The growing incidence of cystic pancreatic tumours has become a major problem in daily clinical practice. These patients usually undergo follow-up programmes of questionable clinical efficacy that put significant strain on endoscopy units. Safe and effective alternatives to surgery are desperately needed in these cases. The aim of this study was to critically review the utility of ablative therapies in cystic pancreatic tumours.
La incidencia creciente de los tumores quísticos de páncreas representa un problema en la práctica clínica diaria. Estos pacientes se ven sometidos a programas de seguimiento de dudosa eficacia clínica, que sobrecargan la actividad asistencial en las unidades de endoscopia. Sería de gran utilidad disponer de tratamientos alternativos a la cirugía para este tipo de pacientes, y que estos fueran seguros y eficaces. En esta revisión se intenta realizar una puesta al día crítica sobre la utilidad de los tratamientos ablativos de los tumores quísticos del páncreas.
The current prevalence of pancreatic cysts in the general population is approximately 2%, with this figure increasing significantly in the older population.1 In recent years, an increase has been reported in the incidence of this type of lesion thanks to greater use of abdominal imaging tests such as computed tomography (CT) and magnetic resonance imaging.1–3
Pancreatic cysts can be divided into mucinous and non-mucinous lesions, with differences in their potential for malignancy.
Among the mucinous cystic tumours of the pancreas, the most common are intraductal papillary mucinous neoplasm (IPMN) and mucinous cystic neoplasm.4,5 IPMN is classified according to the pancreatic duct affected: (1) main duct, or (2) secondary branch.4,5 All mucinous lesions have malignant potential, and their histology and biological behaviour can vary from benign lesions without epithelial dysplasia to malignant lesions with infiltrative character or capacity.
There is no simple treatment algorithm for these lesions. As mentioned above, the incidence of cystic tumours increases with age, with the highest incidence in patients over 75. In view of the high morbidity and mortality rates associated with pancreatic surgery, especially in older patients, and the small likelihood of some of these lesions becoming malignant, there is a need for individualised management. The approach depends on the characteristics of the lesion and the patient. Both factors are important when deciding on whether to opt for aggressive surgical treatment or a more conservative approach of monitoring with periodic imaging tests.
Various consensus guidelines have recently been published setting out management and decision-making recommendations for patients with this type of lesion.4,5 The proposed algorithm is as follows:
- •
Conservative management, involving monitoring with periodic MRI every two years in small lesions (<3cm) with non-thickened walls, without mural nodules or dilated main pancreatic duct (duct of Wirsung <5mm).
- •
Endoscopic ultrasound assessment, in lesions with some sign of risk (size >3cm, wall thickening, mural nodules or dilated main pancreatic duct at 5–9mm). If a solid mural component and dilation of the pancreatic duct is confirmed, endoscopic ultrasound-guided (EUS-guided) fine-needle aspiration biopsy and then considering surgical resection are usually recommended, depending on the clinical context of the patient. However, in selected cases or patients who are high risk for surgery, we can consider annual follow-up with magnetic resonance imaging and ablative treatments (injection of alcohol or ablative drugs, thermo- or cryoablation).
- •
Surgical resection is indicated in lesions classified as having a high risk of malignancy (presence of obstructive jaundice, hyperenhancement of mural nodule in imaging tests, marked dilation of the main duct (>10mm), high-grade dysplasia or cytohistological study showing carcinoma.4,5
In spite of these recommendations, it is often not possible to obtain sufficient evidence from imaging tests and cytopathological analysis to determine which lesions should be treated surgically and which should be periodically monitored. No strategy is perfect, as pancreatic surgery has high morbidity and mortality rates, particularly in older patients, and there is a significant financial cost involved in long-term radiological/ultrasound follow-up. In this context, less invasive treatments have been developed with the aim of treating cystic lesions in patients with high surgical risk, in order to reduce their potential for becoming malignant and not subject the patient to aggressive surgery or costly follow-up programmes.
Most of these treatments are endoscopic ultrasound-guided techniques. Although evidence on their effectiveness is still limited, the best developed are alcohol ablation (ethanol injection), the administration of chemotherapy agents (paclitaxel) and the application of radiofrequency.
Endoscopic ultrasound-guided treatments of cystic tumours of the pancreasAblation by ethanol lavage and injection of chemotherapy agentsThe injection of alcohol (ethanol lavage) into a cystic lesion causes lysis of the epithelial membranes of the cyst, followed by protein denaturation and vascular occlusion. Ablation of cystic lesions by injection of alcohol has for a long time been performed percutaneously in renal cysts with excellent success rates.6,7 However, the retroperitoneal, periduodenal, and partly retrogastric location of the pancreas, as well as the size of the pancreatic cysts, complicates a percutaneous approach, both in terms of performing a puncture and carrying out treatment. This is where endoscopic ultrasound comes into play, as due to the position of the pancreas in relation to the duodenum and stomach, the lesion is close to the endoscopic ultrasound transducer, which makes it possible to carry out treatment under real-time guidance.
