Gisbert115 | Spain | P | 4th° | PPI + C + L | 10 | 2 | 100% |
| Gisbert115 | Spain | P | 4th° | PPI + C + R | 10 | 2 | 50% |
“Intention-to-treat” eradication rates.
Design: P (prospective), R (retrospective).
B: bismuth; C: clarithromycin; PPI: proton pump inhibitor; L: levofloxacin; M: metronidazole; R: rifabutin; RBC: ranitidine bismuth citrate; S: sitafloxacin; T: tetracycline; V: vonoprazan.
Recommendation 10. After the failure of a first treatment with clarithromycin and a second treatment with levofloxacin, a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) is recommended.
100% agreement; votes: totally agree (92.8%); strongly agree (7.1%). GR: strong. QE: low.
Following failure of a triple or quadruple combination with clarithromycin, as previously reviewed, levofloxacin therapy is frequently recommended.150 On occasions, this second treatment also fails, and in these cases a quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) is usually prescribed.15 The choice of rescue treatment depends on the drugs that have been used in the previous eradication attempts. Since repeating the same antibiotic is not recommended (with the well-known exceptions of bismuth, amoxicillin and, to a lesser extent, metronidazole), bismuth quadruple therapy currently seems the most appropriate option, since it fundamentally avoids the re-administration of clarithromycin and levofloxacin.15
A few years ago, a Spanish multicentre study was conducted in which the efficacy of bismuth quadruple therapy as a third-line treatment was evaluated.196 A third eradication therapy was administered with PPI (at standard doses every 12 h), bismuth subcitrate (120 mg/6 h or 240 mg/12 h), tetracycline (between 250 mg/8 h and 500 mg/6 h) and metronidazole (between 250 mg/8 h and 500 mg/6 h) for 7–14 days in 200 patients. Eradication (by ITT) was deficient (65%), probably due, at least in part, to the insufficient dose and duration of the antibiotic treatment.196 Subsequently, other studies have been carried out, using the Pylera® single capsule formulation (and therefore with a homogeneous dose and duration of its components), and the meta-analysis of this as a third-line treatment has calculated an average eradication of 82%.84 Among these studies, one carried out in Spain stands out, which included 101 patients with previous failure of triple therapy with clarithromycin and triple therapy with levofloxacin, with Pylera® being effective in 80% of cases (by ITT).197 More recently, Hp-EuReg data focused on the third line of treatment have confirmed these encouraging results, describing eradication figures of 88% when evaluating 275 patients treated with Pylera®.82 These high eradication figures (of approximately 90%) have been confirmed in various updated analyses of the Hp-EuReg, carried out in 2020 and 2021, on the efficacy of Pylera® as third-line treatment.88,95
One possibility in the event of failure of two eradication therapies is to perform a culture and antibiogram to select the most appropriate third-line antibiotic combination based on bacterial susceptibility. Although this "targeted" treatment option is recommended in some consensus guidelines, its advantage over empirical treatment has not been confirmed.13 The sub-analysis of the studies that included second-line treatments in a recent meta-analysis,11 and a review of the literature comparing the efficacy of empirical treatment versus treatment based on antibiotic susceptibility,12 could not demonstrate statistically significant differences between both strategies. These results coincide with those described in a subsequent meta-analysis.198 No randomised clinical trials were identified that compared empirical versus third-line antibiogram-directed treatment, but the mean eradication rate of studies using the culture-based strategy was only 72%.11 Finally, in an updated meta-analysis carried out in 2020, when all the rescue therapies were included (13 studies), similar results were demonstrated with both strategies – empirical and based on microbial susceptibility –, both when including all studies as well as only the randomised clinical trials.14
It has been previously specified that the comments included in this consensus document are based on the assumption that the antibiotic susceptibility of the particular patient is unknown. In addition, most of the authors of this document believe that there are arguments for not having to systematically perform a culture before indicating a third eradication therapy, but that, on the contrary, it is perfectly feasible and appropriate to administer an empirical treatment after the failure of a second attempt. This recommendation is evidence-based and also takes into account that culturing H. pylori is a technique available in few centres, which requires an invasive test such as upper gastrointestinal endoscopy (since detection by PCR in faeces has not yet been sufficiently evaluated), with an average sensitivity of less than 90% and with considerable discrepancy between the results obtained in vitro and the eradication rate in vivo.13,199 Furthermore, culture provides useful information only about some antibiotics already used in previous first-line (clarithromycin and metronidazole) and second-line (levofloxacin) eradication therapies, which, by definition, should not be reused.
