This case concerned a 44-year-old woman, with no drug allergies or history of tobacco, alcohol or illicit drug use, with seronegative arthritis that commenced at age 18 in the form of asymmetric oligoarthritis, predominantly in the large joints. At age 22, she began to suffer rapidly destructive right coxitis, which required right hip replacement (RHR) at age 42. Rheumatoid factor, anti-citrullinated peptide antibodies, antinuclear antibodies and HLA-B27 tests were negative. She continued with remission induction therapy with hydroxychloroquine, gold salts (discontinued due to inefficacy) and methotrexate (MTX) (discontinued due to intolerance). In 2006, she initiated treatment with adalimumab (discontinued at 3 months due to skin rash) and then with etanercept (2008–2009), with partial response, and required glucocorticoids during the arthritis flares. She then received rituximab every 6 months (2009–2011, interrupted for the RHR surgery). She required infiltration of the right carpus for a new arthritis flare, and recommenced rituximab in June 2014, with a partial response.
In July 2014, the biological treatment was switched after the patient began to lose weight and became anaemic, with deterioration in her general health and arthritis, and elevated acute phase reactants (erythrocyte sedimentation rate [ESR] 64mm/h and C-reactive protein [CRP] 56mg/L (normal value: <5mg/L). As a result of the marked systemic component, intravenous tocilizumab (TCZ) monthly monotherapy (due to intolerance to MTX) was initiated in October 2014.
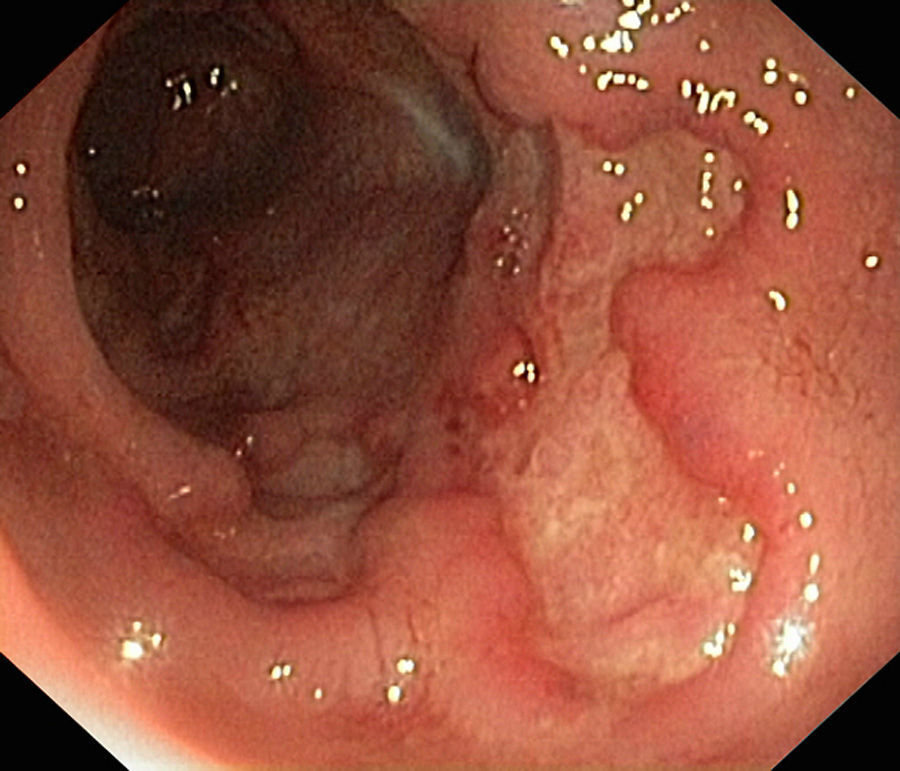
In January 2015, she was admitted to the gastroenterology department for loose stools (5–7 day) with no blood, pus or mucus, abdominal pain and 10-kg weight loss, which had commenced 1 month previously. Physical examination was remarkable for abdominal pain predominantly in the left flank, with no palpable masses or visceromegaly. Laboratory tests showed neither leukocytosis nor anaemia (haemoglobin 14.2g/dL), with CRP 0.24mg/L and albumin 44g/L. To complete the study, she underwent fibrogastroscopy, which was normal, and colonoscopy, which showed extensive, deep ulcers with healthy interlesional mucosa extending from 12cm from the anal margin to the caecum, as well as the terminal ileum; 15cm were affected with minute aphthae (Fig. 1). Histopathology findings were consistent with inflammatory bowel disease (IBD). She later presented high faecal calprotectin (FC) levels of 2714mg/kg faeces (normal value <100mg/kg). This was diagnosed as a severe flare-up of ileocolic Crohn disease (CD), with associated peripheral arthropathy. The patient started treatment with full-dose corticosteroid therapy (40mg/day), azathioprine 150mg/day and total enteral nutrition (TEN). She progressed well, with a decrease in the daily number of stools, as well as resolution of the abdominal and joint pain, and was discharged home.