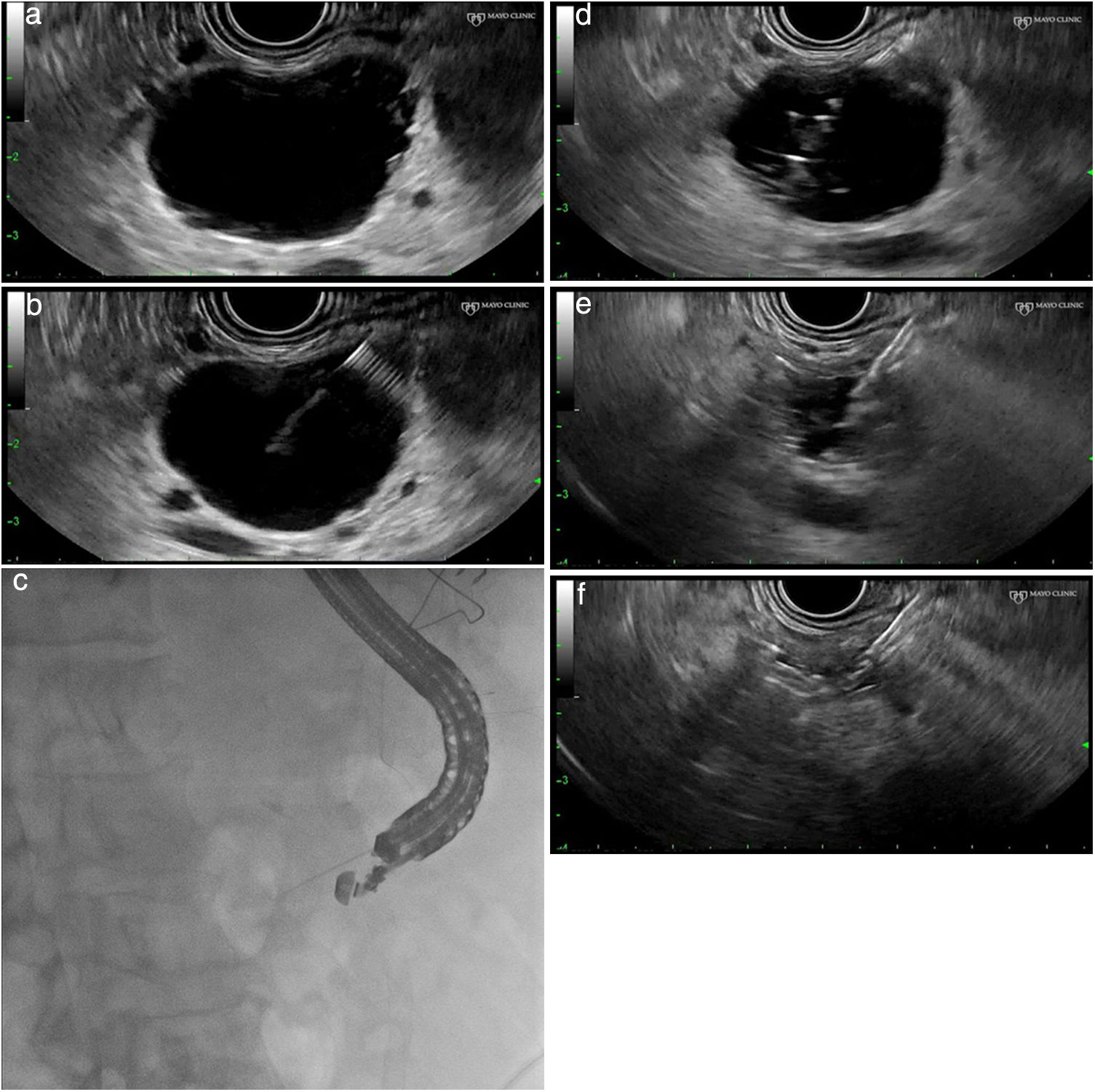
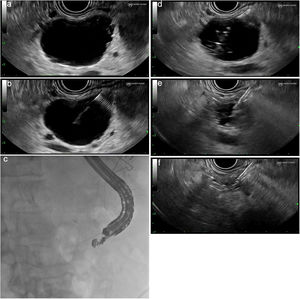
Technical procedure (Fig. 1a–f)This type of treatment is not standardised and varies from one centre to another. It is advisable to perform these procedures in centres with experience in advanced endoscopy and endoscopic ultrasound and, if possible, as part of research protocols. After administration of antibiotic prophylaxis (generally an IV quinolone), a linear ultrasound endoscope is inserted and the pancreatic anatomy is explored from the duodenum and stomach to determine the most suitable access point for the puncture of the lesion.