In summary, it is concluded that the empirical quadruple rescue treatment with bismuth is a valid alternative after failure of a treatment with clarithromycin and another with levofloxacin.
Recommendation 11. After the failure of a first treatment with clarithromycin and a second-line quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole), a quadruple therapy with levofloxacin (PPI, amoxicillin, levofloxacin and bismuth) is recommended.
100% agreement; votes: totally agree (100%). GR: strong. QE: low.
After the failure of triple or quadruple therapy with clarithromycin, quadruple therapy with PPI, bismuth, tetracycline and metronidazole is frequently recommended.5,15 When this second treatment also fails, with the intention of not re-administering clarithromycin or metronidazole, it has been suggested to prescribe a triple regimen with PPI, amoxicillin and levofloxacin.5,15
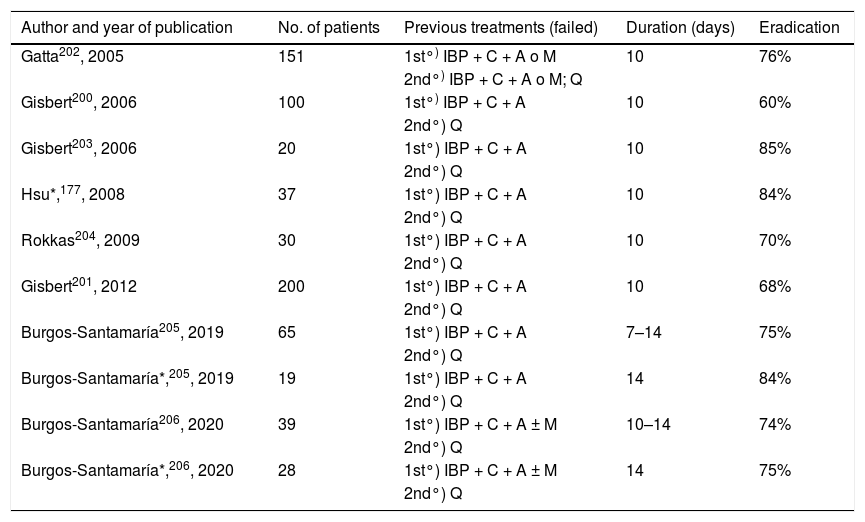
However, there has been little experience in the use of quinolones after the failure of two eradication therapies. A few years ago a Spanish multicentre study was published evaluating the efficacy of triple therapy with levofloxacin as third-line treatment, achieving eradication in approximately 70% of cases.200 These results have recently been confirmed in a larger national multicentre study, including a total of 200 patients.201 Patients were included in whom a first treatment with PPI, amoxicillin and clarithromycin had failed, as well as a second treatment with bismuth quadruple therapy (PPI, bismuth, tetracycline and metronidazole). A third eradication therapy with PPI, amoxicillin and levofloxacin was administered for 10 days. The eradication rate (by ITT) was 68%. Other authors have obtained similar or slightly better results with this third-line treatment with levofloxacin, with eradication rates that have ranged between 60% and 84% (with a mean of 75%), as summarised in Table 7.177,200–206 Among these studies, the best results (with eradication rates of 84%) were obtained when bismuth was added to triple treatment with levofloxacin, making it quadruple,177,205 similar to that previously described for second-line levofloxacin therapy.176
Studies evaluating the efficacy of a third-line combination with PPI, amoxicillin and levofloxacin (± bismuth) for the eradication of H. pylori after two eradication failures.
