Most pancreatic cysts (PCs) found incidentally by CT and MRI scans might not be clinically important according to the Fukuoka guidelines, the American Gastroenterological Association (AGA) guidelines and European guidelines.
AimsTo determine and compare the prevalence of incidental clinically important PCs (CIPCs).
MethodsAbdominal contrast-enhanced CT or MRI scans performed during a one-year period were retrospectively reviewed to identify incidental PCs. CIPCs were defined as those cysts that would be capable of triggering further evaluation with endoscopic ultrasound, immediate surveillance (within 3–6 months) and/or surgery. Prevalence was calculated as the number of patients with CIPCs per 100 subjects imaged (%).
ResultsSixty patients (mean age 70±14 years) out of 565 were found to have incidental PCs, representing a prevalence of 8.7% (95% CI 6.3–11.5) in CT scans and 27.5% (95% CI 16–41) in MRI scans. Seven patients (11.6%, 95% CI 5–22) had CIPCs based on size ≥ 30mm (n=5), size ≥ 30mm and pancreatic duct (PD) dilation (n=1) and PD dilation and presence of solid component (n=1). Based on the Fukuoka guidelines, the prevalence of CIPCs was 1.2% (95% CI 0.4–2.5) in CT scans (6/507) and 1.7% (95% CI 0.1–9) in MRI scans (1/58). Based on the AGA and European guidelines, the prevalence of CIPCs was 0.2% (95% CI 0.1–1) in CT scans (1/507) and 1.7% (95% CI 0.1–9) in MRI scans (1/58). Patients with PCs initially classified as “AGA- or European-positive” had a higher surgical probability and this decision was taken earlier in the follow-up.
ConclusionsIn our cohort, the prevalence of important incidental pancreatic cysts was not negligible at around 1% according to current guidelines.
La mayoría de los quistes de páncreas (PC) hallados incidentalmente en las tomografías (TC) y las resonancias magnéticas (RMN) podrían no ser clínicamente importantes de acuerdo con las actuales guías Fukuoka, American Gastroenterological Association (AGA) y europea.
ObjetivosDeterminar y comparar la prevalencia de PC incidentales clínicamente importantes (CIPCs).
MétodosSe revisaron retrospectivamente las TC de abdomen con contraste y las RMN durante un período de un año para identificar PCs incidentales. Los CIPC se definieron como aquellos quistes que serían capaces de desencadenar una evaluación ulterior con ecoendoscopia, vigilancia en un corto intervalo (3-6 meses) y/o cirugía. La prevalencia se calculó como el número de pacientes con CIPC por cada 100 sujetos estudiados (%).
ResultadosSe encontró que 60 (edad media 70 ± 14 años) de 565 pacientes tenían PC incidentales, lo que representó una prevalencia de 8,7% (IC95% 6,3-11,5) en las TC y 27,5% (IC95% 16-41) en las RMN. Siete pacientes (11,6%, IC95% 5-22) tenían CIPC basados en el tamaño ≥30mm (n=5), tamaño ≥30mm y dilatación del conducto pancreático (PD) (n=1), y dilatación de PD y presencia de componente sólido (n=1). Basándonos en la guía Fukuoka, la prevalencia de CIPC fue de 1,2% (IC95% 0,4-2,5) en las TC (6/507) y 1,7% (IC95% 0,1-9) en las RMN (1/58). Basado en las guías AGA y europea, la prevalencia de CIPC fue de 0,2% (IC95% 0,1-1) en las TC (1/507) y 1,7% (IC95% 0,1-9) en las RMN (1/58). Los pacientes con PC inicialmente clasificados como “AGA o europea positivo” tuvieron una mayor probabilidad quirúrgica y esta decisión se tomó antes en el seguimiento.
ConclusionesEn nuestra cohorte, la prevalencia de quistes pancreáticos incidentales y relevantes no fue despreciable, siendo cercana al 1% según las guías actuales.
Most pancreatic cysts (PCs) are found incidentally in cross sectional imaging. Current high-resolution computed tomography (CT) and magnetic resonance imaging (MRI) allow identification of PCs in patients imaged for diseases unrelated to the pancreas, and its prevalence is supposed to be between 2-20% depending on patient's age and type of scan.1–3
PCs are considered to be a risk factor for pancreatic cancer which is difficult to detect in early stages. In addition, incidental PCs are associated with an increased risk of adenocarcinoma that would be three time higher than controls.4
Although the clinical behavior of the PCs is uncertain and controversial, most of these incidental pancreatic cystic lesions might not be clinically important according to the most common guidelines currently used: the Fukuoka guideline,5 the American Gastroenterological Association (AGA) guideline,6 and the European guideline.7
In 2013, the European experts consensus statement7 established that the presence of symptoms related to the pancreas, mural nodules, dilation of the main pancreatic duct > 6mm, cyst rapidly increasing in size, or elevated serum levels of carbohydrate antigen (CA)19-9 were risk factors for the presence of malignancy in branch duct-intraductal papillary mucinous neoplasias.
In 2015, the AGA guideline6 defined that asymptomatic patients with PCs should have at least two high risk features (size greater than 30mm, dilation of the main pancreatic duct, or solid component) in order to consider them as having relevant alert signs, and recommended further evaluation in the short term or surgery only in this subgroup of patients.
In 2017, the Fukuoka guideline5 was reviewed and updated; it defined the “worrisome features” (cyst size ≥3cm, thickened/enhancing cyst walls, main pancreatic duct size between 5-9mm, enhancing mural nodule < 5mm, abrupt change in caliber of pancreatic duct with distal pancreatic atrophy, lymphadenopathy, elevated serum level of CA 19-9, and rapid rate of cyst growth > 5mm/2 years) and the “high risk stigmata” (obstructive jaundice, enhanced mural nodule ≥ 5mm, main pancreatic duct ≥10mm) as important features in patients with mucinous PCs, and recommended further evaluation (endoscopic ultrasound with fine needle aspiration (EUS-FNA) and surveillance in a short interval with MRI or CT scan) or surgery in patients with at least one of these features.
The presence of any cystic lesion in the pancreas causes concern and anxiety in patients, especially if the characteristics of the lesion demand more evaluations and procedures. Many studies have evaluated the accuracy of these guidelines8,9 to detect advanced lesions and minimally invasive cancer. Considering that these guidelines have different criteria to define the importance of the cystic lesions, the prevalence of relevant or clinically important PCs (CIPCs) at the time of the diagnosis might be different depending on the guideline used.
