Thiopurine therapy can be optimised by determining the concentration of the drug's metabolites.
Patients and methodsRetrospective analysis on a prospective database of 31 patients with inflammatory bowel disease who failed therapy with thiopurines. Thiopurine metabolites (6-thioguanine, 6-TGN and 6-methylmercaptopurine, 6-MMP) were measured by high-performance liquid chromatography (Laboratorios Cerba, Barcelona) and treatment was duly adjusted in accordance with the results. Clinical response was reassessed after six months.
ResultDespite the appropriate theoretical dose of thiopurines being administered, the dose was insufficient in 45.6% of patients (nonadherence to treatment suspected in 6.45%) and 16.2% received an excessive dose or the drug was metabolised by other metabolic pathways. After treatment was optimised based on metabolite levels, only 25.8% (8/31) were prescribed a biological agent, while 74.2% of cases (23/31) were managed through dose optimisation alone.
DiscussionMonitoring thiopurine metabolite levels may help clinicians to assess non-responsive patients before adding or switching to another drug (generally a biological agent), thereby avoiding any additional costs or potential toxicity. This strategy may also help to identify patients receiving an insufficient dose and those with an alternative metabolic pathway, who could be candidates for low-dose AZA with allopurinol, as well as patients who are suspected of being non-adherent. In three out of four patients, switching to a biological agent can be avoided.
El tratamiento con tiopurinas puede optimizarse determinando la concentración de sus metabolitos.
Pacientes y métodosAnálisis retrospectivo sobre una base de datos prospectiva, con inclusión de 31 pacientes con enfermedad inflamatoria intestinal en tratamiento con tiopurinas, que presentaban respuesta insuficiente. Se determinaron los metabolitos de tiopurinas en plasma (6-tioguanina, 6-TGN y 6-metilmercaptopurina, 6-MMP) por cromatografía líquida de alta eficacia (Laboratorios Cerba, Barcelona) ajustando el tratamiento de acuerdo a resultados. Tras 6 meses se reevaluó la respuesta clínica.
ResultadoA pesar de la dosis adecuada teórica de tiopurinas un 45,6% de los pacientes estaba infradosificado (sospechándose falta de adhesión al tratamiento en un 6,45% del total) y un 16,2% sobredosificado o metabolizaba por ruta metabólica alternativa. Tras ajustar a partir de niveles de metabolitos, solo el 25,8% (8/31) requirió biológico, mientras que el 74,2% de los casos (23/31) se manejó mediante optimización.
DiscusiónLa monitorización del tratamiento con tiopurinas mediante determinación de sus metabolitos puede ser utilizada para valorar pacientes no respondedores, antes de sustituir o complementar dichos fármacos con otros alternativos (biológicos, por lo general), con los consiguientes aumentos de toxicidad potencial y coste. Se puede rescatar a pacientes infradosificados, identificar aquellos que presentan una desviación en la ruta metabólica en los que se podría plantear una terapia con dosis bajas de AZA asociada a alopurinol, o aquellos en los que los datos sugieran falta de adhesión al tratamiento. En 3 de cada 4 pacientes puede evitarse la escalada a biológico.
