The use of stress ulcer prophylaxis (SUP) has risen in recent years, even in patients without a clear indication for therapy.
AimTo evaluate the efficacy of an electronic medical record (EMR)-based alarm to improve appropriate SUP use in hospitalized patients.
MethodsWe conducted an uncontrolled before-after study comparing SUP prescription in intensive care unit (ICU) patients and non-ICU patients, before and after the implementation of an EMR-based alarm that provided the correct indications for SUP.
Results1627 patients in the pre-intervention and 1513 patients in the post-intervention cohorts were included. The EMR-based alarm improved appropriate (49.6% vs. 66.6%, p<0.001) and reduced inappropriate SUP use (50.4% vs. 33.3%, p<0.001) in ICU patients only. These differences were related to the optimization of SUP in low risk patients. There was no difference in overt gastrointestinal bleeding between the two cohorts. Unjustified costs related to SUP were reduced by a third after EMR-based alarm use.
ConclusionsThe use of an EMR-based alarm improved appropriate and reduced inappropriate use of SUP in ICU patients. This benefit was limited to optimization in low risk patients and associated with a decrease in SUP costs.
El uso de la profilaxis de úlceras por estrés (PUE) ha aumentado en los últimos años, incluso en pacientes sin indicación.
ObjetivoEvaluar la eficacia de una alarma electrónica en la historia clínica (AEHC) para mejorar el uso apropiado de la PUE en pacientes hospitalizados.
MétodosEstudio no controlado antes-después para comparar la prescripción de la PUE en pacientes de la unidad de cuidados intensivos (UCI) y sala general, antes y después de la implementación de una AEHC que proporcionaba las indicaciones correctas de la PUE.
ResultadosSe incluyeron 1.627 pacientes en la cohorte previa a la intervención y 1.513 pacientes en la cohorte posterior a la intervención. La AEHC mejoró el uso apropiado (49,6 vs. 66,6%; p<0,001) y redujo el uso inapropiado de la PUE (50,4 vs. 33,3%; p<0,001) solo en pacientes de la UCI. Estas diferencias se relacionaron a la optimización del uso de la PUE en pacientes de bajo riesgo. No hubo diferencias en la frecuencia de hemorragia digestiva manifiesta entre ambas cohortes. El uso de la AEHC redujo un tercio del costo injustificado relacionado con la PUE.
ConclusionesEl uso de una AEHC mejoró el uso apropiado de la PUE y redujo el uso inapropiado de la PUE en pacientes de la UCI. Este beneficio fue limitado a la optimización del uso de la PUE en pacientes de bajo riesgo y se asoció a una disminución del costo de la PUE.
Gastrointestinal (GI) stress ulcers represent an important problem in hospitalized patients. The development of clinically significant GI hemorrhage in admitted patients has been associated with an increase in morbidity and mortality.1
Endoscopic studies have demonstrated gastric lesions in 75–100% of intensive care unit (ICU) patients within 24h of admission.2 Additionally, overt bleeding rates have been reported to occur in 5–25% of critically ill patients who do not receive stress ulcer prophylaxis (SUP).3 Mechanical ventilation and coagulopathy have been identified as the main risk factors associated with stress ulcers.4 SUP consists of the administration of acid-suppression therapy (AST), such as proton pump inhibitors (PPI) and histamine-2 receptor blockers (H2RB), to reduce gastric acid secretion and the risk of GI bleeding.4 Despite the lack of evidence in favor of universal SUP, use of AST has markedly increased in recent years. Some studies indicate that approximately 90% of ICU patients and nearly 70% of non-ICU patients receive SUP during admission.5
The use of SUP may have adverse effects, and place patients at a higher risk of hospital-acquired pneumonia and Clostridium difficile (C. difficile) infections.6,7 Moreover, inappropriate use of SUP increases healthcare costs.7 The implementation of training programs or clinical practice guidelines aimed at reducing inappropriate prescription of AST have been shown to have favorable results in several studies.6,8 There are currently no studies investigating the use of electronic medical record (EMR) based alarms to improve the appropriate use of SUP. However, the use of EMR-based alarms have been demonstrated to positively affect provider medication administration, in similar clinical situations.9
In this study, we aim to evaluate the impact of an EMR-based alarm to improve the appropriate SUP use in ICU and non-ICU patients.