Therefore, our aims were to assess the prevalence of PCs in a cohort of consecutive adults undergoing cross-sectional imaging (CT or MRI) for non-pancreatic reasons in a community hospital, and to determine and compare the prevalence of incidental CIPCs among these three guidelines (Fukuoka,5 AGA,6 and European7) at the time of the diagnosis.
MethodsThe study protocol was approved by the human ethics committee from our institution. Our institutional review board approved the review of radiological and clinical data for this study. Informed consent was waived for this retrospective review study.
An extensive and broad search was performed to identify patients with abdominal contrast enhanced-CT or MRI scans in the Radiology Department database. All reports of patients with abdominal contrast enhanced-CT or abdominal MRI scans (with or without gadolinium) performed during a one-year period (between January 1st 2014 and December 31th 2014) were retrospectively reviewed by pairs to identify PCs. Patients who underwent abdominal ultrasounds before these image modalities and had PCs or suspected PCs were excluded. If the indication of the CT or MRI was related to the pancreas (suspected pancreatic disease, history of pancreatitis, pancreatic cancer, pancreatic surgery, known PCs), the patient was also excluded. Only the most recent scan was reviewed in patients with more than one scan performed during the study period; in those cases that a MRI was ordered due to the result of a CT scan (and vice versa), only the index (first) scan was considered.
The following data were gathered for each patient: indication, age, gender, and presence of PCs. In patients with PCs: number, size, location, evidence of communication with pancreatic duct (PD), presence of worrisome features (size ≥30mm, thickened wall, enhancing mural nodule < 5mm, PD dilation between 5-9mm, abrupt change in PD, lymphadenopathy) and presence of high-risk stigmata (enhancing solid component ≥ 5mm, PD dilation ≥10mm). All these features were gathered from the scans in which the diagnosis of the incidental finding was made.
Despite the behavior of PCs in uncertain, CIPCs were defined as those cysts that, according to current guidelines,5–7 would be capable of triggering further evaluation with endoscopic ultrasound, surveillance in a short interval (3-6 months) and/or surgery at the time of the diagnosis. Although the Fukuoka and the European guidelines are focalized in mucinous pancreatic cysts, in clinical practice these guidelines are used to guide management even with the uncertainty of cyst diagnosis. Thus, for the Fukuoka guideline,5 CIPCs were defined as those pancreatic cysts with “worrisome features” or “high risk-stigmata”; for the European guideline,7 CIPCs were defined as those PCs with mural nodules or dilation of the main pancreatic duct over 6mm; and for the AGA guideline,6 as those with at least two positive high risk features (size greater than 30mm, dilation of the main pancreatic duct, or solid component within the cyst). Thereby, PCs were classified at the time of the diagnosis as not clinically important (those Fukuoka, AGA and European negative) and as clinically important (those Fukuoka positive and AGA/European negative, those Fukuoka/European positive and AGA negative, and those Fukuoka/European/AGA positive).
The main outcomes were to determine the prevalence of CIPCs based on these guidelines and to compare the prevalence among them. The prevalence of PCs was calculated as the number of patients with PCs per 100 subjects imaged (%). The prevalence of CIPCs was calculated as the number of patients with CIPCs (for each guideline) per 100 subjects imaged (%). Secondary outcomes were to assess the prognosis and the need of surgery at last follow-up of those patients with PCs incidentally found in this period.