| Author and year of publication | No. of patients | Previous treatments (failed) | Duration (days) | Eradication |
|---|---|---|---|---|
| Gatta202, 2005 | 151 | 1st°) IBP + C + A o M | 10 | 76% |
| 2nd°) IBP + C + A o M; Q | ||||
| Gisbert200, 2006 | 100 | 1st°) IBP + C + A | 10 | 60% |
| 2nd°) Q | ||||
| Gisbert203, 2006 | 20 | 1st°) IBP + C + A | 10 | 85% |
| 2nd°) Q | ||||
| Hsu*,177, 2008 | 37 | 1st°) IBP + C + A | 10 | 84% |
| 2nd°) Q | ||||
| Rokkas204, 2009 | 30 | 1st°) IBP + C + A | 10 | 70% |
| 2nd°) Q | ||||
| Gisbert201, 2012 | 200 | 1st°) IBP + C + A | 10 | 68% |
| 2nd°) Q | ||||
| Burgos-Santamaría205, 2019 | 65 | 1st°) IBP + C + A | 7–14 | 75% |
| 2nd°) Q | ||||
| Burgos-Santamaría*,205, 2019 | 19 | 1st°) IBP + C + A | 14 | 84% |
| 2nd°) Q | ||||
| Burgos-Santamaría206, 2020 | 39 | 1st°) IBP + C + A ± M | 10–14 | 74% |
| 2nd°) Q | ||||
| Burgos-Santamaría*,206, 2020 | 28 | 1st°) IBP + C + A ± M | 14 | 75% |
| 2nd°) Q |
“Intention-to-treat” eradication rates.
A: amoxicillin; C: clarithromycin; PPI: proton pump inhibitor; M: metronidazole; Q: quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole).
Therefore, it is concluded that the empirical quadruple rescue treatment with PPI, amoxicillin, levofloxacin and bismuth constitutes a third-line alternative after the failure of two previous eradication therapies containing key antibiotics such as amoxicillin, clarithromycin, metronidazole and tetracycline.
Recommendation 12. After the failure of a first quadruple therapy with bismuth (PPI, bismuth, tetracycline and metronidazole) and a second-line treatment with levofloxacin, a concomitant quadruple therapy (PPI, amoxicillin, clarithromycin and metronidazole) is suggested.
100% agreement; votes: totally agree (92.8%); strongly agree (7.1%). GR: weak. QE: very low.
In this case (failure of a first quadruple therapy with bismuth and a second-line treatment with levofloxacin), since clarithromycin has not been used previously, it is suggested to use a concomitant quadruple therapy, which is precisely one of the first-line treatments of choice in our setting. It should be mentioned that this recommendation is not based on any direct evidence, but is established according to theoretical assumptions, and due to the absence of other effective therapeutic alternatives. Thus, although it has previously been argued that concomitant therapy would probably not be the best option after the failure of a quadruple therapy with bismuth (see recommendation 8), in this case we do not have a better option (especially if initially opting for bismuth quadruple therapy and not concomitant quadruple therapy was not due to a high rate of resistance to clarithromycin).
As an alternative, the combination of PPI, clarithromycin, amoxicillin and bismuth could be suggested, with which good results have been obtained in some studies,23 although experience in our setting is very limited.97 Another option is to use rifabutin, although this antibiotic is usually reserved, as detailed below, for a fourth line of treatment.207,208
Recommendation 13. After the failure of a third treatment, it is suggested to carefully reevaluate the need to eradicate the infection and, where appropriate, to indicate a fourth-line treatment with rifabutin.
Agreement 92.8%; votes: totally agree (92.8%); somewhat disagree (7.1%). GR: weak. QE: very low.
Occasionally, H. pylori infection persists despite having administered three eradication therapies.199 Since it is unknown whether the benefit obtained from the potential eradication of H. pylori outweighs the safety problems with more complex lines of treatment, in these patients the indication for eradication therapy and the possibility of stopping (if necessary) maintenance antisecretory treatment, should be individually reevaluated. Obviously, the decision to prescribe a fourth line of treatment will be clearer when the benefit of H. pylori eradication is greater, as is the case in patients with a peptic ulcer (especially if they have suffered previous complications) or a gastric MALT lymphoma.