MethodsThis study was conducted at a tertiary-care academic hospital located in Córdoba, Argentina. The hospital has 210 beds and averages 13,000 admissions annually. The study protocol was approved by the institutional review board of the hospital.
Study design and populationWe developed an uncontrolled before-after study that included two, four-month study periods: a pre-intervention period (June 2015 to September 2015) and a post-intervention period (March 2016 to June 2016). During the first period, prescription of AST for SUP was recorded to obtain a pre-intervention baseline. Six months after the first stage, a digital alarm was incorporated into the EMR of all admitted patients and all physicians were instructed about the use of this alarm. This alarm consisted of a chart that listed the main risk factors for SUP and specified indications for AST. This alarm appeared on the physician's computer screen every time they prescribed AST medications. During the second period, prescription of AST for SUP was also recorded to evaluate the intervention impact. There was no other intervention related to SUP in the post-intervention period.
We included patients older than 18 years-old who were admitted to the hospital (both ICU and non-ICU admitted patients) for more than 24h. Pregnant and puerperal women, patients in the oncology isolation ward, patients diagnosed with GI bleeding within the last 48h or 24h after admission, and patients on AST prior to the day of admission were excluded. In-patients who were prescribed AST with an indication other than SUP (i.e. gastroesophageal reflux disease, peptic ulcer disease, dyspepsia) were also excluded from the study.
EvaluationWe collected data regarding sex, age, reason for hospitalization, hospital ward of admission (ICU vs. non-ICU), SU risk factors and AST administration. For patients who received AST during their hospitalization, data were collected at the time of AST administration. For patients who did not receive AST during their hospitalization, data were collected when they were considered to be at highest risk for SU.
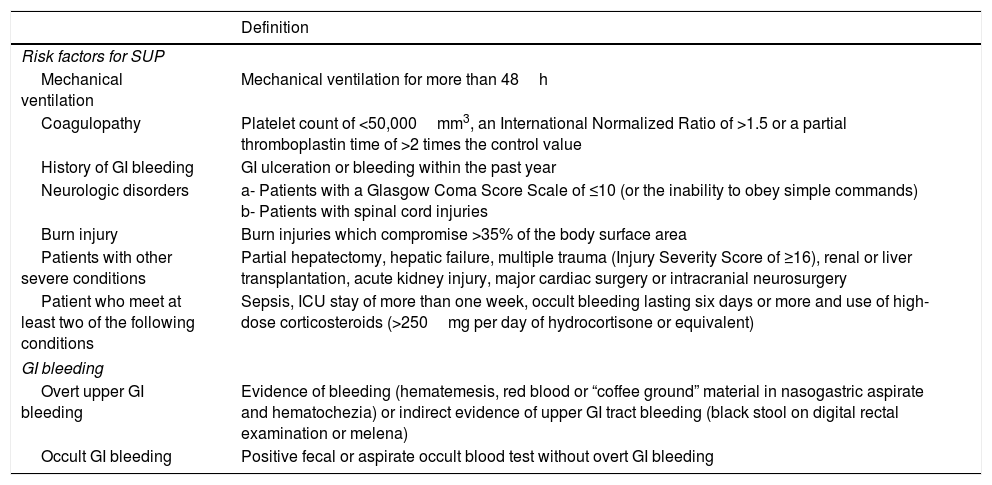
DefinitionsSUP was defined as the administration of AST for at least one day.4 The appropriate indications for SUP were determined by internal hospital guidelines that were based on the American Society of Health-System Pharmacists guidelines.10 Accepted indications for SUP are summarized in Table 1.4,10–13 GI bleeding definitions are also described in Table 1.4 Nosocomial pneumonia was considered when there was a new radiographic infiltrate that persisted for at least 48h with at least two of the following criteria: temperature above 38.5°C or below 35.0°C, a leukocyte count of more than 10,000cells/mm3 or less than 3000cells/mm3, purulent sputum, or isolation of pathogenic bacteria from an endotracheal aspirate.14C. difficile diarrhea was defined as liquid diarrhea plus a positive test for A or B toxins by enzyme-linked immunosorbent assay.15
Definition of indications for stress ulcer prophylaxis and gastrointestinal bleeding.