Statistical analyses. Statistical analyses to assess differences between continuous variables (age, size of cystic lesions) were performed using Student¿s T-test. Statistical analyses to assess differences between dichotomous variables were performed using the Fisher exact test. All statistical analyses were carried out using the SPSS Statistical software, version 22 (IBM, Armonk, NY). P values of less than 0.05 were considered significant. A regression analysis based on curvilinear estimation was carried out to assess the influence of age on PCs prevalence. The need of surgery for each PCs during the follow-up was gathered looking into the medical records; it depended on the criteria of the doctor in charge. Then, the surgical probability over time (expressed as the probability of being free of surgery) was calculated by Kaplan Meier analysis comparing these factor levels: PCs Fukuoka/European/AGA negative, PCs Fukuoka positive/both European and AGA negative, PCs both Fukuoka and European positive/AGA negative, and PCs Fukuoka/European/AGA positive.
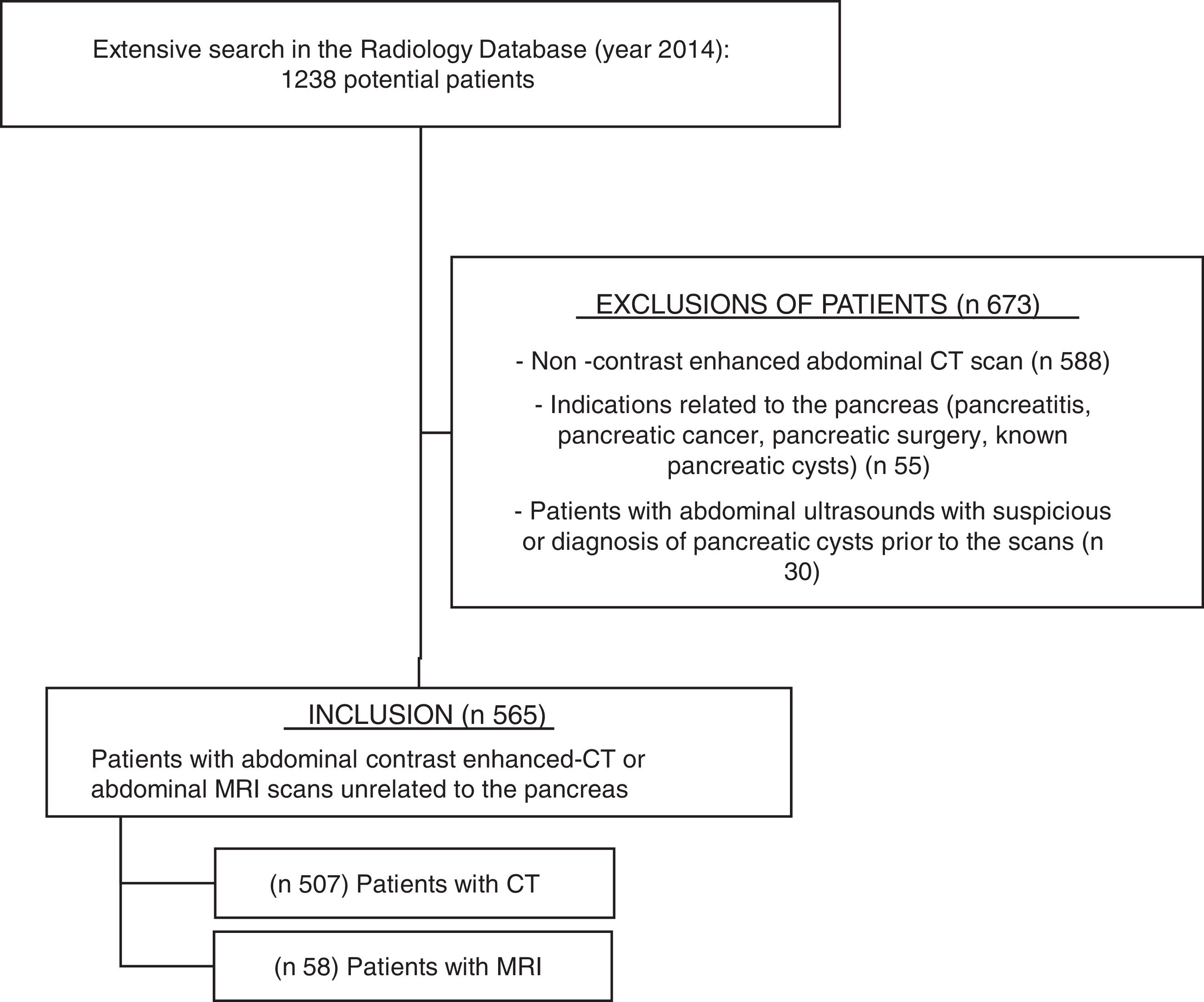
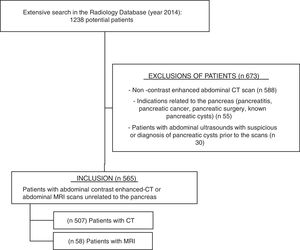
ResultsAfter searching in the Radiology Department database (Figure 1), 1238 potential patients were analyzed during this 12-month period. Six hundred seventy-three patients were excluded because of non-contrast enhanced abdominal CT scan (n 588), indications related to the pancreas (n 55, mostly pancreatitis and surveillance of pancreatic cysts), or abdominal ultrasounds showing PCs or suspicious of PCs prior to the scan (n 30). Finally, five hundred sixty-five patients fulfilled the inclusion criteria and their scans were reviewed: 507 abdominal contrast enhanced-CTs and 58 abdominal MRIs (50 of them with gadolinium).
The mean age of patients included was 63 years old (range 18-94), without statistical differences between the CT and MRI scans (63±14 years old vs. 61±15, respectively; p=0.22). Most common indications were related to gastrointestinal symptoms (50%; mainly abdominal pain), non-pancreatic neoplasms (30%), and genitourinary or vascular problems. The term abdominal pain in the indications of the scans referred to a spectrum of diseases (such as appendicitis, diverticulitis, biliary colic, ischemic colitis, inguinal hernia, refractory irritable bowel syndrome); in no case the abdominal pain was considered to be caused by a pancreatic cyst.
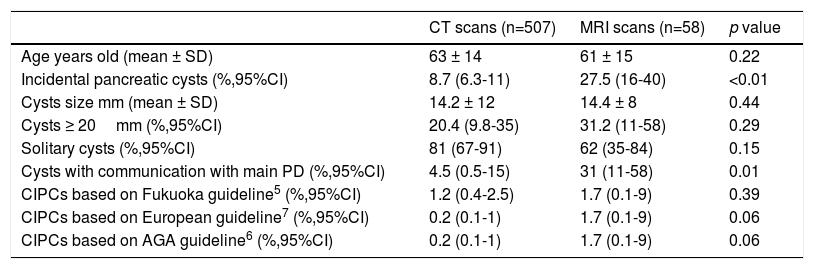
Prevalence of pancreatic cystsSixty patients (mean age 70±14 years, range 28-91; 50% male) out of the 565 patients imaged were found to have incidental PCs: 44 patients in the CT scans group and 16 patients in the MRI group. The prevalence of PCs was 8.7% (95%CI 6.3-11.5) in the CT scans and 27.5% (95%CI 16-41) in the MRI scans; it was significantly higher in the MRI (p<0.01). See Table 1.
Summary of findings.
| CT scans (n=507) | MRI scans (n=58) | p value | |
|---|---|---|---|
| Age years old (mean ± SD) | 63 ± 14 | 61 ± 15 | 0.22 |
| Incidental pancreatic cysts (%,95%CI) | 8.7 (6.3-11) | 27.5 (16-40) | <0.01 |
| Cysts size mm (mean ± SD) | 14.2 ± 12 | 14.4 ± 8 | 0.44 |
| Cysts ≥ 20mm (%,95%CI) | 20.4 (9.8-35) | 31.2 (11-58) | 0.29 |
| Solitary cysts (%,95%CI) | 81 (67-91) | 62 (35-84) | 0.15 |
| Cysts with communication with main PD (%,95%CI) | 4.5 (0.5-15) | 31 (11-58) | 0.01 |
| CIPCs based on Fukuoka guideline5 (%,95%CI) | 1.2 (0.4-2.5) | 1.7 (0.1-9) | 0.39 |
| CIPCs based on European guideline7 (%,95%CI) | 0.2 (0.1-1) | 1.7 (0.1-9) | 0.06 |
| CIPCs based on AGA guideline6 (%,95%CI) | 0.2 (0.1-1) | 1.7 (0.1-9) | 0.06 |
CT: computed tomography; MRI: magnetic resonance imaging; PD: pancreatic duct; CIPCs: clinically important pancreatic cysts; AGA: American of Gastroenterology Association.