(a) Identification and characterisation of the lesion. (b) An EUS-guided needle for puncture is inserted. (c) Fluoroscopic control of the procedure. (d) Aspiration and injection 80–90% alcohol until full. (e) Complete aspiration of the ethanol volume injected. (f) Complete collapse of the cystic lesion.
Using an endoscopic ultrasound guide, a fine 19–22G needle (fine needle aspiration) is introduced into the cyst cavity, and the content of the lesion is aspirated and sent for biochemical and cytological study. Some authors recommend that, after emptying, contrast is injected into the interior of the cyst through the needle in order to use radioscopy to rule out possible communication with the main pancreatic duct and/or the presence of a fistula with the pancreatic parenchyma. This manoeuvre could reduce the risk of post-ethanol lavage pancreatitis.
Once the cyst cavity is empty (complete aspiration of the contents of the cyst), as many millilitres of ethanol (usually at 80%) are injected as fluid was aspirated from the cyst and, after 5min of lavage, the contents of the cyst are again aspirated, sending the aspirated material for cytological and biochemical analysis.8–14 To date no variant of the technique has been described for septated cysts; the presence of septa may reduce the effectiveness of the technique, by preventing adequate diffusion of the alcohol inside the cyst. Most authors recommend hospital observation (12–24h) after the treatment.
Imaging tests, usually CT, are used to check the response to this ablative technique, with the response criterion being a change in the size of the lesion.
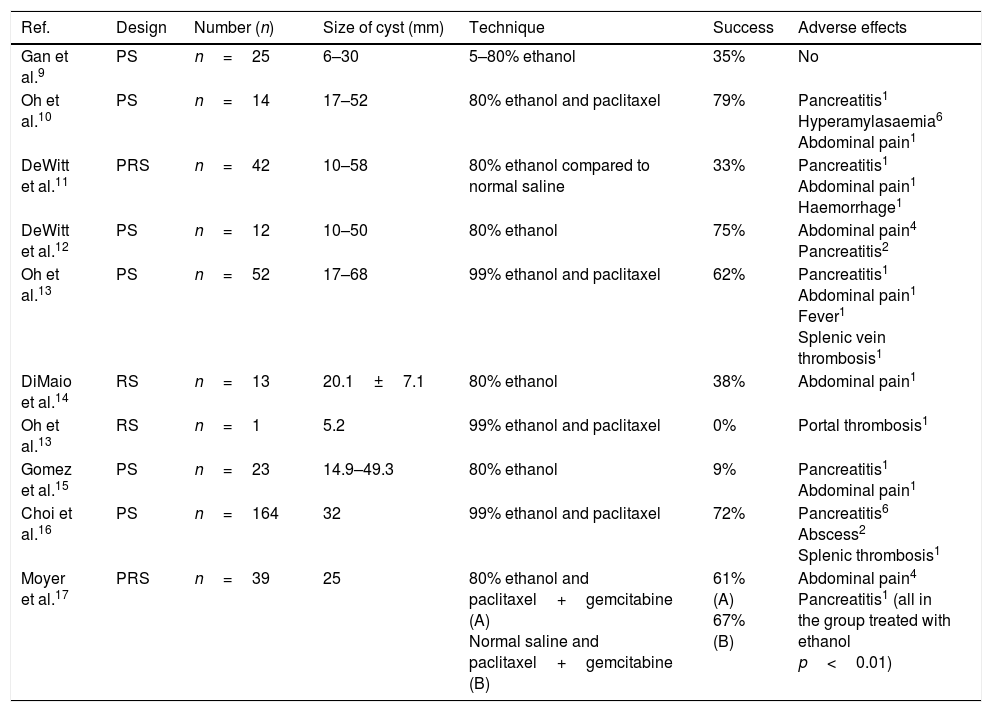
ResultsThis type of treatment is still in the research phase, and the results of the different studies should still be considered as preliminary. Acceptable safety has been demonstrated in both animal and human models.9 However, the clinical utility of ethanol lavage in the treatment of pancreatic cystic lesions remains open to debate. The rate for complete resolution of the lesions (based on the CT images before and after the ethanol lavage±local injection of a chemotherapy agent) varies from 0% to 79% in the different studies (Table 1).9–17
Summary of published studies on the ablation of pancreatic cystic tumours by endoscopic ultrasound-guided ethanol lavage.