| Definition | |
|---|---|
| Risk factors for SUP | |
| Mechanical ventilation | Mechanical ventilation for more than 48h |
| Coagulopathy | Platelet count of <50,000mm3, an International Normalized Ratio of >1.5 or a partial thromboplastin time of >2 times the control value |
| History of GI bleeding | GI ulceration or bleeding within the past year |
| Neurologic disorders | a- Patients with a Glasgow Coma Score Scale of ≤10 (or the inability to obey simple commands) b- Patients with spinal cord injuries |
| Burn injury | Burn injuries which compromise >35% of the body surface area |
| Patients with other severe conditions | Partial hepatectomy, hepatic failure, multiple trauma (Injury Severity Score of ≥16), renal or liver transplantation, acute kidney injury, major cardiac surgery or intracranial neurosurgery |
| Patient who meet at least two of the following conditions | Sepsis, ICU stay of more than one week, occult bleeding lasting six days or more and use of high-dose corticosteroids (>250mg per day of hydrocortisone or equivalent) |
| GI bleeding | |
| Overt upper GI bleeding | Evidence of bleeding (hematemesis, red blood or “coffee ground” material in nasogastric aspirate and hematochezia) or indirect evidence of upper GI tract bleeding (black stool on digital rectal examination or melena) |
| Occult GI bleeding | Positive fecal or aspirate occult blood test without overt GI bleeding |
SUP: stress ulcer prophylaxis, GI: gastrointestinal, ICU: intensive care unit.
Patients were classified into four groups according to their SU risk (high or low), and AST status (drug given or not). Appropriate SUP use was defined as providing AST to high risk patients, and withholding AST from low risk patients. Inappropriate SUP use was defined as failing to provide AST to high risk patients, or providing AST to low risk patients.
End points of the studyThe primary endpoint of our study was SUP prescription (appropriate and inappropriate) both in ICU and non-ICU patients. The secondary endpoints of the study were overt GI bleeding, nosocomial pneumonia and C. difficile diarrhea, within a 30-day follow-up period.
Cost-analysisFor the economic evaluation, considering the lack of evidence of any difference favoring the EMR-based alarm, a cost-minimization strategy was chosen. Only direct medical costs were considered for the analysis, which included for each patient the cost according to the route of administration (oral or IV infusion) of PPI and H2RB and the length of SUP. Direct nonmedical costs and indirect costs were not accounted for because they were assumed to be similar between both periods and because they are not usually a concern under the selected perspectives. Costs were presented as International Dollars (Int$). Conversion from Argentine Pesos into International Dollars was performed using purchasing power parity conversion factor, as provided by the World Bank for the year of 2015 and 2016 (http://data.worldbank.org). Medical costs were estimated based on the hypothetical hospitalization of an average patient requiring SUP, in a private Argentine tertiary care hospital. Medical expenditures were calculated using the cost of the drugs and equipment needed for SUP administration. Costs related to the personnel (salaries and all related charges) were not considered.
Statistical analysisContinuous variables were expressed as means [standard deviations (SD)] or as medians (interquartile range or range), per their homogeneity. Categorical variables were compared using Pearson's chi-square test or Fisher's exact test, as appropriate. Continuous variables were compared using the Student T test or Mann–Whitney U test, per their homogeneity. A 2-sided probability value <0.05 was taken to be significant. Statistical analysis was performed with the SPSS V22.0 statistical package (IBM Corp, Armonk, NY).