A recent review of the literature has evaluated the role of rifabutin – an antibiotic that has high in vitro activity against H. pylori – in the treatment of this infection.208 The mean rate of resistance of H. pylorito rifabutin (calculated from 39 studies and almost 10,000 patients) was less than 1%. When only those studies that included patients without prior eradication therapy were considered, this figure was even lower (0.07%). Overall, the mean eradication rate (by ITT) with the combinations that included rifabutin (3000 patients analysed) was 73%. Specifically, the corresponding figures for fourth-line rifabutin therapies were approximately 70%.115,205,209–215 For the treatment of H. pylori infection, most studies have prescribed 300 mg/day of rifabutin, a dose that appears to be more effective than 150 mg/day. The optimal duration of treatment has not been established, but it is generally recommended to administer it for 10−12 days. The mean incidence of adverse effects was 15%; myelotoxicity was the most relevant, although exceptional. To date, all patients have recovered from leukopenia within a few days after completing treatment, and no infections or other complications associated with myelotoxicity have been reported.208
These results have been confirmed in a large Spanish multicentre study, in which the efficacy of a fourth empirical rescue treatment with rifabutin was evaluated in patients in whom three eradication attempts had previously failed (the first with PPI, clarithromycin and amoxicillin; the second with a quadruple therapy with PPI, bismuth, tetracycline and metronidazole; and the third with PPI, amoxicillin and levofloxacin).213 A fourth eradication therapy with PPI, amoxicillin (1 g/12 h) and rifabutin (150 mg/12 h) was administered for 10 days to 100 patients, and the eradication rate (by ITT) was 50%. Adverse effects were reported in 30% of patients; myelotoxicity (always mild) was found in 4% of patients and resolved spontaneously in all cases after completion of treatment. Lastly, the most recent data from the Hp-EuReg have confirmed somewhat higher eradication figures, of 65% in the fourth line of treatment.216
Finally, although the evidence in this respect is very limited,217–219 in the face of multiple eradication failures, combining bismuth with rifabutin could be considered,217–219 with the intention of increasing eradication efficacy. In this regard, Ciccaglione et al. reported that the addition of bismuth to a triple therapy that included a PPI, amoxicillin and rifabutin in patients receiving a third line of treatment, resulted in a therapeutic gain of 30% compared to rifabutin-based triple therapy.219 Finally, this rifabutin regimen with bismuth has recently been evaluated in the context of the Hp-EuReg, where various empirical third-line and subsequent rescue therapies were analysed, obtaining an efficacy (by ITT) with PPI, rifabutin, amoxicillin and bismuth of approximately 60%.206
Finally, as a fourth line of treatment, a combination of a PPI and amoxicillin could also be suggested, both at high doses (e.g. omeprazole 40 mg/8 h and amoxicillin 1 g/8 h for 14 days). However, although this dual combination has obtained good results in some countries (mostly Asian),220,221 its efficacy has not been confirmed in our setting.
In summary, after the failure of a third treatment, it is recommended to carefully evaluate the need for eradication and the possibility of stopping maintenance antisecretory therapy. If eradication is considered appropriate, it is recommended to individually assess the need for a fourth line of treatment, for example, with rifabutin (probably together with bismuth, in addition to amoxicillin), with strict control and monitoring of the patient.
Recommendation 14. In patients with uncomplicated duodenal ulcer who do not require non-steroidal anti-inflammatory drugs/aspirin, it is not recommended to continue antisecretory therapy after completing the H. pylori eradication therapy.
100% agreement; votes: totally agree (100%). GR: strong. QE: high.
Initially, most authors who used PPI in eradication therapies for uncomplicated duodenal ulcer disease (without gastrointestinal bleeding or perforation) extended these drugs for 2–4 weeks after the conclusion of antibiotic treatment, with the aim of "ensuring" ulcer healing.222 However, it has been proven that to obtain a high rate of ulcer healing in these patients, the use of a PPI limited to the period of administration of antibiotics is sufficient in the context of eradication therapy. Thus, a systematic review of the medical literature identified 24 studies, including a total of 2378 patients, in which ulcer healing with a PPI plus two antibiotics for 7 days was evaluated.223 The mean healing rate (by ITT) was 86% when considering all patients (both with successful and failed eradication of H. pylori). This figure rose to 95% when only those patients in whom the infection had been eradicated were included. The meta-analysis of the six randomised studies224–229 that compared efficacy in terms of ulcer healing when administering a PPI plus two antibiotics for 7 days versus the same treatment combined with a PPI for a further 2–4 weeks,223 confirmed the healing of the ulcer in 91% and 92% of cases, respectively. The odds ratio (OR) for this comparison was 1.11 (95% confidence interval [CI] 0.71–1.74), with the results being homogeneous. Finally, when a sub-analysis was performed including only patients with duodenal ulcer, the results were similar (OR 1.14, with a 95% CI of 0.71–1.84). After the publication of this meta-analysis, two additional randomised studies have been carried out, with similar results.230,231
In summary, it is concluded that to obtain a high healing rate of uncomplicated duodenal ulcer in patients who do not take non-steroidal anti-inflammatory drugs (NSAIDs) or aspirin, the use of PPI during the period of administration of antibiotics is sufficient. However, in the case of complicated duodenal ulcers (gastrointestinal bleeding, perforation), it is prudent to administer antisecretors until eradication of H. pylori is confirmed.