| Ref. | Design | Number (n) | Size of cyst (mm) | Technique | Success | Adverse effects |
|---|---|---|---|---|---|---|
| Gan et al.9 | PS | n=25 | 6–30 | 5–80% ethanol | 35% | No |
| Oh et al.10 | PS | n=14 | 17–52 | 80% ethanol and paclitaxel | 79% | Pancreatitis1 Hyperamylasaemia6 Abdominal pain1 |
| DeWitt et al.11 | PRS | n=42 | 10–58 | 80% ethanol compared to normal saline | 33% | Pancreatitis1 Abdominal pain1 Haemorrhage1 |
| DeWitt et al.12 | PS | n=12 | 10–50 | 80% ethanol | 75% | Abdominal pain4 Pancreatitis2 |
| Oh et al.13 | PS | n=52 | 17–68 | 99% ethanol and paclitaxel | 62% | Pancreatitis1 Abdominal pain1 Fever1 Splenic vein thrombosis1 |
| DiMaio et al.14 | RS | n=13 | 20.1±7.1 | 80% ethanol | 38% | Abdominal pain1 |
| Oh et al.13 | RS | n=1 | 5.2 | 99% ethanol and paclitaxel | 0% | Portal thrombosis1 |
| Gomez et al.15 | PS | n=23 | 14.9–49.3 | 80% ethanol | 9% | Pancreatitis1 Abdominal pain1 |
| Choi et al.16 | PS | n=164 | 32 | 99% ethanol and paclitaxel | 72% | Pancreatitis6 Abscess2 Splenic thrombosis1 |
| Moyer et al.17 | PRS | n=39 | 25 | 80% ethanol and paclitaxel+gemcitabine (A) Normal saline and paclitaxel+gemcitabine (B) | 61% (A) 67% (B) | Abdominal pain4 Pancreatitis1 (all in the group treated with ethanol p<0.01) |
PRS: prospective randomised study; PS: prospective study; RS: retrospective study.
Initially, Gan et al.9 conducted a pilot study including 25 patients, where a complete response rate of 35% was observed, without reporting significant complications associated with the technique. Based on these promising results, DeWitt et al.11 conducted a double-blind controlled study, where the patients were randomised to receive ethanol versus saline, and demonstrated a response rate of 33% with ethanol (based on the CT images). In that study, there were no differences between the two groups in terms of complications.
In view of the poor efficacy of the ethanol lavage of cystic lesions in some of the published studies, some authors tried to improve the results through co-administration with intracystic local chemotherapy. In 2008, Oh et al. combined the administration of 80–90% ethanol with 3mg/ml of paclitaxel (chemotherapy used in other types of tumours such as breast, ovarian and non-small cell lung cancer), and complete resolution of the cystic lesion was observed in 79% of patients.10 Although promising, these results need to be corroborated in controlled, randomised studies with a larger sample size before we can accept the possible improvement in efficacy by combining paclitaxel with ablative treatment.
One recently published prospective study16 analysed the long-term effect of pancreatic cyst ablation using ethanol plus paclitaxel. They included 164 patients and assessed the outcomes in mucinous cysts, serous cysts and cysts of undetermined aetiology. The rate of adverse effects was 9.8%, with only one serious side effect. Complete resolution of the cyst was achieved in 72.2%, while in 19.6% the resolution was partial. Only in 8.2% did the cyst remain unchanged after ablation. There were only two recurrences (1.7%) among the lesions that had completely resolved. The multivariate analysis showed that the absence of intracystic septa (OR 7.12, 95% CI 2.72–18.67) and cyst size less than 35mm (OR 2.39, 95% CI 1.11–5.16) were independent predictors of complete resolution of the cyst after treatment. The authors reported that 98.3% of cystic lesions completely ablated with alcohol plus paclitaxel continued to be in remission after a long period of six years and they considered this treatment to be an effective and lasting alternative to surgery.
Another recently published, prospective, double-blind study17 compares the infusion of alcohol plus chemotherapy agents (paclitaxel plus gemcitabine) to saline plus the same chemotherapy agents for the ablation of mucinous cystic lesions of the pancreas. Although the number of patients assessed was small (10 patients), partial and complete resolution rates were similar between the two groups, suggesting that alcohol infusion may not be necessary when applying chemotherapy ablation. Moreover, the only serious side effect (acute pancreatitis) occurred in the alcohol ablative treatment arm.
Other ablative substances have also been assessed. One prospective study included 29 patients and, using lauromacrogol, a sclerosing agent with mild anaesthetic effects, achieved complete resolution rates of 37.9% at nine months; the rates were slightly lower in uncinate process and head of pancreas lesions than in body and tail lesions.18 The only significant adverse effects occurred in patients with head of pancreas/uncinate process lesions (two mild pancreatitis and one moderate fever).