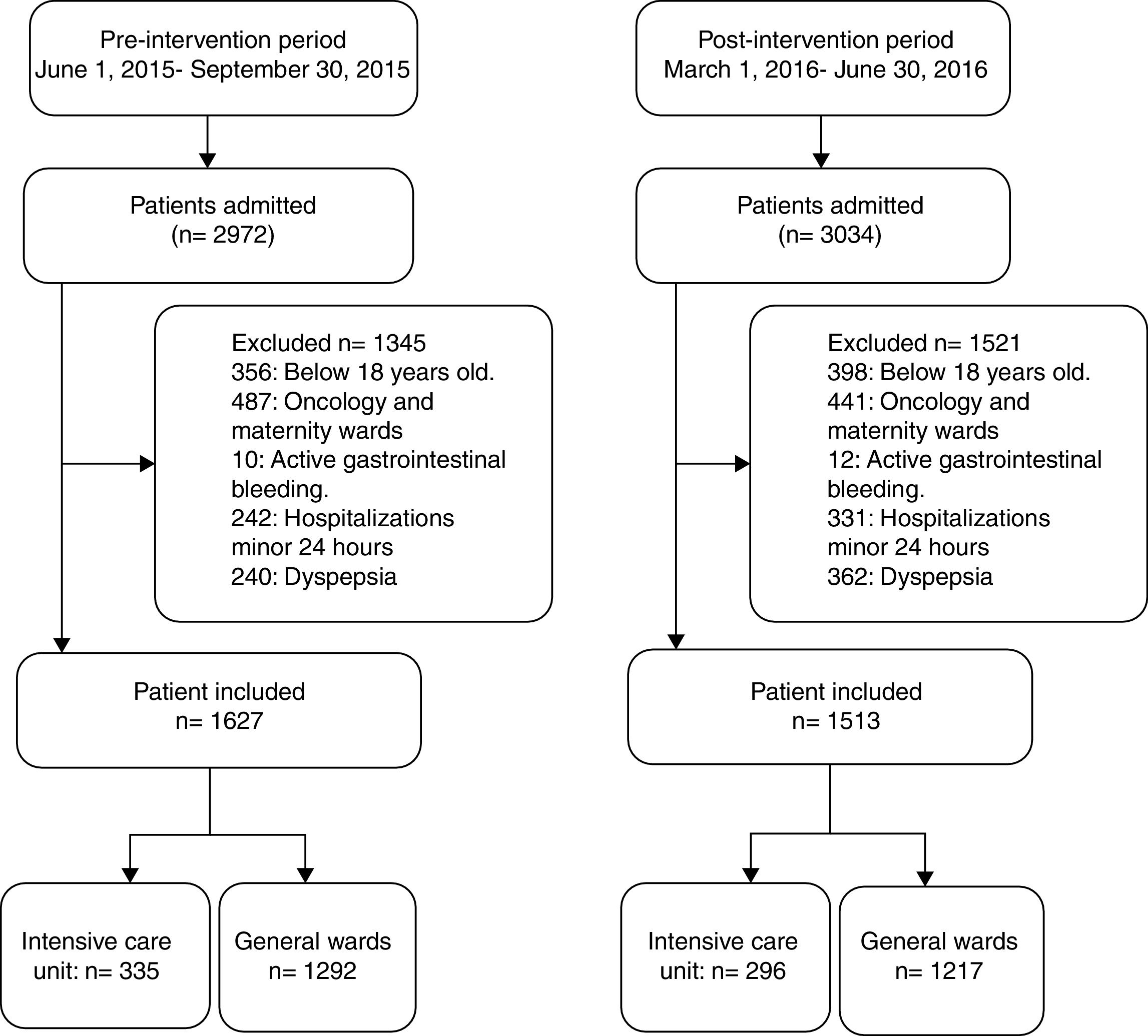
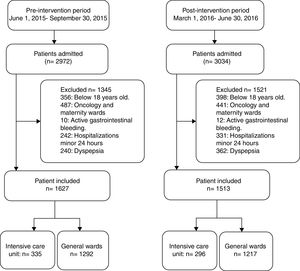
ResultsOf the 2972 patients admitted during the pre-intervention study period, 1627 (54.7%) patients met the inclusion criteria and were included in the study (335 ICU and 1292 non-ICU). In the post- intervention study period, 1513 (49.9%) met the inclusion criteria and were included (296 ICU and 1217 non-ICU) in the study (Fig. 1). A 30-day follow-up evaluation was performed in all patients included in both study periods.