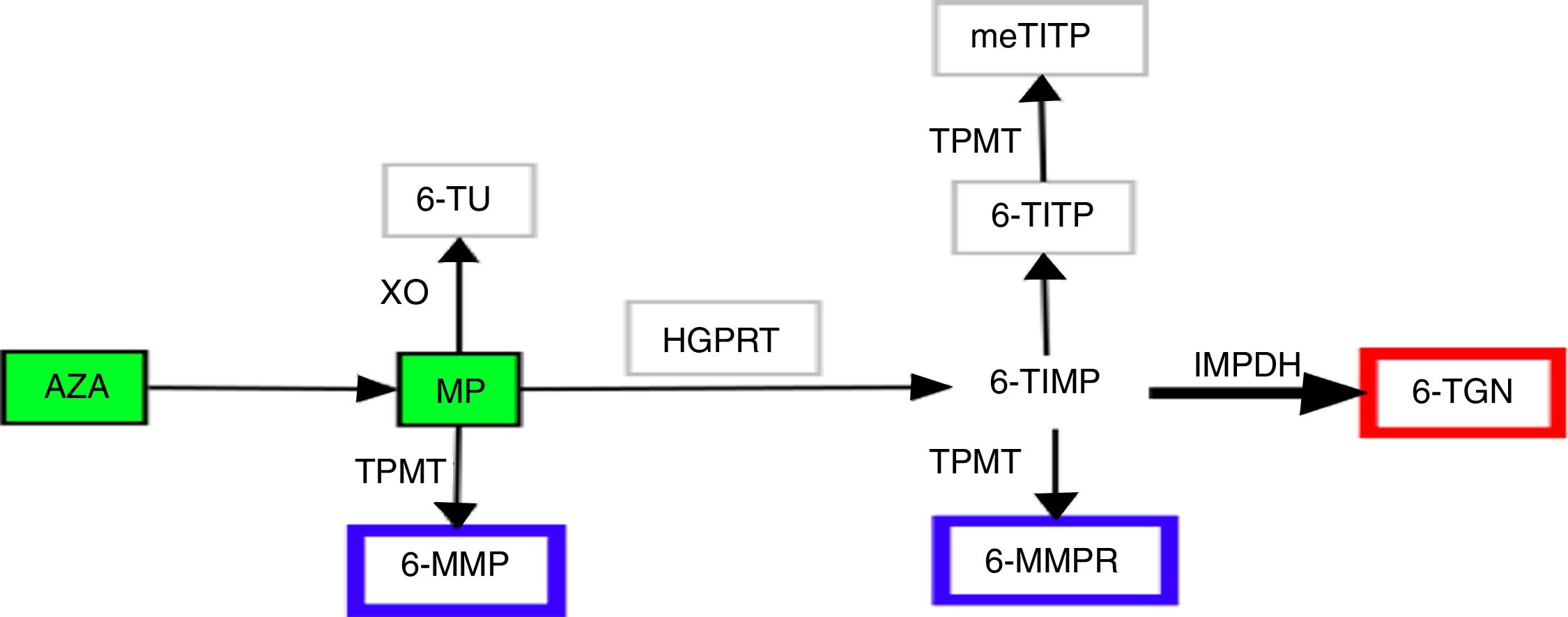
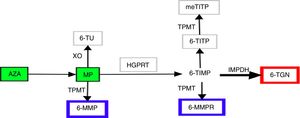
For half a century, thiopurines have been one of the main pillars of immunosuppressive therapy for patients with inflammatory bowel disease (IBD).1,2 Their principal uses2 are maintenance of remission in steroid-dependent disease3 or after control of a severe flare-up of ulcerative colitis,3 prevention of postoperative recurrence in Crohn's disease4 and in combined therapy with biological drugs. Thiopurines exert their immunomodulatory action inside the cell. In view of their similarity to endogenous purines, they are incorporated into nucleic acids as abnormal bases, interfering with the synthesis of proteins and nucleic acids, they inhibit lymphocyte proliferation and activate cell apoptosis via Rac1.5,6 The therapeutic power of thiopurines derives from these cytotoxic and immunosuppressive properties, but so does their most common side effect, myelotoxicity. Achieving successful treatment outcomes, while also keeping the toxic effects to a minimum, has always been a challenge for clinicians in the management of these patients. In recent years, improved understanding of the complex metabolism of thiopurines has made it possible to specifically design strategies aimed at optimising their use.7 Azathioprine (AZA) and 6-mercaptopurine (MP) are prodrugs, chemically analogous to endogenous guanines, with low and variable bioavailability. They are metabolised by at least four different pathways until the final molecules are obtained, which we generically call nucleotide metabolites. Although the metabolic steps are highly complex and still not fully understood, we can summarise them as follows (Fig. 1). The AZA to MP step occurs thanks to glutathione5 in a reaction not mediated by enzymes. The MP can then be catabolised by xanthine oxidase to thiouric acid; xanthine oxidase's high level of activity is the main factor responsible for only 16% of the drug being available in the systemic circulation. That 16% fraction may be anabolised by thiopurine methyltransferase (TPMT) to obtain 6-methyl-mercaptopurine (6-MMP) and ribonucleotides from 6-MMP ribonucleotides or catabolised by hypoxanthine-guanine phosphoribosyltransferase to obtain 6-thioinosine-5′-monophosphate. Next, thanks to inosine monophosphate dehydrogenase, this last intermediate product is transformed into 6-thioguanine nucleotides (6-TGN), mono/di/tri-phosphate. It is this final group of metabolites that exerts the immunomodulatory effect, whereas 6-MMP and 6-MMPR are the inactive and potentially toxic metabolites.5,7,8