We consider this clinical case to be of particular interest, firstly because of the sequence of clinical presentation, with erosive peripheral arthropathy 20 years before the intestinal manifestation of CD, a situation that accounts for only 10% of cases. Secondly, the normality of the biological parameters is remarkable, despite having significant mucosal involvement on the colonoscopy. Numerous cases of severe infections with normal biological reactants have been described in patients with rheumatoid arthritis on treatment with TCZ, but none in patients with CD and normal biological reactants in the context of anti-interleukin-6 (anti-IL-6) therapy.1–4
Joint symptoms are the most common extra-intestinal manifestation of CD (10–35%), either in the form of axial spondylarthropathy (sacroilitis and spondylitis) or peripheral arthritis, acute or chronic. Peripheral arthritis is usually related with the IBD activity, and generally appears simultaneously with IBD flares. Involvement is often pauciarticular, asymmetric, transient and self-limiting, although there are forms with persistent chronic activity, as in the case described. Although these manifestations respond to IBD treatment, it is often necessary to add remission induction drugs or administer local glucocorticoid injections.5
The normality of the biological reactants, despite the major ileocolic involvement as a result of CD, could be explained by the effect of the anti-IL-6 therapy. Interleukin 6 is a pro-inflammatory cytokine produced by various cell types, including monocytes, lymphocytes and fibroblasts, which participate in multiple biological processes such as formation of acute phase proteins and hepcidine in the liver, increased megakaryocyte levels with systemic thrombocytosis, differentiation of osteoclasts and consequently osteoporosis, and activation of macrophages, B- and T-cells, which cause chronic inflammation. All these pro-inflammatory actions link it to the pathophysiology of many autoimmune inflammatory diseases, such as CD.
TCZ is a humanised monoclonal antibody that targets both the soluble and membrane-bound forms of the interleukin-6 receptor. It has been approved in Europe as monotherapy or in combination with MTX for the treatment of adults with moderate-severe rheumatoid arthritis who respond inadequately to, or are intolerant to, one or more disease-modifying anti-rheumatic drugs or anti-tumour necrosis factor-alpha agents.6 However, it is not effective for CD, as interleukin-6 does not appear to be directly implicated in the pathophysiological mechanism of this disease. In fact, the results of different studies in patients with IBD treated with TCZ have shown a clinical response in more than 50% of cases, but no endoscopic or histological remission when compared with the placebo group.7–9
In this case, a simple, useful tool to clarify the diagnosis is FC. Calprotectin is an antimicrobial protein that accounts for 60% of the cytosolic proteins in granulocytes; its presence in stool is directly proportional to neutrophil activity in the intestinal lumen.
FC has been proposed as a biomarker of intestinal inflammation, as it correlates better with the degree of inflammation than serological markers and clinical indices, and has a sensitivity for detecting mucosal activity that varies from 70% to 100% and a specificity of 44–100%, according to the cut-off point in used in different studies.10
In conclusion, normal biological reactant levels cannot rule out IBD in a patient with clinical suspicion of the disease. In this case, FC seems to be an excellent diagnostic test to select those patients requiring further study.
Conflict of interestsThe authors declare that they have no conflict of interests.
Please cite this article as: Zacarías Martínez L, Mateo Soria L, Mañosa Círia M, Clos Parals A, Cabré Gelada E, Domènech Morral E. Mala correlación entre reactantes de fase aguda y afectación intestinal en paciente con comienzo de la enfermedad de Crohn bajo tratamiento con inhibidor de IL-6 por artropatía seronegativa. Gastroenterol Hepatol. 2016;39:670–672.