The arterio-portal fistula (APF) is a rare, treatable and reversible cause of pre-sinusoidal portal hypertension (PPH).
We describe a case of a patient with APF following an ultrasound-guided percutaneous liver biopsy (PLB) in which endovascular treatment was given by Surgical Radiology, together with a literature review.
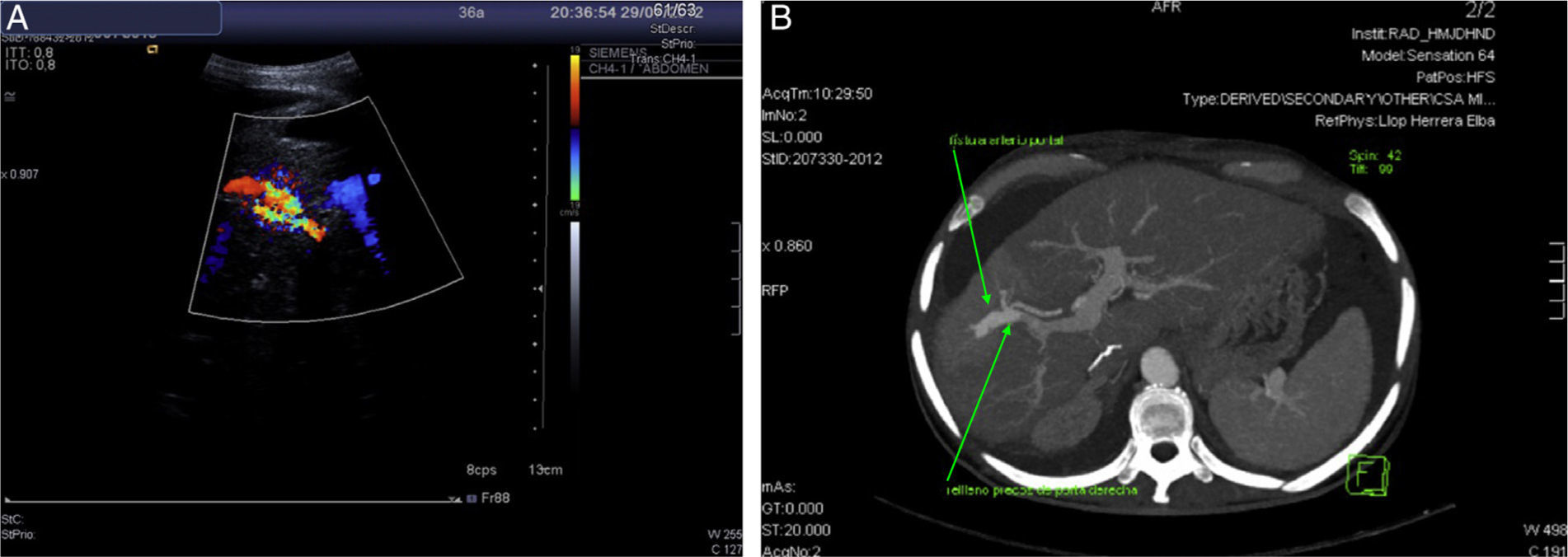
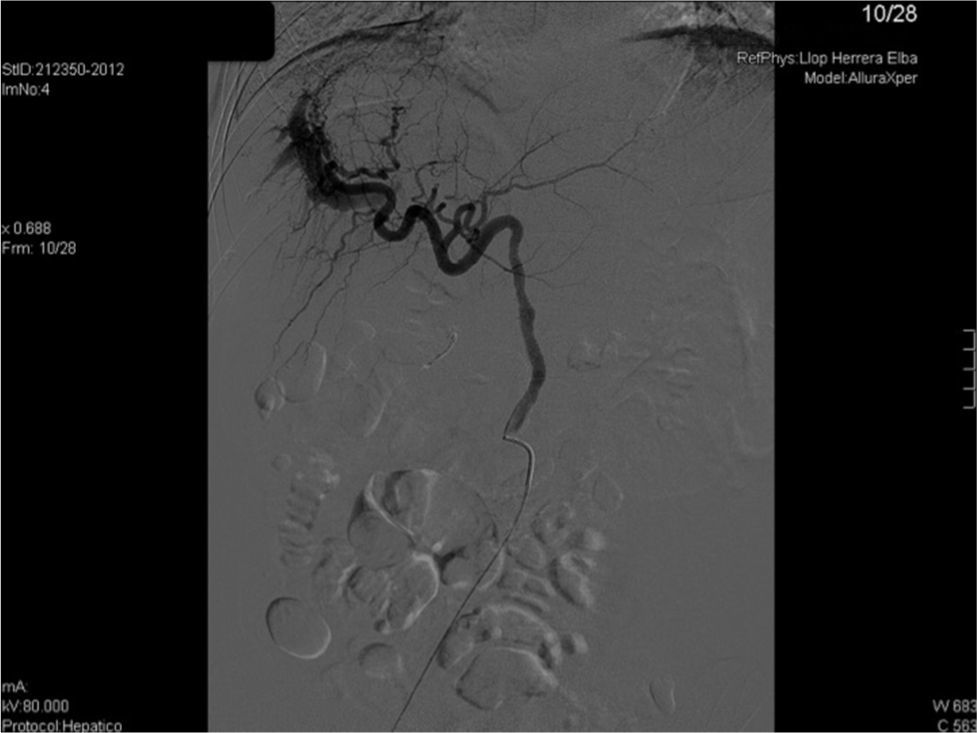
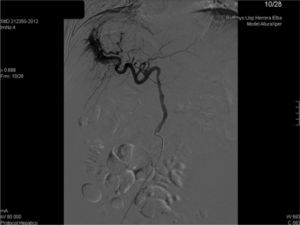
We present the case of 35-year-old woman who received a liver transplant in 2007 due to acute fulminant autoimmune hepatitis under immunosuppressive treatment with Sirolimus (1mg/24h orally). She presented with 2 weeks of development of asthenia and abdominal distension. Upon physical exploration, clinical signs of ascites were found. Analysis showed increasing transaminases and cholestasis enzymes (ALT 65U/L, AST 57U/L, GGT 356U/L) and 56% prothrombin activity, with remaining parameters within normality. She was admitted with the diagnosis of hydropic decompensation in probable relation to pre-existing hepatopathy at cirrhosis stage of the transplanted liver. However, the patient had a PLB one month prior to the onset of symptoms, with F2 liver fibrosis. In view of the discrepancies in findings, a Doppler ultrasonography was performed, which showed an aliasing phenomenon in the right lobe of the liver, with high velocity flow in the right branch of the portal vein, splenomegaly and ascites (Fig. 1a). CT angiography confirmed the presence of an APF (Fig. 1b). In view of findings, the APF was embolised with a copolymer named Onyx® (Fig. 2). Clinical and analytical improvement was progressively noted, with a reduction of the ascites with no need for diuretic treatment. The patient was discharged with the diagnosis of hydropic decompensation secondary to PPH and secondary to APF due to the PLB.
APF is an abnormal arteriovenous communication between any splanchnic artery and the portal vein, most frequently the hepatic artery (65%), followed by the splenic artery (11%) and the superior mesenteric artery (10%).1 It is a rare cause of PPH.