Various studies have examined the relationship between 6-TGN levels in red blood cells and clinical response to thiopurines. Although this area is still subject to debate,9,10 there is evidence to suggest that 6-TGN levels above 230pmol/8×108 erythrocytes are related to clinical success,11–14 with these levels more commonly found in patients in remission than patients with activity, while remission is more likely when the patient's levels are above that figure. However, we must stress that reaching those levels does not guarantee remission. Some authors suggest that levels above 400pmol/8×108 erythrocytes may lead to an increased risk of myelotoxicity, although the development of myelotoxicity involves multiple factors, such as the existence of two low TPMT activity alleles in homozygosis (TPMTLL).15,16 Concentrations of 6-MMP above 5400pmol/8×108 erythrocytes have been related to the development of hepatotoxicity.17 Some authors go as far as to suggest that if such values are found, even without evidence of liver damage, the dose should automatically be reduced.18
Our study describes the clinical practice outcomes, for the first time in our area, of the optimisation of treatment with thiopurines through measurement of their metabolites and subsequent adjustment, optimising the use of drugs, treatment escalation, clinical response and identification of subjects at risk of toxicity.
Material and methodsPopulationAt our centre at the time of the study, 1297 patients with IBD were under follow-up. They had been diagnosed according to the usual criteria and treated in line with the international clinical practice guidelines. Of these patients, 690 (53.19%) had been exposed to thiopurines on some occasion. From January to June 2017 we set out to examine the role of optimisation of thiopurine treatment. In that period, we selected 31 patients on thiopurine treatment with an insufficient response, defined by the treating physician with the information from clinical, biological (high CRP or calprotectin) or endoscopic findings. We collected clinical information regarding gender, age, type of disease, dose of thiopurine, concomitant treatments, and disease activity index scores at each visit (Harvey-Bradshaw19 for Crohn's disease and Walmsley20 for ulcerative colitis). We also collected analytical information, including blood count and bilirubin and transaminase levels. At our centre, prior to the start of treatment, a determination of TPMT activity is routinely requested and no patient with less than 5units/ml is eligible to receive thiopurines.