We therefore need to consider the fact that the variable response rate found in the published studies may be the result of differences in the size and characteristics of the lesions included, the concentration of alcohol or ablative substances used, the combined use of other drugs, the variable follow-up time and the absence of standardised and reliable response criteria.
One problematic issue is the method of assessing treatment response; all the studies base this on the size of the lesion post-treatment using imaging tests. However, the risk of malignancy depends on the integrity of the epithelium and not on the size of the lesion. The studies where surgery was performed after the ablation and which therefore had material from pathology samples available show a complete epithelial ablation rate ranging from 50% to 100%, but they all had small sample sizes.9–12 The same problem occurs when paclitaxel is administered locally with alcohol (50% of patients will have epithelium of the cyst in the surgical sample).10
In summary, in our opinion, the endoscopic ultrasound-guided ethanol lavage of pancreatic cysts cannot currently be considered an effective treatment for the complete ablation of pancreatic cystic tumours. Nevertheless, it could have some utility as a palliative treatment option in patients with high surgical risk, who have cystic tumours <3cm, which are not communicated with the main pancreatic duct and show no signs of risk in imaging tests (septa, mural nodules). However, this possibility would need to be corroborated by prospective and controlled studies.
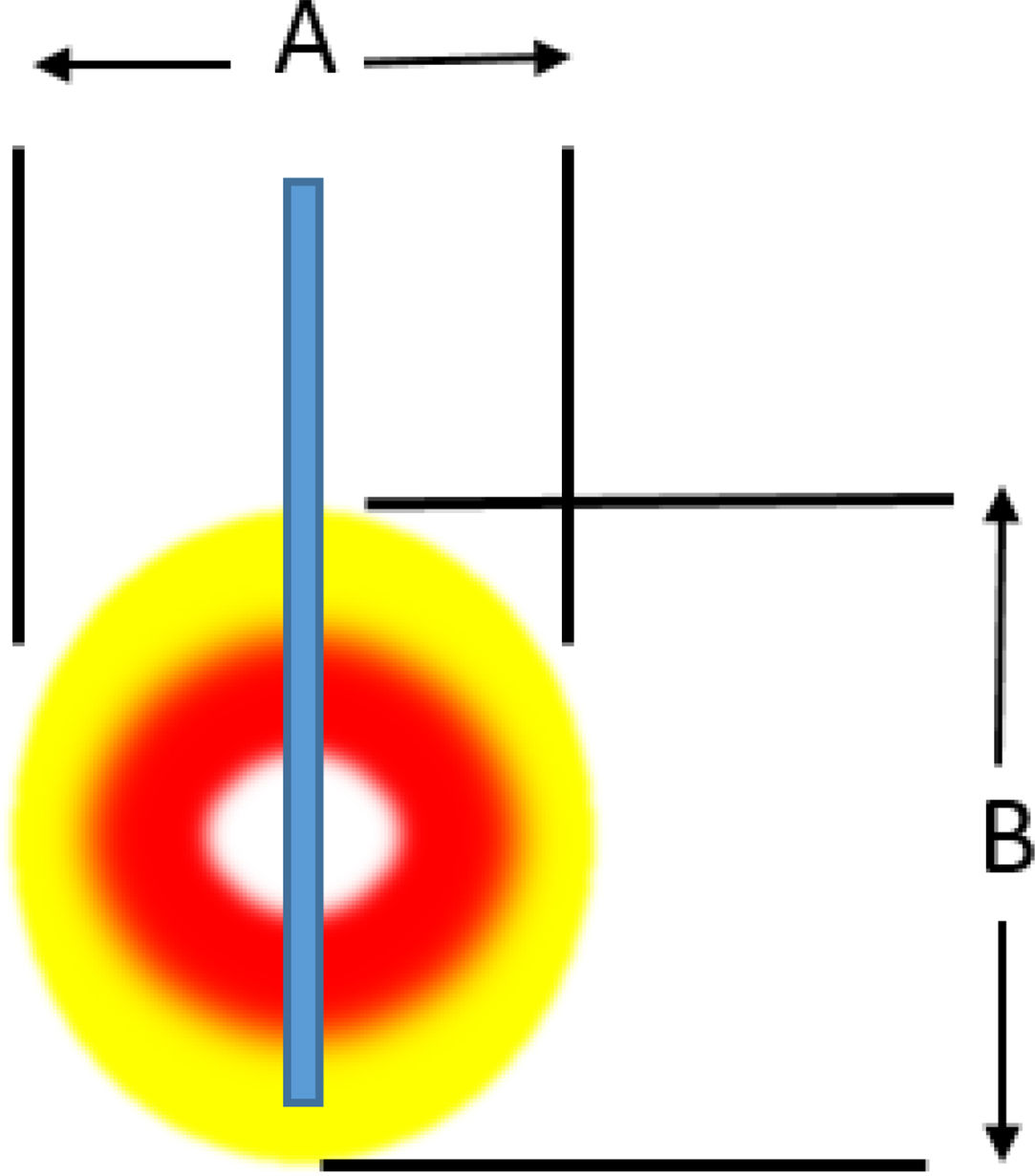
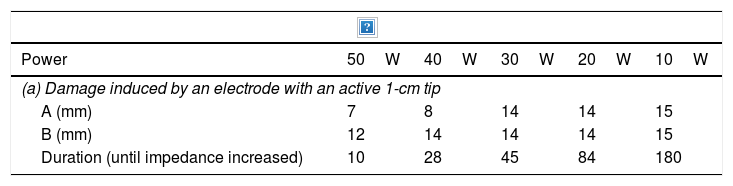
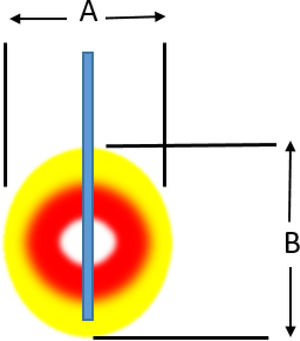
Radiofrequency ablationRadiofrequency (RF) ablation causes tissue destruction through the application of a high frequency alternating current that generates a local increase in the temperature, which induces coagulative necrosis. The physical principle of RF is based on the induction of heat through the production of an electrical field between the patient and two electrodes: the active RF electrode and the reference electrode. The electric field induces oscillatory movement of ions and the production of energy as a consequence of the friction associated with the ionic current. RF bands between 300 and 3000kHz, which correspond to average RF waves are used in medical applications. The temperature ranges reached depend on a number of factors, the most important being the power of the RF (in watts) and the tissue exposure time. The biological