Recommendation 15. In patients with gastric ulcers who do not require NSAIDs/aspirin, it is recommended to maintain the antisecretory therapy for 4−8 weeks after the end of the H. pylori eradication therapy.
100% agreement; votes: totally agree (92.8%); strongly agree (7.1%). GR: strong. QE: moderate.
There are two reviews of the medical literature published more than a decade ago that provide arguments in favour of not prolonging antisecretory therapy in patients with gastric ulcer, in keeping with the attitude adopted with duodenal ulcer. The first concludes that “eradication of H. pylori induces greater healing of ulcerative lesions regardless of whether they are duodenal or gastric”.232 The other systematic review concludes that “eradication of H. pylori heals both duodenal and gastric ulcers, with a similar percentage of healing”.233 From these data, it would appear that the healing rate with H. pylori eradication therapy would be similar for gastric and duodenal ulcers.
However, more recent studies suggest the need to prolong antisecretory therapy, especially in gastric ulcers larger than 1 cm. A first study documented the healing of 100% of gastric ulcers smaller than 1 cm at 8 weeks after the administration of an eradication therapy for 2 weeks.234 This healing rate was considerably reduced when the ulcers were greater than 1 cm, and it is important to note that all the unhealed lesions at 8 weeks did so after additional treatment with PPI. One of the studies, with a randomised design, which provides very relevant information on this topic, evaluated gastric ulcer healing after eradication therapy for one week or a PPI for 8 weeks.235 It was possible to verify how the healing rate at 8 weeks of eradication therapy decreased exponentially according to the size of the ulcer (89% for ulcers <1 cm, 54% for ulcers between 1 and 1.4 cm, and 5% for those equal to or greater than 1.5 cm); while it was significantly higher in the group treated with a PPI for 8 weeks (100% for ulcers < 1 cm, 77% for ulcers between 1 and 1.4 cm, and 77% for those equal to or greater than 1.5 cm).235
More recently, another randomised study was published comparing the administration of eradication therapy on its own versus this being followed by PPI for 3 weeks.236 Gastric ulcer healing at 4 weeks was lower in patients treated with triple therapy alone, without a subsequent PPI (64% vs. 82%). Likewise, in patients with unhealed gastric ulcers, an additional 4-week course of a PPI (esomeprazole) increased the percentage of healing to 89–96 %. It should be noted that this study suffers from certain methodological deficiencies, such as the exclusion of patients with ulcers of a size >2 cm and an excessively early endoscopic control of healing (at 4 weeks) for a gastric ulcer.
Finally, a recent Japanese study randomised 115 gastric ulcer patients stratified according to the size of the lesion (<0.5 cm, 0.5–1.5 cm and >1.5 cm) to receive eradication therapy (for one week) plus 7 weeks of a PPI versus eradication therapy plus 7 weeks of a cytoprotective agent.231 Ulcer healing rates at 8 weeks were significantly higher in the eradication plus PPI group. In agreement with previous studies, the results of ulcer healing with eradication therapy plus PPI were superior for gastric ulcers >1.5 cm (85% vs. 43%).
In summary, the available evidence points to a higher rate of ulcer healing with eradication therapy followed by PPI for gastric ulcers associated with H. pylori infection. In all the previously reported studies,231,235,236 an absence of healing of up to 20% was found with the isolated eradication therapy, this failure being higher for larger ulcers. Therefore, it is recommended that, after finishing the eradication therapy, antisecretory therapy be extended between 4 and 8 weeks in gastric ulcers, especially in those larger than 1 cm.
Recommendation 16. In patients with gastrointestinal bleeding from peptic ulcer, the eradication of H. pylori eliminates practically all relapses. Therefore, once eradication is confirmed and if the patient is not taking non-steroidal anti-inflammatory drugs/aspirin, it is recommended not to administer maintenance treatment with antisecretory drugs.
100% agreement; votes: totally agree (92.8%); strongly agree (7.1%). GR: strong. QE: high.