Measurement of metabolitesThe levels of 6-TG and 6-MMP (pmol/8×108 erythrocytes, pmol/8×108 erythrocytes) were determined in plasma by high-performance liquid chromatography at Laboratories Cerba, Barcelona, Spain.21
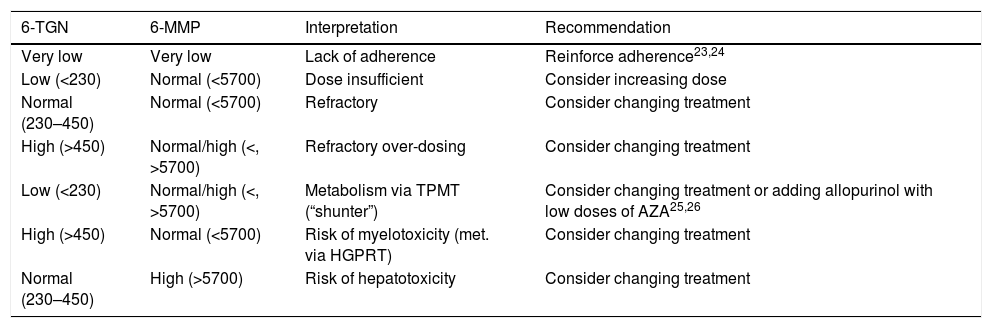
Interpretation of the resultsAccording to the laboratory values, 6-TGN levels below 230pmol/8×108 erythrocytes were considered to be suboptimal, those from 230 to 450pmol/8×108 erythrocytes within therapeutic range, and those above 450pmol/8×108 erythrocytes overdosed. For 6-MMP, levels below 5700pmol/8×108 erythrocytes were considered optimal and levels above that figure, overdosed. The aim was therefore to obtain 6-TGN levels in the range of 230–450pmol/8×108 erythrocytes and 6-MMP below 5700pmol/8×108 erythrocytes. Combining the different values for the two measurements, a number of possibilities were obtained; interpretation and recommended actions in patients with no response to treatment are shown in Table 1.
Interpretation of metabolite levels (measured in pmol/8×108 erythrocytes) and recommended approach.
| 6-TGN | 6-MMP | Interpretation | Recommendation |
|---|---|---|---|
| Very low | Very low | Lack of adherence | Reinforce adherence23,24 |
| Low (<230) | Normal (<5700) | Dose insufficient | Consider increasing dose |
| Normal (230–450) | Normal (<5700) | Refractory | Consider changing treatment |
| High (>450) | Normal/high (<, >5700) | Refractory over-dosing | Consider changing treatment |
| Low (<230) | Normal/high (<, >5700) | Metabolism via TPMT (“shunter”) | Consider changing treatment or adding allopurinol with low doses of AZA25,26 |
| High (>450) | Normal (<5700) | Risk of myelotoxicity (met. via HGPRT) | Consider changing treatment |
| Normal (230–450) | High (>5700) | Risk of hepatotoxicity | Consider changing treatment |
HGPRT: hypoxanthine-guanine phosphoribosyltransferase; TPMT: thiopurine methyltransferase; 6-MMP: 6-methylmercaptopurine; 6-TGN: 6-thioguanine.
Mean, standard deviation and range were calculated for continuous variables, while the percentage was provided for categorical variables. The Chi-square statistic was used for the comparison of samples. A p value >0.05 was considered as statistically significant.
ResultsMetabolite measurement was requested in 31 cases (74% males), 16 (51.6%) of whom had Crohn's disease, 14 (45.2%) ulcerative colitis and one (3.7%) indeterminate colitis. Mean age was 43.2±14 (22–74). The mean dose of AZA at the time of testing was 2.33±0.5 (1.1–2.9)mg/kg/d, while that of MP was 1.13±0.49 (0.5–1.7)mg/kg/d.
Tables 2 and 3 show the mean metabolite levels and interpretation of those levels.
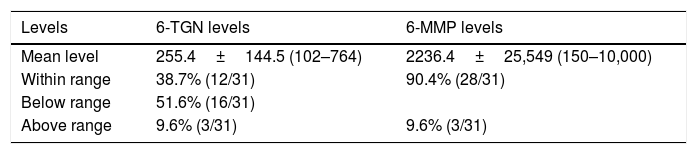
Metabolite levels. Mean, standard deviation and range of values of 6-thioguanine (6-TGN), 6-methylmercaptopurine (6-MMP) measured in pmol/8×108 erythrocytes.
