Tofacitinib is a Janus kinase (JAK) inhibitor, particularly of JAK 1 and JAK 3, that works at an intracellular level blocking the activity of multiple cytokines. Unlike other biological drugs, which act by blocking a specific cytokine, tofacitinib is also administered orally, making it an easier and possibly preferred route of administration for patients. It has other advantages, such as its short half-life, speed of action and low molecular weight which means that it does not induce immunogenicity.
We carried out a descriptive retrospective study at Hospital Torrecárdenas, analysing the seven patients with ulcerative colitis treated to date with tofacitinib. The main characteristics of these patients are shown in Table 1.
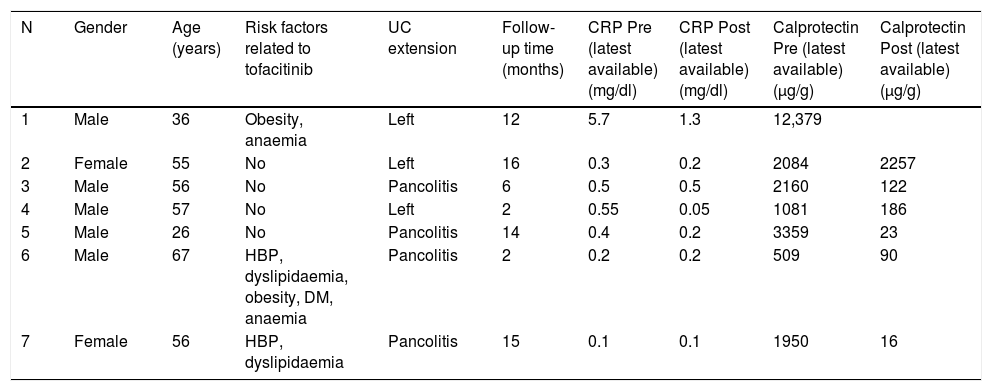
Characteristics of the patients with ulcerative colitis treated with tofacitinib at Hospital Torrecárdenas.
| N | Gender | Age (years) | Risk factors related to tofacitinib | UC extension | Follow-up time (months) | CRP Pre (latest available) (mg/dl) | CRP Post (latest available) (mg/dl) | Calprotectin Pre (latest available) (μg/g) | Calprotectin Post (latest available) (μg/g) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male | 36 | Obesity, anaemia | Left | 12 | 5.7 | 1.3 | 12,379 | |
| 2 | Female | 55 | No | Left | 16 | 0.3 | 0.2 | 2084 | 2257 |
| 3 | Male | 56 | No | Pancolitis | 6 | 0.5 | 0.5 | 2160 | 122 |
| 4 | Male | 57 | No | Left | 2 | 0.55 | 0.05 | 1081 | 186 |
| 5 | Male | 26 | No | Pancolitis | 14 | 0.4 | 0.2 | 3359 | 23 |
| 6 | Male | 67 | HBP, dyslipidaemia, obesity, DM, anaemia | Pancolitis | 2 | 0.2 | 0.2 | 509 | 90 |
| 7 | Female | 56 | HBP, dyslipidaemia | Pancolitis | 15 | 0.1 | 0.1 | 1950 | 16 |
Of the seven patients treated, five were male (75%) and two female (25%). They were aged from 26 to 67 (mean age 50.4, median 56). Four patients had pancolitis and three had left-sided colitis. None of them presented extraintestinal manifestations.
All the patients had previously been treated with at least three biologic drugs (2 anti-TNFs and vedolizumab). All patients had severe endoscopic activity (Mayo endoscopic subscore of 3) and histological activity (Rutter grade 4).
All were initially treated with doses of 10 mg/12 h, and all of them showed a clinical response within the first eight weeks, with a significant decrease in the number of daily stools, the number of bloody stools and abdominal discomfort. Five of them were in clinical remission, which we defined as a return to normal bowel habit and the absence of blood in their stools. Five of the patients had already reported a response within the first two weeks of treatment, one in week four and the last in week eight. In five patients the dose was reduced to 5 mg/12 h between eight and 16 weeks after starting treatment, and two patients are close to reaching the eighth week of treatment and so are still on the induction dose at present. One patient had to go back onto the dose of 10 mg/12 h due to clinical worsening, but responded well to the increase in dose. The follow-up of these patients varies from two to 16 months, with a median of 12 and a mean of 9.57 months. Three patients have reported adverse effects: two had an increase in cholesterol with the need to start statin therapy, and one developed C. jejuni enterocolitis, which responded well to antibiotic therapy. There were no other significant adverse effects in the rest of the patients.
In tests, calprotectin had previously been found to be elevated in all seven cases. Levels were determined again in six of the patients after taking tofacitinib and were found to have returned to normal in five (we consider calprotectin normal when not significantly elevated, with levels <200 μg/g) and remained elevated in one. However, despite not having achieved clinical remission, that particular patient showed noticeable clinical improvement. C-reactive protein had only been elevated in two patients, returning to normal in both. Only two patients subsequently had a colonoscopy, and both were in endoscopic remission.
From our results, we are able to conclude that tofacitinib is an effective drug for the treatment of ulcerative colitis, having achieved a clinical response in all treated patients, plus an improvement in biomarkers in most of them, all in a very short period of time and without any serious adverse effects. We would like to stress the difficult scenario in which we have been using the drug, in that these were patients with severe disease activity who had previously suffered failure or loss of response to all the other biologic drugs available. One particular patient was a 67-year-old man with cardiovascular risk factors, with previous failure of two anti-TNFs, vedolizumab and ustekinumab. After he rejected the alternative of surgery, he was offered treatment with tofacitinib, and informed of the restrictions included in the drug's summary of product characteristics and the potential risks. Our series only covers a short time and continued efficacy in the longer term remains to be assessed, but four of the patients have already exceeded a year of treatment.
Please cite this article as: López González J, Hernández Martínez Á, Lázaro Sáez M, Vega Sáenz JL. Experiencia local con tofacinitib en pacientes con colitis ulcerosa refractaria. Gastroenterol Hepatol. 2021;44:300–301.