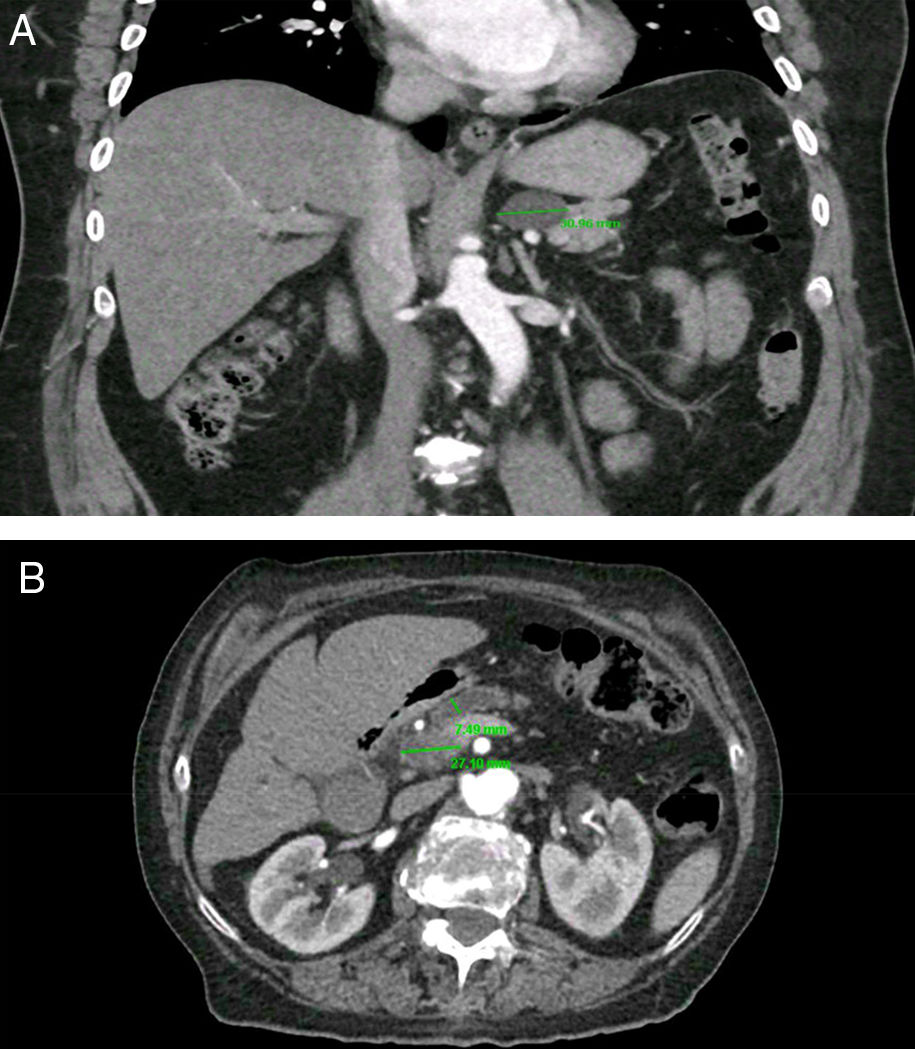
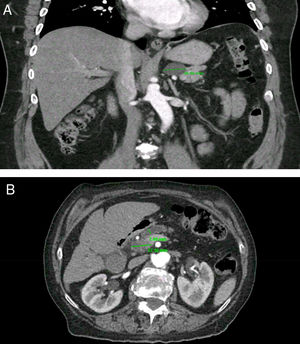
PCs were visualized as solitary lesions in most cases (75%, 95%CI 62-85) and the mean size in diameter was 14.4±11mm (range 3-61); most PCs were smaller than 20mm (76%, 95%CI 64-86). PCs were more frequent in the head (53%), followed by the body (23%), tail (19%) and neck (5%). In the CT scans, two PCs (4.5%, 95%CI 0.5-15) had clear communication with the main PD; in the MRI scans, five PCs (31%, 95%CI 11-58) were noted to have clear communication with main PD. Seven patients (1.2%, 95%CI 0.5-2.5) were found to have CIPCs based on size ≥30mm (n=5), size ≥30mm and PD dilation (n=1), and PD dilation and presence of solid component (n=1). See Figure 2. Only 11.6% (95%CI, 5-22) of the patients with PCs were considered to have CIPCs (7/60).
Clinically important pancreatic cysts (CIPCs). A) CIPCs based on Fukuoka guideline: Pancreatic cyst larger than 30mm in the body/tail of the pancreas. B) CIPCs based on AGA guideline: Pancreatic cyst smaller than 30mm in the neck of the pancreas with a solid component and pancreatic duct dilation.
When considering the subgroup of patients who presented with gastrointestinal symptoms (mainly abdominal pain), there weren¿t significant differences in relation to the size of the cysts (p=0.85), the presence of worrisome features or high risk stigmata (p=0.21), or surgical indication (p=0.75).
Prevalence of clinically important pancreatic cysts (CIPCs)Based on the Fukuoka guideline, the prevalence of CIPCs was 1.2% (95%CI 0.4-2.5) in CT scans (6/507) and 1.7% (95%CI 0.1-9) in MRI scans (1/58). Based on the European and the AGA guidelines, the prevalence of CIPCs was 0.2% (95%CI 0.1-1) in CT scans (1/507) and 1.7% (95%CI 0.1-9) in MRI scans (1/58). See Table 1.
The prevalence of CIPCs in the CT scans at the time of the diagnosis was significantly higher using the Fukuoka criteria (1.2% vs 0.2%, p<0.01).