| Levels | 6-TGN levels | 6-MMP levels |
|---|---|---|
| Mean level | 255.4±144.5 (102–764) | 2236.4±25,549 (150–10,000) |
| Within range | 38.7% (12/31) | 90.4% (28/31) |
| Below range | 51.6% (16/31) | |
| Above range | 9.6% (3/31) | 9.6% (3/31) |
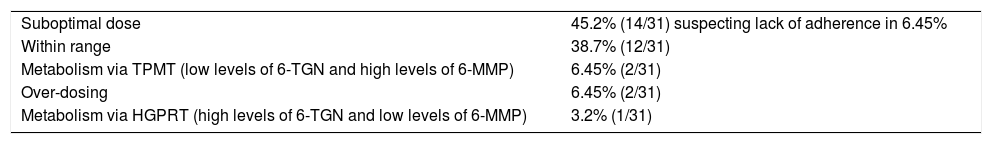
Interpretation of metabolite levels.
| Suboptimal dose | 45.2% (14/31) suspecting lack of adherence in 6.45% |
| Within range | 38.7% (12/31) |
| Metabolism via TPMT (low levels of 6-TGN and high levels of 6-MMP) | 6.45% (2/31) |
| Over-dosing | 6.45% (2/31) |
| Metabolism via HGPRT (high levels of 6-TGN and low levels of 6-MMP) | 3.2% (1/31) |
HGPRT: hypoxanthine-guanine phosphoribosyltransferase; TPMT: thiopurine methyltransferase; 6-TGN: 6-thioguanine; 6-MMP: 6-methylmercaptopurine.
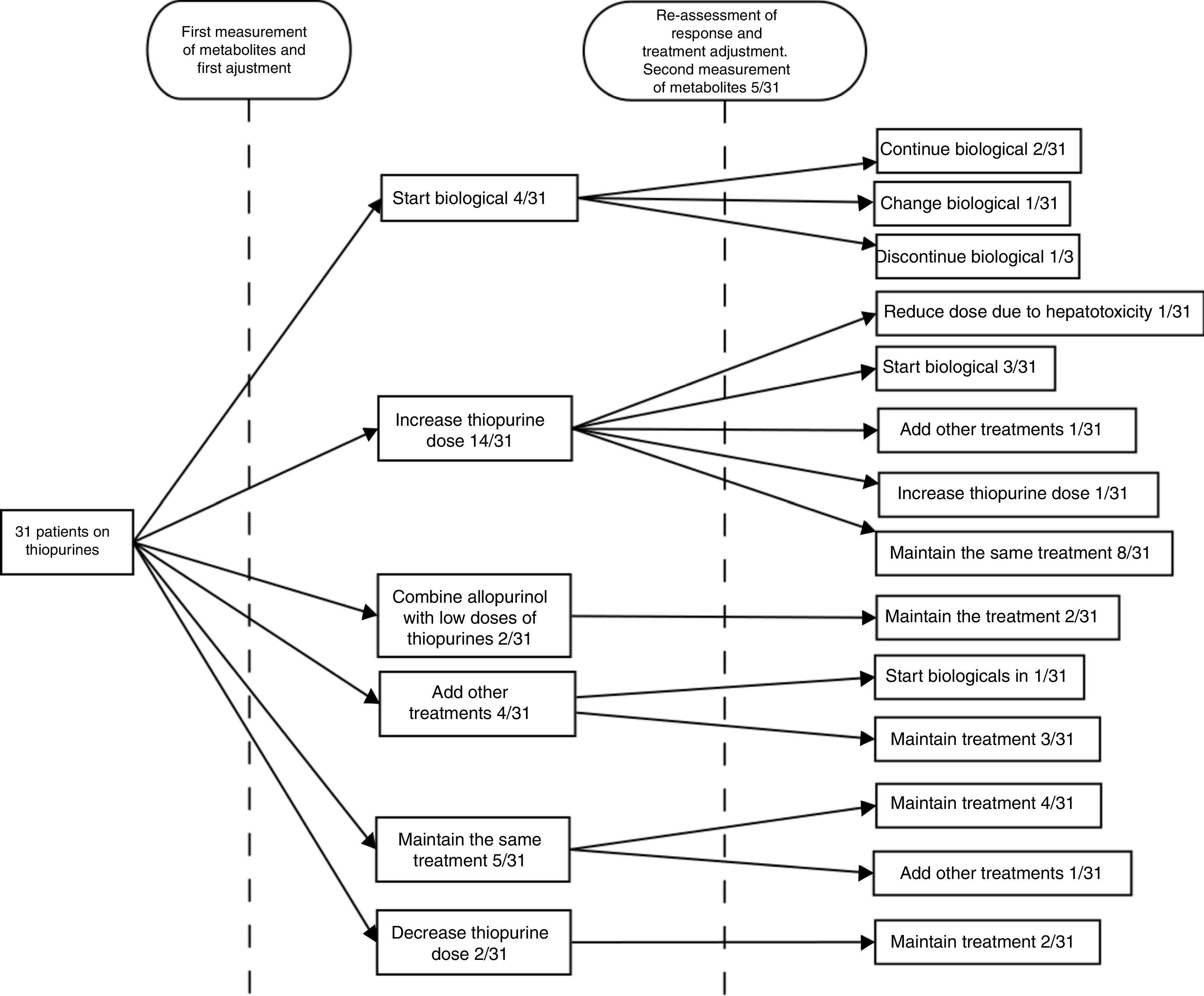
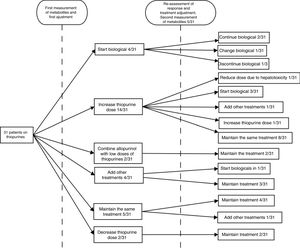
After obtaining the measurement results, the next step was to escalate treatment with a biological drug in four (12.9%) cases, increase the dose of thiopurines in 14 (45.2%), combine allopurinol with low doses of thiopurines in two (6.45%), prescribe a cycle of corticosteroids in two (6.45%), add mesalazine in two (6.45%), maintain the same treatment in five (16.1%) and decrease the dose in two (6.45%). Lack of adherence to treatment was suspected in one (3.2%) of the cases.
The patients were followed up for the six months after the adjustment, at the end of which the clinical response was re-assessed. At the discretion of the treating physician, at this second assessment, new levels were requested in five (16.6%) cases, with one (20%) being within range and three (60%) under-dosed (one was asymptomatic and lack