characteristics of the tumour (solid or cystic), its location and orientation and the organ it is embedded in are also factors affecting the volume of ablation achieved. Last of all, the “heat sink” effect, heat dissipation induced by vascular flow and cooling as a result of adjacent tissues absorbing a part of the heat induced, also has to be taken into account. Table 2a and b shows the type of damage that occurs according to the heat applied.
Induced damage.
| Power | 50W | 40W | 30W | 20W | 10W |
|---|---|---|---|---|---|
| (a) Damage induced by an electrode with an active 1-cm tip | |||||
| A (mm) | 7 | 8 | 14 | 14 | 15 |
| B (mm) | 12 | 14 | 14 | 14 | 15 |
| Duration (until impedance increased) | 10 | 28 | 45 | 84 | 180 |
| (b) Damage induced according to the temperature reached in the tissue | |
| 36–40°: | Cell homeostasis preserved. |
| 42–45° | Hyperthermia-cells susceptible to damage; e.g. RT/CT. |
| 46° | Irreversible cell damage with exposure >60min. |
| 50–52° | Cell damage with less exposure, at 4–6min. |
| 60–100° | Instantaneous induction of protein coagulation: |
| Irreversible damage to mitochondrial/cytosolic enzymes, nucleic acids. | |
| Coagulative necrosis: time to onset variable. | |
| >105° | Boiling, vaporisation, carbonisation (not ions). |
This technique has been widely used in different types of solid malignancies and is currently a part of the standard therapy for hepatocellular carcinoma.19
The first applications of EUS-guided RF were in porcine pancreas in 1999.20 It had previously been used in pancreatic cancer, almost always intraoperatively, in combination with palliative bypass surgery.21
Although it had been shown to be a feasible and safe technique in animal studies, the first clinical applications in pancreatic tumours were associated with very high serious complication and mortality rates from inadvertent damage to adjacent structures (non-cancerous pancreatic tissue, duodenum, biliary tree and peripancreatic vasculature). In these initial studies, very high temperatures (above 90°C) were applied, with repeated applications in a single session to treat large tumours, especially in the head of pancreas.22–25 However, in general terms, based on the findings of both animal and human studies, the technique is considered safe.26,27