Peptic ulcer is the first cause of upper gastrointestinal bleeding and H. pylori infection is the main aetiological factor in gastroduodenal ulcer disease.237 Long-term maintenance antisecretory therapy was traditionally the standard treatment to prevent recurrent bleeding in patients with a previous episode of gastrointestinal bleeding from peptic ulcer. Although it is widely known that the eradication of H. pylori is associated with a drastic reduction in ulcer recurrence, until relatively recently the efficacy of eradication therapy in preventing recurrent bleeding from peptic ulcer was unknown.
A meta-analysis has been published, following the Cochrane Collaboration methodology, in which the efficacy of H. pylori eradication therapy was compared with antisecretory therapy for the prevention of recurrent bleeding from peptic ulcer.238,239 In a first sub-analysis, seven studies were included, with a total of 578 patients: the average percentage of recurrent bleeding in the eradication therapy group was 2.9% and in the group without eradication therapy or maintenance antisecretory drugs it was 20% (OR 0.17; 95% CI 0.10–0.32). In a second sub-analysis, three studies were included, with a total of 470 patients: the average percentage of recurrent bleeding in the group that received eradication therapy was 1.6% and in the group in which eradication therapy was not prescribed, but maintenance antisecretory drugs were prescribed, it was 5.6% (OR 0.24; 95% CI 0.09–0.67).
Several studies have evaluated the incidence of recurrent bleeding in patients with confirmed H. pylori eradication and who were followed up without administering maintenance antisecretory therapy.240–259 Given that the duration of follow-up varies considerably between the different studies, the follow-up periods of each one have been taken into account and we have calculated the respective annual incidence of recurrent bleeding (per patient and year of follow-up). As such, the global follow-up was 1913 patient years, and 13 episodes of recurrent bleeding were detected among the patients in whom the bacterium was eradicated. Thus, annual recurrence is only 0.88% per patient and year of follow-up.6
These favourable results have been confirmed in a Spanish multicentre study, which prospectively included 1000 patients with gastrointestinal bleeding due to gastroduodenal ulcer in whom H. pylori infection was eradicated and, subsequently, antisecretory therapy was not prescribed.260 Three episodes of recurrent bleeding were detected at one year of follow-up (in two cases after ingestion of NSAIDs and in the other after reinfection by H. pylori), and another two episodes at two years (one after ingestion of NSAIDs and the other after reinfection by H. pylori). The cumulative incidence of rebleeding was 0.5% and the incidence rate was 0.15% per patient year.
The above results indicate that the treatment of H. pylori infection is more effective than antisecretory treatment (either with or without maintenance antisecretory drugs) in preventing recurrent bleeding from peptic ulcer. Consequently, the presence of H. pylori infection should be evaluated in all patients with gastrointestinal bleeding from peptic ulcer (early,261 ideally during the hospital admission itself262) and eradication therapy prescribed to those who are infected. Once eradication has been confirmed (which should always be done, and without delay263), it is not necessary to administer maintenance treatment with antisecretory drugs (if the patient does not require NSAIDs), since the eradication of H. pylori eliminates practically all recurrent bleeding. However, it seems prudent in the case of a peptic ulcer with complications (e.g. gastrointestinal bleeding) to administer antisecretory therapy until eradication of H. pylori is confirmed.
Conflicts of interestJ. Alcedo, J. Amador, L. Bujanda, X. Calvet, M. Castro-Fernández, E. Gené, A. Lanas, A.J. Lucendo, J. Molina-Infante, A. Pérez-Aisa and I. Puig declare that they have no conflict of interest.
L. Fernández-Salazar has carried out teaching activities for Janssen, Norgine, Salvat and Tillots.
J.P. Gisbert has provided scientific advice and support for research and/or training activities for Mayoly, Allergan, Diasorin, Gebro Pharma and Richen.
O.P. Nyssen has provided research support for Mayoly and Allergan.
Please cite this article as: Gisbert JP, Alcedo J, Amador J, Bujanda L, Calvet X, Castro-Fernández M, et al. V Conferencia Española de Consenso sobre el tratamiento de la infección por Helicobacter pylori. Gastroenterol Hepatol. 2022;45:392–417.
This article is published simultaneously in [Gastroenterología y Hepatología – [https://doi.org/10.1016/j.gastre.2021.07.001] and [Revista Española de Enfermedades Digestivas – [https://doi.org/10.17235/reed.2021.8358/2021], with the consent of the authors and editors.