It is normally secondary to an iatrogenic cause (representing more than 50% of all published cases2), such as percutaneous or transjugular liver biopsy, performance of TIPS, transhepatic biliary drainage or surgery, although it also may occur following trauma, as part of liver cirrhosis, due to the rupture of a hepatic aneurism and linked to hepatocellular carcinoma (HCC) or benign growths. Congenital fistulas (such as Osler–Weber–Rendu syndrome) are rare; according to existing literature, only 18 cases of congenital intrahepatic APF have been reported.3
Preger was the first to describe APFs following PLB in 1967,4 and, although they continue to be rare, their rate of occurrence is rising due to the increase in invasive procedures and improved survival rates. The current rate of APF following PLB is 5.4%,5–7 although with the performance of PLB guided by ultrasonography damage to vessels can be reduced and, as such, the number of APFs drops.
The majority of APFs occurring following PLB are small and peripheral, and tend to spontaneously resolve themselves within the first month, and thus rarely require treatment.5,8 It has been proven that the time interval between formation of the APF and its discovery may vary from 1h to up to 43 years.2 Accordingly, it is recommendable to follow-up on patients after performance of a PLB.
The physiopathology of the APF is similar to other causes of non-cirrhotic PPH. Initially there is a dilation of sinusoids and branches of the portal vein. Long-term fistulas with persistent PPH show hyperplasia in the vein intima and the arterialisation of the branches of the portal vein, making it difficult to distinguish them from their counterpart arteries. These morphological changes are known as hepatoportal sclerosis, and contribute to progressive increase in pressure in the portal system. Arterial blood flows directly into the portal vein, thus firstly producing PPH and then increasing cardiac output.