Ex vivo studies of the thermokinetic characteristics of RF have determined that optimal specifications for application in the pancreas to prevent damage to neighbouring organs would be temperatures of around 90–105°C applied for less than 5min. Active cooling systems with cold normal saline in the vessels and duodenum around the area of application also reduce the number of complications. As the complications which have generally been associated with death in intraoperative applications have been uncontrollable bleeding resulting from thermal damage to large peripancreatic vessels, some authors have recommended limiting the use of RF solely to tumours of the body and tail of the pancreas.28–31
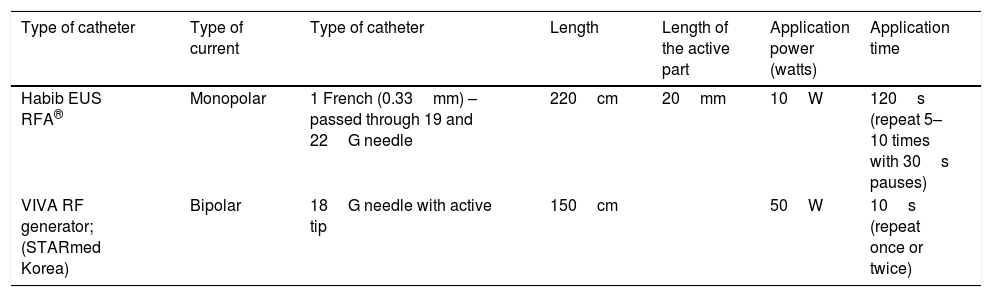
Technical procedureThe procedure basically consists of locating the lesion to be treated by endoscopic ultrasound. The lesion is punctured with a fine needle (19 or 22G), or with needles specifically designed for RF application, through which a radiofrequency catheter is inserted. Depending on the type of catheter, monopolar current (Habib EUS RF catheter) or bipolar current (VIVA RF system) is applied (Table 3).32 In cystic lesions, the contents of the cyst should be aspirated first before applying the radiofrequency, in order to make it as effective as possible. Once the catheter is inside the lesion, radiofrequency energy is applied. The time duration and intensity in watts will depend on the type of catheter used. Table 3 shows the characteristics of the two types of RF application catheters currently available.
Types of catheters for application of endoscopic ultrasound-guided radiofrequency.
| Type of catheter | Type of current | Type of catheter | Length | Length of the active part | Application power (watts) | Application time |
|---|---|---|---|---|---|---|
| Habib EUS RFA® | Monopolar | 1 French (0.33mm) – passed through 19 and 22G needle | 220cm | 20mm | 10W | 120s (repeat 5–10 times with 30s pauses) |
| VIVA RF generator; (STARmed Korea) | Bipolar | 18G needle with active tip | 150cm | 50W | 10s (repeat once or twice) |
RF: radiofrequency; RFA: radiofrequency ablation; W: watts.
The intensity of the current and the most appropriate number of pulses/sessions have yet to be defined, but, as shown in Table 4, a number of authors have published different intervention protocols. The intervention is usually performed guided by radioscopy and endoscopic ultrasound to ensure correct placement of the radiofrequency electrode. For large lesions, the needle should be withdrawn and relocated inside the cyst, repeating the process as many times as necessary until the lesion is ablated.33
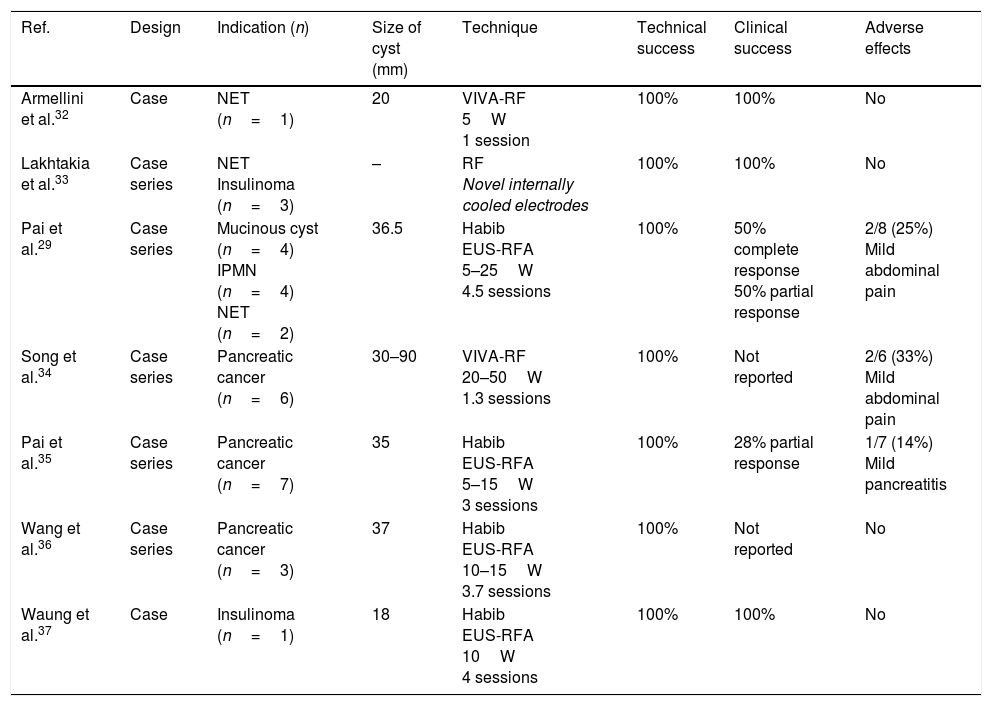
Summary of published studies on the ablation of pancreatic lesions by endoscopic ultrasound-guided radiofrequency ablation catheters.