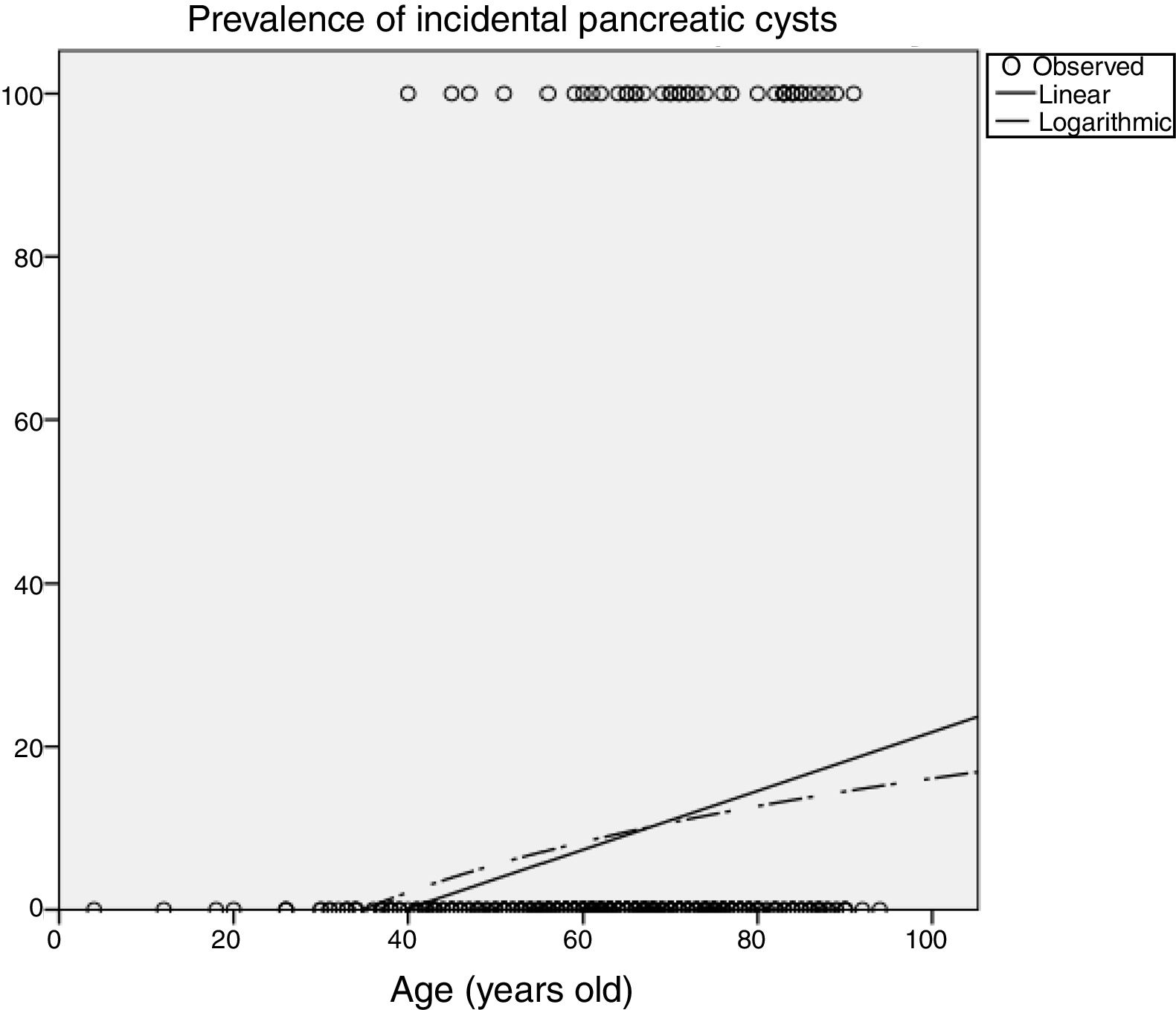
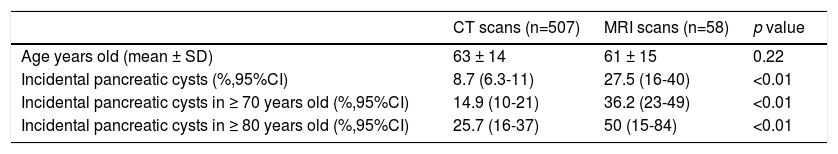
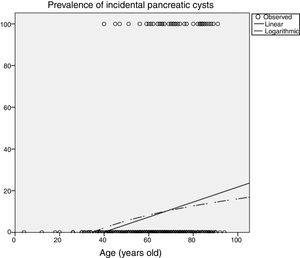
Influence of age in pancreatic cyst prevalencePrevalence of PCs was significantly influenced by age (Table 2), increasing up to 14.9% (95%CI 10-21) in patients older than 70 years imaged with CT, and up to 25.7% (95%CI 16-37) in patients older than 80 years. In the MRI scans, 50% (95%CI 15-84) of patients older than 80 years had incidental PCs. Figure 3 shows an estimation of the influence of age on the PCs prevalence by curvilinear regression analyses. In our cohort, approximately 20% of the population could have some type of PC at the age of 100.
Influence of age in the prevalence of pancreatic cysts.
| CT scans (n=507) | MRI scans (n=58) | p value | |
|---|---|---|---|
| Age years old (mean ± SD) | 63 ± 14 | 61 ± 15 | 0.22 |
| Incidental pancreatic cysts (%,95%CI) | 8.7 (6.3-11) | 27.5 (16-40) | <0.01 |
| Incidental pancreatic cysts in ≥ 70 years old (%,95%CI) | 14.9 (10-21) | 36.2 (23-49) | <0.01 |
| Incidental pancreatic cysts in ≥ 80 years old (%,95%CI) | 25.7 (16-37) | 50 (15-84) | <0.01 |
CT: computed tomography; MRI: magnetic resonance imaging.
Prevalence of CIPCs based on Fukuoka guideline was also significantly influenced by age, increasing up to 2.1% (95%CI 0.6-5.3) in patients older than 70 years imaged with CT scans.
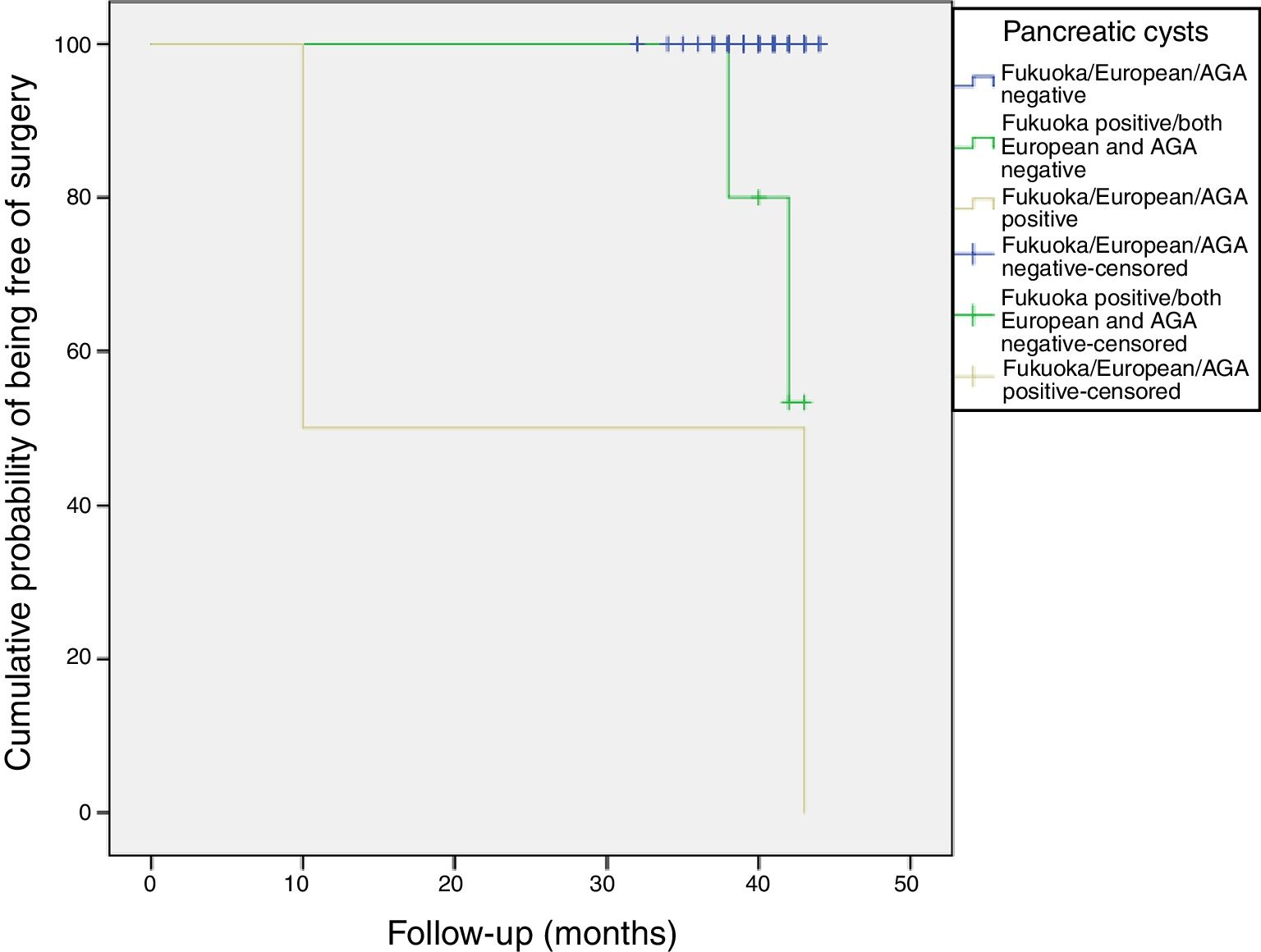
Follow-up of patients with CIPCsThe sixty patients with incidental PCs were followed for a mean of 40 months (range: 32–44 months). Most PCs remained stable without changes during the follow-up (Figure 4).