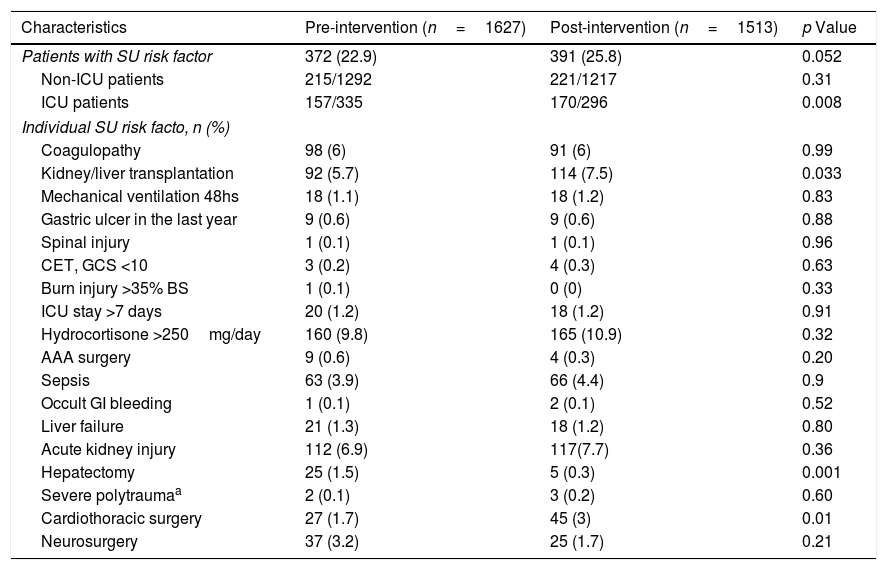
The mean age was 56.5±18.6 years and 56.1±18.8 years in the pre- and post-intervention periods, respectively (p=0.47). Male gender was more frequent in both study periods (pre-intervention: 53.3% vs. post-intervention: 52.1%; p=0.5). The most common risk factors for SUP during both study periods were acute kidney injury, kidney and/or liver transplantation and coagulopathy. The only difference between study periods was the presence of major cardiovascular surgery (pre-intervention: 1.7% vs. post-intervention: 3%; p<0.001), hepatectomy (pre-intervention: 1.5% vs. post-intervention: 0.3%; p<0.001) and kidney and/or liver transplants recipients (pre-intervention: 5.7% vs. post-intervention: 7.5%; p<0.001). All other measured risk factors were distributed similarly in each study period (Table 2).
Baseline characteristics in both periods.
| Characteristics | Pre-intervention (n=1627) | Post-intervention (n=1513) | p Value |
|---|---|---|---|
| Patients with SU risk factor | 372 (22.9) | 391 (25.8) | 0.052 |
| Non-ICU patients | 215/1292 | 221/1217 | 0.31 |
| ICU patients | 157/335 | 170/296 | 0.008 |
| Individual SU risk facto, n (%) | |||
| Coagulopathy | 98 (6) | 91 (6) | 0.99 |
| Kidney/liver transplantation | 92 (5.7) | 114 (7.5) | 0.033 |
| Mechanical ventilation 48hs | 18 (1.1) | 18 (1.2) | 0.83 |
| Gastric ulcer in the last year | 9 (0.6) | 9 (0.6) | 0.88 |
| Spinal injury | 1 (0.1) | 1 (0.1) | 0.96 |
| CET, GCS <10 | 3 (0.2) | 4 (0.3) | 0.63 |
| Burn injury >35% BS | 1 (0.1) | 0 (0) | 0.33 |
| ICU stay >7 days | 20 (1.2) | 18 (1.2) | 0.91 |
| Hydrocortisone >250mg/day | 160 (9.8) | 165 (10.9) | 0.32 |
| AAA surgery | 9 (0.6) | 4 (0.3) | 0.20 |
| Sepsis | 63 (3.9) | 66 (4.4) | 0.9 |
| Occult GI bleeding | 1 (0.1) | 2 (0.1) | 0.52 |
| Liver failure | 21 (1.3) | 18 (1.2) | 0.80 |
| Acute kidney injury | 112 (6.9) | 117(7.7) | 0.36 |
| Hepatectomy | 25 (1.5) | 5 (0.3) | 0.001 |
| Severe polytraumaa | 2 (0.1) | 3 (0.2) | 0.60 |
| Cardiothoracic surgery | 27 (1.7) | 45 (3) | 0.01 |
| Neurosurgery | 37 (3.2) | 25 (1.7) | 0.21 |
SU: stress ulcer. ICU: intensive care unit. CET: craneo encephalic trauma. GCS: Glasgow Coma Score, BS: body surface, AAA surgery: abdominal aort aneurysm surgery. GI: gastrointestinal.