| Ref. | Design | Indication (n) | Size of cyst (mm) | Technique | Technical success | Clinical success | Adverse effects |
|---|---|---|---|---|---|---|---|
| Armellini et al.32 | Case | NET (n=1) | 20 | VIVA-RF 5W 1 session | 100% | 100% | No |
| Lakhtakia et al.33 | Case series | NET Insulinoma (n=3) | – | RF Novel internally cooled electrodes | 100% | 100% | No |
| Pai et al.29 | Case series | Mucinous cyst (n=4) IPMN (n=4) NET (n=2) | 36.5 | Habib EUS-RFA 5–25W 4.5 sessions | 100% | 50% complete response 50% partial response | 2/8 (25%) Mild abdominal pain |
| Song et al.34 | Case series | Pancreatic cancer (n=6) | 30–90 | VIVA-RF 20–50W 1.3 sessions | 100% | Not reported | 2/6 (33%) Mild abdominal pain |
| Pai et al.35 | Case series | Pancreatic cancer (n=7) | 35 | Habib EUS-RFA 5–15W 3 sessions | 100% | 28% partial response | 1/7 (14%) Mild pancreatitis |
| Wang et al.36 | Case series | Pancreatic cancer (n=3) | 37 | Habib EUS-RFA 10–15W 3.7 sessions | 100% | Not reported | No |
| Waung et al.37 | Case | Insulinoma (n=1) | 18 | Habib EUS-RFA 10W 4 sessions | 100% | 100% | No |
Clinical success: defined as disappearance of the lesion/necrotic appearance of the lesion in imaging tests or persistence of the lesion but with reduction in its size (partial response).
IPMN: intraductal papillary mucinous neoplasm; NET: neuroendocrine tumour; RF: radiofrequency; RFA: radiofrequency ablation; W: watts.
Very few studies have been conducted to date using RF for the ablation of cystic or solid lesions of the pancreas, and they have only included a small number of patients. These studies have demonstrated technical success, i.e. 100% ability to apply RF to the target lesion.29,33–37
With respect to clinical success, however, the results vary, and are not yet clearly established (Table 4). In a study consisting of a case series of cystic and neuroendocrine tumours, the findings of Pai et al.29 were from complete response (100%) to a 50% reduction in the size of the lesion. Although they were not cystic tumours, a number of studies with small numbers of patients have also been published which have opened up the possibility of conducting pilot studies to evaluate this technique in the treatment of adenocarcinoma-type solid tumours of the pancreas. In cases where EUS-guided RF has been applied in solid tumours, the rate of side effects has been low, and there has been some reduction in tumour size and in Ca19-9 levels, indirectly supporting the possible efficacy of this type of treatment.33–37
In terms of side effects, both animal studies and published case series in humans suggest that the procedure is safe, with no major clinical complications. In the five case series published on experience of pancreatic tumour ablation using EUS-guided RF, the most common adverse effect was mild-to-moderate abdominal pain after the intervention, with an estimated incidence of 25–33%.29,33–37 In one of the studies, the incidence of secondary acute pancreatitis was 14%.35
In summary, the application of RF in cystic and solid tumours can only currently be recommended in the context of pilot studies or controlled clinical trials.
In cystic lesions, further studies are required to determine whether or not the neoplastic epithelium is actually eradicated, as this could mean a reduction in the potential for malignancy and so make long-term follow-up unnecessary.
ConclusionsThe increase in the use of abdominal imaging tests has led to a significant increase in incidental cystic pancreatic lesions. The lack of adequate markers to predict which lesions are likely to become malignant very often obliges us to consider surgery or carry out long-term follow-up, with the consequent impact on quality of life and costs.
The EUS-guided ablative methods for this type of tumour are still unreliable in terms of efficacy and safety. However, as has recently been suggested,38 although there is still a long way to go, in view of the promising preliminary data, such methods could eventually become an attractive alternative in the management of cystic lesions.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Guerrero García A, González-Huix F, Levy MJ, García de Paredes AG, Vázquez-Sequeiros E. Tratamiento ablativo de lesiones quísticas pancreáticas. Gastroenterol Hepatol. 2019;42:43–50.