of adherence was suspected in another), and new levels within range of hepatotoxicity being obtained in the remaining one (20%). After the initial adjustment, 67.7% had achieved good control of their disease. In the follow-up after that therapeutic decision, four (13%) patients were started on a biological drug, in one (3.2%) patient biological treatment was discontinued following an adverse reaction to it, two (6.5%) patients required a cycle of steroids, in one (3.2%) patient, new levels were obtained within range of hepatotoxicity so the dose of AZA was decreased, and in one (3.2%) patient, new suboptimal dose levels were obtained, so the dose was increased. During the follow-up period overall, a lack of adherence was suspected in 6.45% of the steps; in one patient (3.2%) at the first assessment and in a second patient (3.2%) at the second assessment. In line with our centre's routine clinical practice, patients who received treatment with biological drugs continued on low doses of AZA (50mg/day) as optimisation strategy. No further determinations of thiopurine metabolite levels were requested in any of these patients.22
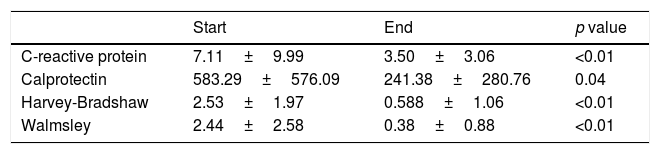
Although the treating physician's opinion about the existence of remission is a parameter used in numerous studies (for example, the Physician General Assessment is part of the Mayo Disease Activity Index for ulcerative colitis), to reinforce the assessment of our therapeutic approach we decided to analyse the changes in more objective parameters, such as CRP, faecal calprotectin and the respective activity indexes, with a decrease in the initial levels observed in all cases (Fig. 2 and Table 4).