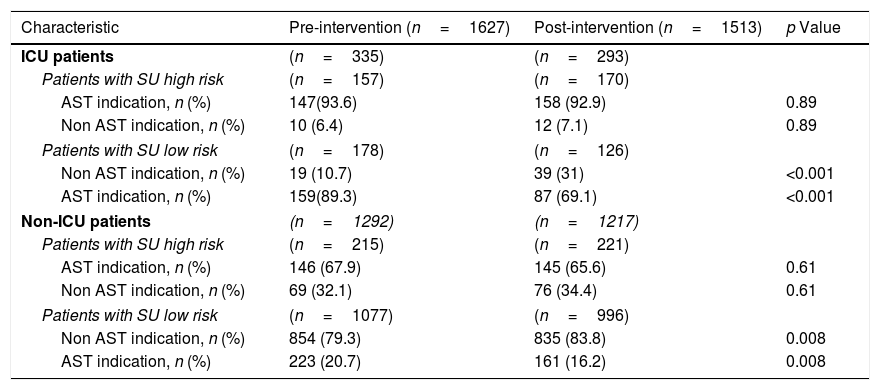
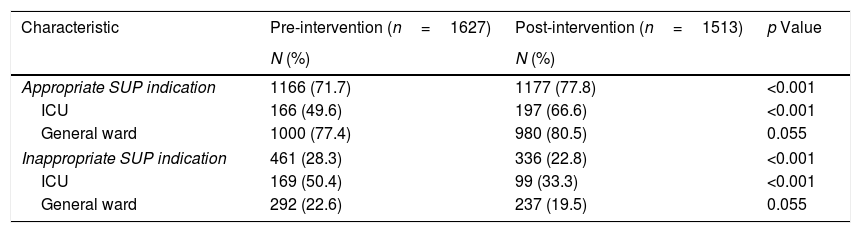
In patients at high risk for SU, AST indication was similar in both study periods, both ICU patients (pre-intervention: 93.6% vs. post-intervention: 92.9%; p=NS) and non-ICU patients (pre-intervention: 67.9% vs. post-intervention: 65.6%; p=NS). On the other hand, in low risk patients AST indication decreased significantly after the introduction of the EMR-based alarm both in ICU (pre-intervention: 89.3% vs. post-intervention: 69.1%; p<0.001) and non-ICU wards (20% vs. 16%; p=0.008) (Table 3). When appropriate SUP use (AST administration in high-risk patients and no AST administration for low-risk patients) was analyzed, we found a significant improvement between the pre- and post-intervention periods only in ICU patients (49.6% vs. 66.6%, p<0.001) (Table 4). Similarly, inappropriate SUP use (high risk patients without AST indication and low risk factor patients with AST prescription) was reduced in ICU patients (50.4% vs. 33.3%, p<0.001) (Table 4). These differences were limited to the optimization of SUP prescription in low risk patients without impact in high risk patients (Table 3).
Relationship between SU risk and AST indication in ICU and general ward patients in both stages.
| Characteristic | Pre-intervention (n=1627) | Post-intervention (n=1513) | p Value |
|---|---|---|---|
| ICU patients | (n=335) | (n=293) | |
| Patients with SU high risk | (n=157) | (n=170) | |
| AST indication, n (%) | 147(93.6) | 158 (92.9) | 0.89 |
| Non AST indication, n (%) | 10 (6.4) | 12 (7.1) | 0.89 |
| Patients with SU low risk | (n=178) | (n=126) | |
| Non AST indication, n (%) | 19 (10.7) | 39 (31) | <0.001 |
| AST indication, n (%) | 159(89.3) | 87 (69.1) | <0.001 |
| Non-ICU patients | (n=1292) | (n=1217) | |
| Patients with SU high risk | (n=215) | (n=221) | |
| AST indication, n (%) | 146 (67.9) | 145 (65.6) | 0.61 |
| Non AST indication, n (%) | 69 (32.1) | 76 (34.4) | 0.61 |
| Patients with SU low risk | (n=1077) | (n=996) | |
| Non AST indication, n (%) | 854 (79.3) | 835 (83.8) | 0.008 |
| AST indication, n (%) | 223 (20.7) | 161 (16.2) | 0.008 |
ICU: intensive care unit, SU: stress ulcer. AST: acid suppressor therapy.