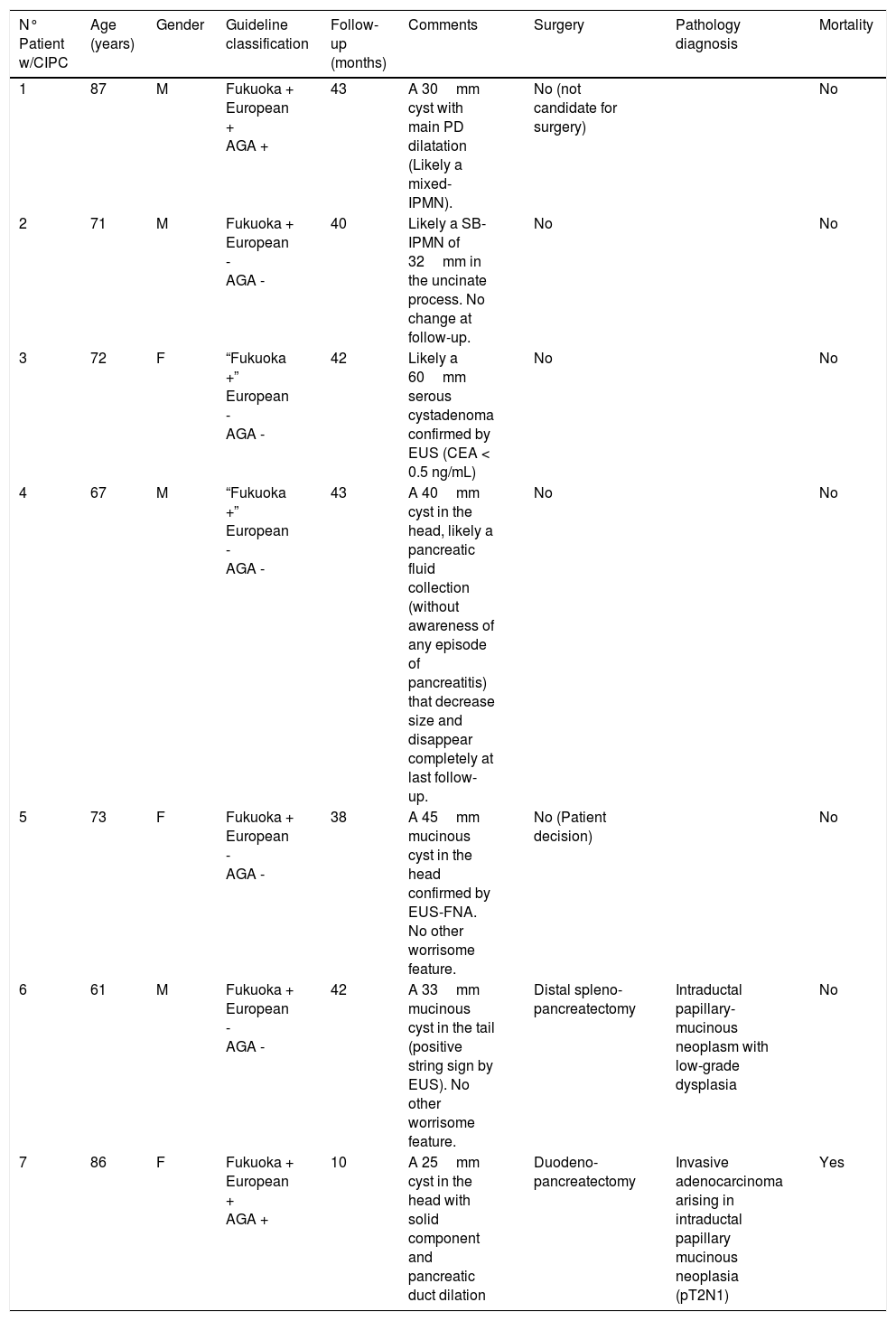
Table 3 summarizes the patients with CIPCs at the time of diagnosis and their follow-up. Four of these patients (6.7% of the PCs; 57% of the CIPCs) were determined to require surgery in the follow-up, but in the end only two patients underwent surgery (one patient was not candidate because of comorbidities and the other did not accept the surgery). One patient had a duodenopancreatectomy due to a PC with solid component and pancreatic duct dilatation in the head of the pancreas. Pathology showed an invasive adenocarcinoma arising in an intraductal papillary mucinous neoplasia (pT2N1) and patient died 10 months later. The other patient underwent a distal splenopancreatectomy due to a mucinous 33mm PC in the pancreatic tail previously confirmed by EUS-FNA. Pathology showed an intraductal papillary-mucinous neoplasm with low-grade dysplasia.