Parameters assessed.
| Start | End | p value | |
|---|---|---|---|
| C-reactive protein | 7.11±9.99 | 3.50±3.06 | <0.01 |
| Calprotectin | 583.29±576.09 | 241.38±280.76 | 0.04 |
| Harvey-Bradshaw | 2.53±1.97 | 0.588±1.06 | <0.01 |
| Walmsley | 2.44±2.58 | 0.38±0.88 | <0.01 |
C-reactive protein measured in mg/l and faecal calprotectin measured in mcg/g. Mean and standard deviation.
Our data corroborate the fact that, despite receiving a dose of thiopurines considered a priori adequate, 45.6% of patients receive suboptimal doses (with lack of adherence to treatment suspected in 6.45% of cases) and 16.2% are overdosed or metabolise by an alternative metabolic pathway. After adjusting the treatment based on assessment of the drug metabolite levels obtained, only eight out of 31 patients (25.8%) were started on treatment with a biological agent, while 23 (74.2%) were managed through optimisation. In our experience therefore, measuring the concentration of thiopurine metabolites could be used as an additional tool in clinical practice to optimise and individualise treatment with these drugs. This would allow us to assess non-responders before replacing or combining thiopurines with other alternative treatments (generally biological agents), with the consequent increases in both potential toxicity and cost. The advantages of this strategy, which we observed in our study, include being able to identify possible causes of non-response to treatment. We would therefore be able to detect patients who would benefit from receiving a higher drug dose, those who have an abnormal metabolic pathway in whom therapy with low doses of AZA combined with allopurinol might be an option, or those in whom the data suggest a lack of adherence to treatment, a potential problem in all patients, particularly adolescents, and distinguish them from patients who are refractory to treatment with thiopurines who would be candidates for a change in treatment strategy. Although lack of adherence to treatment with thiopurines may be a potential problem in the follow-up of patients with IBD, in our study we did not find high rates of non-adherence (6.45%), with our figures being similar to those previously found by measuring metabolites reported in the literature.27 Among the range of therapeutic options for the optimisation of treatment with thiopurines, combining low doses with allopurinol is one strategy to increase both the efficacy and tolerability of the thiopurines, mainly in patients with a diversion in the metabolic pathway that favours the synthesis of inactive rather than active metabolites.25,26
We feel we need to explain why it was decided that adjustment was necessary in patients with clinical inactivity in whom markers remained elevated. Within the aims of treatment in IBD, clinicians are in a position to be increasingly ambitious, seeking not only improvement in symptoms, but also the normalisation of markers and, ultimately, probably also mucosal healing. In this sense, close monitoring seems to be coupled with a better quality of life and better clinical and endoscopic outcomes.28
Our study does have important limitations, most deriving from its retrospective nature and the small number of patients included. Having said that, among its main strengths is the lack of previous analyses in clinical practice in our area. Nevertheless, given the complexity of thiopurine metabolism and the huge variety of pharmacokinetic and pharmacogenomic factors involved, further studies are needed to more accurately specify the utility and interpretation of the metabolite levels of these drugs.
In conclusion, our results support the use of measurement of 6-TGN and 6-MMP concentrations to optimise treatment with thiopurines.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Sánchez Rodríguez E, Ríos León R, Mesonero Gismero F, Albillos A, Lopez-Sanroman A. Experiencia en práctica clínica de optimización de tiopurinas mediante determinación de sus metabolitos en la enfermedad inflamatoria intestinal. Gastroenterol Hepatol. 2018;41:629–635.