Appropriate and inappropriate indication of SUP in ICU and non-ICU patients according to the study period.
| Characteristic | Pre-intervention (n=1627) | Post-intervention (n=1513) | p Value |
|---|---|---|---|
| N (%) | N (%) | ||
| Appropriate SUP indication | 1166 (71.7) | 1177 (77.8) | <0.001 |
| ICU | 166 (49.6) | 197 (66.6) | <0.001 |
| General ward | 1000 (77.4) | 980 (80.5) | 0.055 |
| Inappropriate SUP indication | 461 (28.3) | 336 (22.8) | <0.001 |
| ICU | 169 (50.4) | 99 (33.3) | <0.001 |
| General ward | 292 (22.6) | 237 (19.5) | 0.055 |
Appropriate SUP indication includes patients with SU risk factors and AST prescription, or patients without SU risk factor nor AST prescription.
Inappropriate SUP indication includes patients with SU risk factors without AST indication, or patients without SU risk factor with AST prescription.
ICU: intensive care unit, SUP: stress ulcer prophylaxis. AST: acid suppressor therapy.
There was no significant difference regarding overt GI bleeding episodes between the two study periods [three cases in the pre-intervention period (peptic ulcer n=2; esophagitis, n=1) and two cases in the post-intervention period (peptic ulcer n=2); p=0.93]. Occult GI bleeding occurred in two patients both in the pre- and pos- intervention periods. The distribution of these patients was similar in ICU or non-ICU patients. All patients with overt GI bleeding episodes had received SUP. Twenty-seven patients were diagnosed with nosocomial pneumonia during the study (12 in the pre- and 15 in the post-intervention period; p=0.44). In patients taking AST, there was only one episode in the pre-intervention group and four episodes in the post-intervention group. On the other hand, there were only five diagnosed cases of C. difficile associated diarrhea (3 in the pre- and 2 in the post-intervention period) and only two episodes occurred in patients not taking AST (one in each study period).
Cost analysisIn patients who received SUP, the mean AST treatment length was 7.18 days and 8.46 days in ICU and non-ICU patients, respectively. Table 4 summarizes the main findings of the cost analysis. The total cost of AST treatment was Int $34,124.98 in the pre-intervention study period and Int $32,324.74 in the post-intervention study period. The total justified costs, as determined by appropriately prescribed AST, were Int $16,812.55 and Int $21,188.29, in the pre- and post-intervention study periods, respectively. The total unjustified cost was reduced from Int $17,312.43 to Int $11,136.45 after the implementation of the EMR-based alarm.
DiscussionDespite a lack of evidence supporting universal SUP in hospitalized patients, use of AST has markedly increased in recent years, in both ICU and non-ICU settings. Several studies have shown that an overuse of SUP in hospitalized patients has resulted in an increase in adverse events associated with AST and an increase in health care costs. Nardino et al. found that 54% of all patients admitted to an internal medicine service received AST, and in 65% of those patients, prophylaxis was later deemed inappropriate.16 A recent review found that inappropriate use of SUP in general medicine wards was 71%.17 Furthermore, hospital-acquired bleeding is very uncommon in non-critically ill patients suggesting that routine prophylaxis might be unnecessary in most hospitalized patients.18 There are no published clinical trials that demonstrate benefit with proton pump inhibitors (PPI) therapy for SUP in low-risk patients. Nevertheless, the utilization of PPI for this indication is widespread. Survey data reveal that up to 40% of clinicians administer SUP to low-risk, non-ICU patients.19,20 Another study demonstrated that 88.5% AST prescriptions for non-critically patients were given inappropriately to patients who were at extremely low risk of GI bleeding.21
There are currently no studies investigating the use of EMR-based alarm to improve the appropriate use of SUP. However, the benefit of EMR-based alarms have been described in different clinical situations as polymedicated users, interval of laboratory test, and tromboembolism prophylaxis prescrption.9,22,23