Follow-up and correlation with final pathologic findings in patients with CIPCs.
| N° Patient w/CIPC | Age (years) | Gender | Guideline classification | Follow-up (months) | Comments | Surgery | Pathology diagnosis | Mortality |
|---|---|---|---|---|---|---|---|---|
| 1 | 87 | M | Fukuoka + European + AGA + | 43 | A 30mm cyst with main PD dilatation (Likely a mixed-IPMN). | No (not candidate for surgery) | No | |
| 2 | 71 | M | Fukuoka + European - AGA - | 40 | Likely a SB-IPMN of 32mm in the uncinate process. No change at follow-up. | No | No | |
| 3 | 72 | F | “Fukuoka +” European - AGA - | 42 | Likely a 60mm serous cystadenoma confirmed by EUS (CEA < 0.5 ng/mL) | No | No | |
| 4 | 67 | M | “Fukuoka +” European - AGA - | 43 | A 40mm cyst in the head, likely a pancreatic fluid collection (without awareness of any episode of pancreatitis) that decrease size and disappear completely at last follow-up. | No | No | |
| 5 | 73 | F | Fukuoka + European - AGA - | 38 | A 45mm mucinous cyst in the head confirmed by EUS-FNA. No other worrisome feature. | No (Patient decision) | No | |
| 6 | 61 | M | Fukuoka + European - AGA - | 42 | A 33mm mucinous cyst in the tail (positive string sign by EUS). No other worrisome feature. | Distal spleno- pancreatectomy | Intraductal papillary-mucinous neoplasm with low-grade dysplasia | No |
| 7 | 86 | F | Fukuoka + European + AGA + | 10 | A 25mm cyst in the head with solid component and pancreatic duct dilation | Duodeno- pancreatectomy | Invasive adenocarcinoma arising in intraductal papillary mucinous neoplasia (pT2N1) | Yes |
Fukuoka + (presence of any worrisome feature or high risk stigmata); European + (presence of mural nodules/solid component or pancreatic duct dilation); AGA + (presence of at least two high risk features: size > 30mm, pancreatic duct dilation or solid component).
CIPC (clinically important pancreatic cyst)
M (male)/F (female)
AGA (American Gastroenterological Association)
SB-IPMN (side branch-intraductal papillary mucinous neoplasia)
During the follow-up of the other three patients initially considered to have CIPCs at the time of the diagnosis, it was shown that one patient probably had a serous cystadenoma, another probably had a pancreatic fluid collection that completely disappeared, and the latter likely a side branch-IPMN without any other worrisome feature rather than the size.
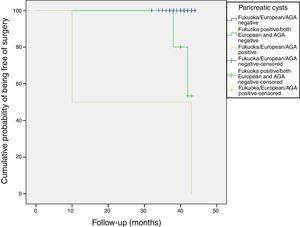
All patients were alive at the last follow-up, except for the patient who underwent the Whipple's surgery. Figure 4 shows the cumulative probability of being free of surgery based on these guidelines criteria. Patients with PCs initially classified as AGA or European positive had a higher surgical probability and this decision was taken earlier in the follow-up. PCs that were initially cataloged as “Fukuoka, AGA and European negative” remained stable without changes during the follow-up.
Taking into account the final diagnosis and excluding the two patients initially classified as “Fukuoka positive” that were not mucinous cysts at last follow-up, the prevalence of CIPCs was between 0.2% (95%CI 0.1-1) and 0.8% (95%CI 0.2-2) depending on the guideline chosen.
DiscussionMost patients with PCs are referred because of an incidental finding in a CT or MRI. Nowadays, it is relatively frequent to find PCs incidentally due to the current high-resolution cross-sectional images. The finding of PCs in an asymptomatic patient represents an immediate challenge to the radiologist and the clinician.1 PCs have variable potential malignancy and this is a source of discomfort for many physicians. Differentiating which PCs are or could be malignant is the key question. The pancreatobiliary EUS-FNA has become a powerful tool for the differential diagnosis of pancreatic cysts,10,11 however, it is still not a perfect tool. The best way to manage the patients with PCs is still controversial and different guidelines5–7 support for different approaches.
The Fukuoka,5 the AGA6 and the European7 guidelines are mostly chosen to manage these patients. The Fukuoka guideline5 defined the “worrisome features” and the “high risk stigmata” as important features in PCs, and recommended further evaluation (EUS-FNA and close surveillance with MRI or CT scans) or even surgery (if clinically appropriate and in young fit patients) if the patient has any of these features. The AGA guideline6 defined that asymptomatic patients with PCs should have at least two high risk features (size greater than 30mm, dilation of the pancreatic duct, or solid component) in order to consider them as having relevant alert signs, and recommended further evaluation or surgery only in these patients. And the European guideline7 established that the presence of symptoms related to the pancreas, mural nodules, dilation of the main pancreatic duct > 6mm, rapidly increasing size, or elevated serum levels of CA 19-9 were risk factors for the presence of malignancy in branch duct-intraductal papillary mucinous neoplasias.
In our study, we found a prevalence of PCs of 8.7% (95%CI 6.3-11.5) in the CT and 27.5% (95%CI 16-41) in the MRI, increasing up to 14.9% (95%CI 10-21) in patients older than 70 years imaged with CT scans, and up to 25.7% (95%CI 16-37) in patients older than 80 years.
Our crude prevalence of PCs was slightly higher than reported by other authors.2,12,13 Laffan et al.2 reported that the prevalence of unsuspected pancreatic cysts identified on multidetector CT (MDCT) was 2.6%, Chang YR et al.12 found that pancreatic cystic neoplasms were identified in 457 cases (2.1%) among 21745 individuals who underwent MDCT, and Zanini et al.13 reported that thirty-five patients out of 650 patients who underwent contrast-enhanced CT had at least one pancreatic cyst (5.4%).
We found a prevalence of relevant incidental PCs close to 1%. At the time of the initial diagnosis of the PCs, the prevalence of CIPCs were significantly higher using the Fukuoka criteria (1.2% vs 0.2%, p<0.01). This difference among the guidelines was mainly given by the size criteria when comparing Fukuoka vs European, and due to the two features criteria when comparing Fukuoka vs. AGA. However, after correcting those PCs that were not mucinous during the follow-up, the crude prevalence of CIPCs was between 0.2% (95%CI 0.01-1) and 0.8% (95%CI 0.2-2) depending on the guideline chosen.