The majority are asymptomatic and require close monitoring, since only in rare cases does the fistula grow in size and become clinically symptomatic.2 Most frequent manifestations include gastrointestinal bleeding (33%), ascites (26%), congestive heart failure (4.5%) and diarrhoea (4.5%),1 due to the PPH caused by the APF. The majority of gastrointestinal bleeding occur due to oesophageal varices. Abdominal pain and diarrhoea may be caused by mesenteric vascular congestion, even causing intestinal ischaemia in extreme cases.
It can also be linked to haemobilia, cholecystitis and acute pancreatitis.6,9
In patients with liver cirrhosis, diagnosis may be underestimated and simply attributed to the normal course of the illness. Liver function does not often change, and patients often conserve lobe architecture.7
Main symptoms include liver murmur in 33% of patients and liver thrill when the fistula exceeds 4mm in diameter.7
Initial diagnosis can be made by B-mode and Doppler ultrasonography and is confirmed with a CT angiography or MRI angiography of the abdominal veins.
The portal vein is shown to be dilated, with increased velocity and turbulence with or without inversion of flow, as well as arterial waves, comparable with the adjacent hepatic artery, which may also be dilated. At times, a fistulous route can be found between the artery and the vein.2
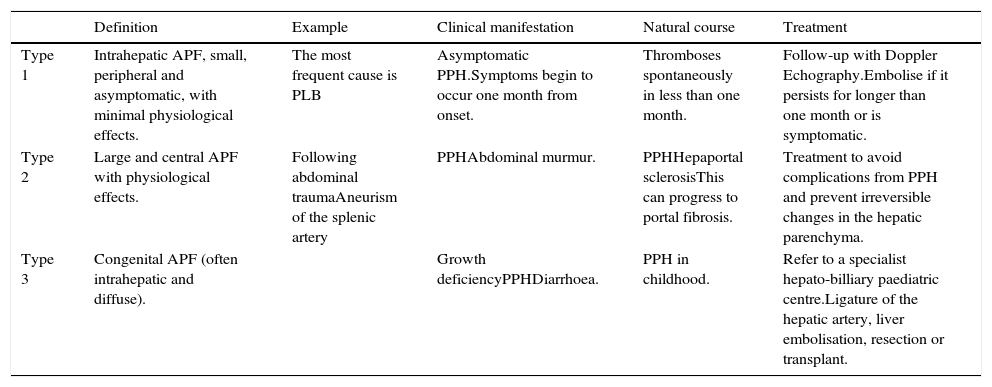
Treatment type depends on the size, location and number of APFs (Table 1). Guzman et al.7 proposed a classification system for APFs, taking into account location, causation and size of the APF in order to determine the appropriate treatment, although this should be developed early in order to avoid the risk of development of PH. Both surgical and endovascular occlusion of the fistula can be attempted. Endovascular treatment has thus currently emerged as a minimally invasive reliable treatment option in such individuals.2
Proposed classification of arterioportal fistulas (APFs).
| Definition | Example | Clinical manifestation | Natural course | Treatment | |
|---|---|---|---|---|---|
| Type 1 | Intrahepatic APF, small, peripheral and asymptomatic, with minimal physiological effects. | The most frequent cause is PLB | Asymptomatic PPH.Symptoms begin to occur one month from onset. | Thromboses spontaneously in less than one month. | Follow-up with Doppler Echography.Embolise if it persists for longer than one month or is symptomatic. |
| Type 2 | Large and central APF with physiological effects. | Following abdominal traumaAneurism of the splenic artery | PPHAbdominal murmur. | PPHHepaportal sclerosisThis can progress to portal fibrosis. | Treatment to avoid complications from PPH and prevent irreversible changes in the hepatic parenchyma. |
| Type 3 | Congenital APF (often intrahepatic and diffuse). | Growth deficiencyPPHDiarrhoea. | PPH in childhood. | Refer to a specialist hepato-billiary paediatric centre.Ligature of the hepatic artery, liver embolisation, resection or transplant. |
APFs often have a good prognosis, due to the minimal effects of a single APF and the effectiveness of current treatments.
In the case at hand, microcoils were used to embolise the fistula. The final outcome has been positive, with the patient being free of ascites.
Conflict of interestNone.