Different studies have proposed several interventions to optimize appropriate SUP use in both ICU and non-ICU settings. In one study, the implementation of an educational program showed a decrease in the prescription of AST from 81% to 47%, but no difference was demonstrated in the proportion of appropriate or inappropriate SUP use.6 A similar study evaluated a different educational program, and demonstrated a decrease of inappropriate use of SUP from 59% to 29%, one month after the intervention.8 Since then, two other studies demonstrated a decrease in inappropriate use of AST by nearly half, after the implementation of clinician education programs.24,25 In our study we found a significant improvement in the appropriate and a significant reduction in the inappropriate prescription of SUP in ICU patients after the introduction of an EMR-based alarm. In this subgroup, the improvement both in appropriate and inappropriate use of SUP was mainly related to the optimization of SUP prescription in low risk patients. Despite there was a 22% reduction of SUP use in low risk ICU patients compared to baseline, 69% of those patients still received AST after EMR-based alarm implementation. The same situation occurred in non-ICU patients, although only 16% low risk finally received AST. On the other hand, the implementation of the EMR-based alarm showed no benefit over the optimization of SUP in high risk patients both in ICU and non-ICU settings and about 7% of high risk ICU patients and 34% of high risk non-ICU patients did not received SUP in the post-intervention period. Finally, considering the differential impact of this single intervention according to risk stratification for SUP, a strategy that includes stewardship and educational program together with an EMR-based alarm could improve SUP use in low and high risk patients.
Qadeer et al. reported a low rate of nosocomial GI bleeds (0.41%) among 17,707 non-ICU patients.18 In accordance with previous studies,26-28 our study found very few episodes of overt GI bleeding in both the pre- and post-intervention cohorts. This highlights the importance of using SUP as indicated by patient risk stratification, rather than simply by etiology of hospitalization. This suggests that decreasing the inappropriate use of AST did not increase the bleeding risk of in this population.
AST-related side effects such as C. difficile associated diarrhea, and hospital-acquired pneumonia, are well documented.29–31 In the present study there were low rates of these complications before and after the study intervention. This indicates that decreasing the use of AST did not negatively affect the safety of the patients at our hospital, with regards to these complications.
Heidelbaugh et al. reported that the inappropriate use of SUP in 1769 patient admissions, incurred in a total cost of $11,024 over 4 months, and $44,096 in an annualized analysis.7 In our study, we found a significant reduction in the unjustified cost related to SUP, after the implementation of an EMR-based alarm. According to these findings, the appropriate prescription of AST can be expected to produce a significant cost-saving benefit for institutions that adopt an EMR-based alarm.
One limitation of our study was that the same alarm was used in both ICU and non-ICU settings. This is a controversial issue, as there are few studies or guidelines that address SUP use in non-ICU settings. However, due to the heavy use of AST in non-ICU settings in our hospital, we decided to include this population in our study. It should be mentioned that as there was no control group in the post-intervention period, any observed changes in SUP may have been due to general trends physicians’ behavior, or even due to the Hawthorn effect of their participation in the study. Finally, the low number of bleeding episodes present in our data may not allow us to draw any significant conclusions regarding bleeding risk in this study. It is possible that we underestimated the number of clinically significant bleeds because we did not perform endoscopy in every patient.
In conclusion, we found that the introduction of an EMR-based alarm improved the appropriate and reduced the inappropriate use of SUP in ICU patients. This benefit was limited to the optimization of SUP in low risk patients. A reduction of the unjustified cost was observed without increasing overt GI bleeding. Further studies are needed to confirm these results and to identify new strategies to optimize the use of SUP.
Authors’ contributionAll authors included in the authorship list approved the final version of the article.
Saad EJ, Bedini M, Becerra AF, Balderramo D, Albertini RA: contributed to the design of the study, performed the research, collected and analysed the data, and wrote the paper. Martini GD, Gonzalez JG, Bolomo A, Castellani L, Quiroga S and Morales C: collected and analysed the data.
FundingOwn Institutional funds.
Conflict of interestsThe authors of this manuscript have no conflicts of interest to disclose as described by Gastroenterologia y Hepatología.
The authors would like to thank Dr. Jose Debes for the critical review of the manuscript.