Our definition of “clinically important or relevant” was based on the algorithms of the three most common guidelines used, and it was focused on the patient¿s needs, referring to the need of surveillance in a short interval (≤ 6 months), the need of more procedures (EUS-FNA) or even the need of surgery. It makes sense to think that if an invasive or aggressive approach is needed it will be clinically important to patients and physicians. We know this definition was somehow arbitrary given the variable potential malignancy of PCs (it is difficult to predict the behavior of PCs and PCs not considered clinically important could become clinically important during the follow-up), but we considered it was the best definition to highlight how many PCs might be clinically important at the time of the finding.
The sixty patients with incidental PCs were followed for a mean of 40 months (range: 32–44 months). PCs that were initially cataloged as “Fukuoka, AGA and European negative” remained stable without changes during the follow-up (Figure 4). However, we consider that 40 months (a little more than 3 years) is a short period to predict the real behavior of the cysts. Pergolini et al.14 recently published a retrospective analysis of patients with BD-IPMNs under surveillance, and they found that their overall risk of malignancy, almost 8%, lasted for 10 years or more, supporting continued surveillance after 5 years.
Another relevant topic to discuss is that PCs are more prevalent with aging. Similar to other findings,2,12,13 in our cohort, we found that the prevalence of PCs was significantly influenced by age (Table 2). We estimated that approximately 20% of the our population could have some type of pancreatic cyst at the age of 100 years (Figure 3). Prevalence of CIPCs at the time of the diagnosis based on Fukuoka guideline was also significantly influenced by age, increasing up to 2.1% (95%CI 0.6-5.3) in patients older than 70 years imaged with CT scans. With the aging of population and the arrival of new imaging technologies, we will probably see an increased number of patients with PCs to pay attention to, and we will likely have to monitor them for a longer period of time.
Beyond the differences among the mentioned guidelines, Kimura et al.15 published a very interesting study in 1995, given that they estimated a prevalence of PCs similar to actual publications, but, above all, they indirectly foretold the variable potential malignancy of PCs. The authors investigated the prevalence of PCs in 300 consecutive autopsy cases at the Department of Pathology, Tokyo Metropolitan Geriatric Hospital, from 1984 to 1986. They defined pancreatic cystic lesions as restricted dilatations of the pancreatic duct which were grossly recognizable and therefore greater than 2mm in diameter. Of the 300 autopsy cases, 186 cystic lesions were found in 73 pancreas (this represents a prevalence of 24.3%). Sixty-four percent of the cystic lesions were less than 4mm in diameter, 27% were 4-7mm, and 9% were larger than 7mm. Only 6 cases had carcinoma in situ inside the cysts; four (67%) of the six cystic lesions with carcinoma in situ were less than 4mm in diameter, and no invasive carcinomas were observed in the cysts. Although it is difficult to draw any conclusions and considering that the features proposed by these guidelines are surrogate outcomes for high grade dysplasia/cancer in situ and minimally invasive cancer, Kimura et al.15 showed a prevalence of incidental CIPCs of 2% (95%CI 0.7-4.3). The majority of these CIPCs reported by Kimura et al. were less than 4mm. Although it is hard to predict the malignant behavior of these cysts, they would have been likely missed by the current guidelines.
It seems that we have a long way ahead in order to understand the variable behavior of PCs. In addition, according to Chernyak et al.,4 incidental pancreatic cysts could be a risk factor to have metachronous pancreatic adenocarcinoma. Chernyak et al.4 designed a retrospective cohort study to assess the effect of incidental pancreatic cysts found on cross-sectional imaging (MR and CT scans) on the incidence of pancreatic adenocarcinoma. They analyzed 8052 patients (2034 patients in the cyst cohort and 6018 patients in the no-cyst cohort) and found that 72 patients were diagnosed with a pancreatic neoplasm after 6 months of the index date (38 patients in the cyst cohort and 34 in the no-cyst cohort). They concluded that incidental pancreatic cysts were associated with an overall increased risk of pancreatic adenocarcinoma (hazard ratio of 3.0, 95%CI 1.3-6.9, after adjusting for age, sex, and race). However, it is worthwhile mentioning that most of the adenocarcinoma arose from a portion of the pancreas different from the cyst location.
Limitations/weaknesses and strengths.As it is a retrospective study, a selection bias may be present. Radiologists were not aware of this study at that time, and small cysts considered irrelevant by them may have not been recorded. This fact could have caused an underestimation of the prevalence of PCs and, indirectly, a probable overestimation of the prevalence of CIPCs. However, one of the strengths of our study was that an extensive, broad and exhaustive search was performed in the Radiology Database with strict inclusion and exclusion criteria.
Other highlight point of our study is the original idea of considering the prevalence of CIPCs based on mostly used guidelines instead of focusing only on the prevalence of PCs. CIPCs are what really matters to physicians and patients. Unfortunately, our sample size in the MRI group was too small to show any significant difference among guidelines.
CONCLUSIONSThe prevalence of relevant incidental pancreatic cyst is not negligible according to current guidelines. In our cohort it was close to 1%, being slightly higher using the Fukuoka criteria. PCs that were initially cataloged as “Fukuoka, AGA and European negative” remained stable without changes during the follow-up. Only 11.6% (95%CI, 5-22) of the patients with PCs were considered to have CIPCs (7/60). Patients with PCs initially classified as AGA or European positive had a higher surgical probability and this decision was taken earlier in the follow-up.
Which guideline is the best to use is still controversial. Guideline selection should be based on local resources and the risks of the population. A well-conducted prospective cost-benefit analysis of these guidelines would clarify the best selection.
InstitutionGastroenterology and Endoscopy Unit, Internal Medicine Department, Hospital Alemán, Av. Pueyrredón 1640, CABA, CP 1118, Buenos Aires, Argentina
Author contributionsAll the authors solely contributed to this paper, helping to conduct the study, analyzing the data and writing the manuscript.
Supportive foundationsAuthors declared that there isn¿t any supportive foundation.
Institutional review board statementThe human ethics committee from our institution approved the protocol. Our hospital institutional review board approved the review of radiological and clinical data for this study. Informed consent was waived for this retrospective review study.
Informed consent statementInformed consent was waived for this retrospective review study.
Conflict-of-interest statementAll the Authors have no conflict of interest related to the manuscript.
Data sharing statementThe original anonymous dataset is available on request from the corresponding author at josemmella@hotmail.com
All authors approved the final version of the manuscript. Authors declared that there isn¿t any supportive foundation. Authors have no conflict of interest related to the manuscript.