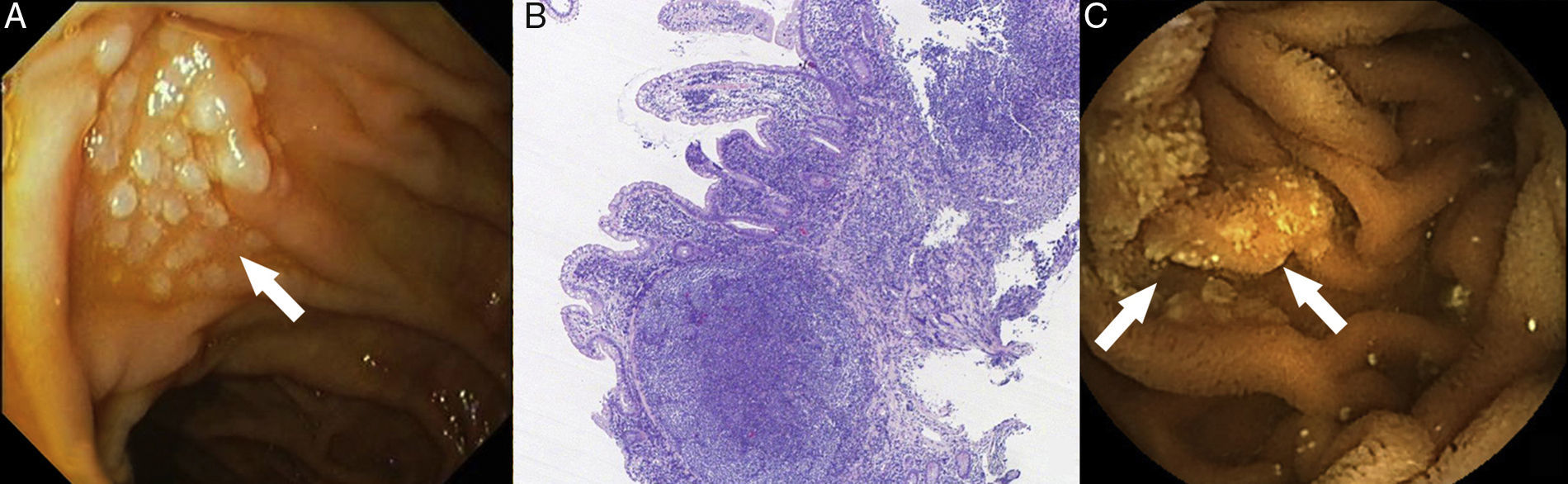
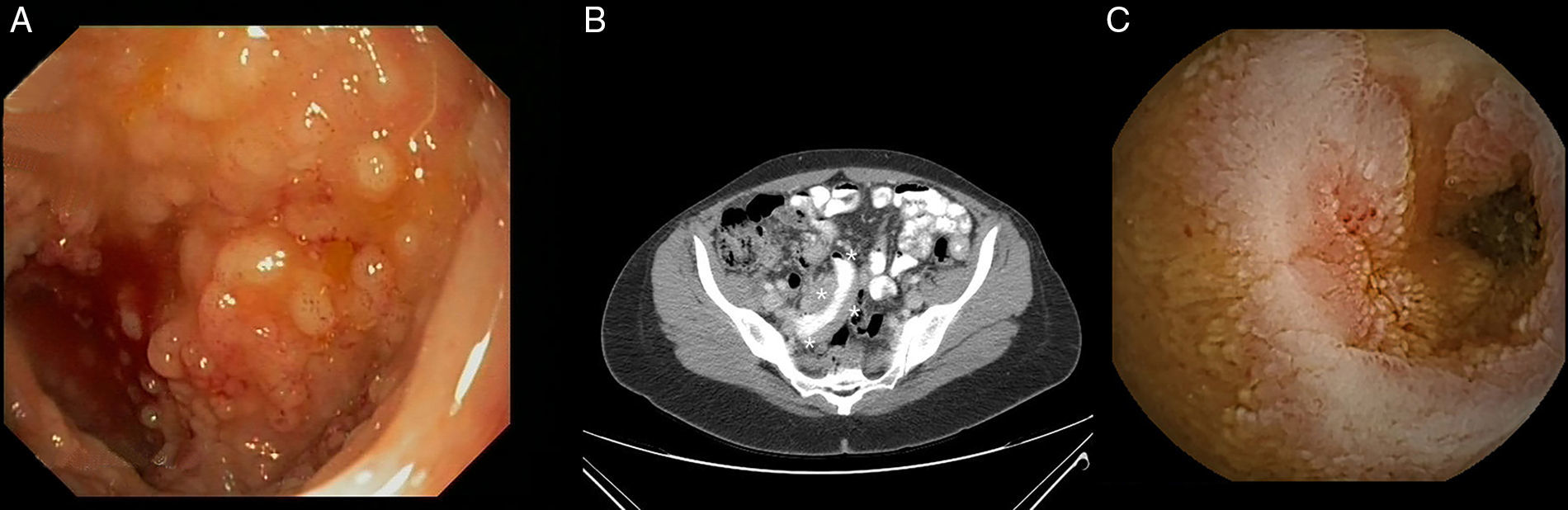
Primary lymphoma of the gastrointestinal tract is rare and is most commonly caused by secondary gastrointestinal invasion. Diffuse large cell lymphoma, MALT lymphoma and mantle cell lymphoma are the most common histological subtypes. Since 2008, the World Health Organisation has recognised a new lymphoma subtype, primary gastrointestinal follicular lymphoma (GI-FL). We present the cases of two patients diagnosed with primary GI-FL at our centre. The first case is a 56-year-old female carrier of an exon 12 mutation in the hMSH-2 gene that was discovered after surgery (hysterectomy with double adnexectomy) for ovarian adenocarcinoma 12 years previously (T1N0M0; TNM classification, 7th edition). Three years ago, she was diagnosed by chance with primary GI-FL of the duodenum after detecting a whitish nodular and elevated lesion close to the major duodenal papilla during a routine gastroscopy (Fig. 1A). The biopsies revealed B-cell lymphoid proliferation in the lamina propria comprising small and irregular lymphocytes and some isolated centroblasts. The immunohistochemical study was positive for the markers CD20, CD10, BCL-2 and BCL-6 and negative for CD3, CD5 and cyclin-D1 (Fig. 1B). The extension study (CT scan of the chest and abdomen, bone marrow biopsy and PET scan) ruled out distant cancer, although three further lesions of similar characteristics were found in the middle and distal jejunum and the terminal ileum during the capsule endoscopy (CE) (Fig. 1C). The patient achieved complete remission after four cycles of treatment with rituximab. A subsequent check-up two years later revealed endoscopic and histological recurrence, but without radiological progression. Given the patient's refusal to receive new treatment, conservative treatment was administered. Twelve months later, the patient remained stable and with no evidence of tumour progression. The second case concerns a 48-year-old woman who was admitted for a six-month history of asthenia, vomiting and abdominal pain caused by menstruation. A conventional endoscopic examination was performed (gastroscopy and ileocolonoscopy), which revealed some polypoid mucosal lesions in the terminal ileum consistent with lymphoid follicular hyperplasia (Fig. 2A). Given the persistent symptoms, an abdominal CT scan was performed, which found thickening of a short segment of the terminal ileum wall as well as regional and retroperitoneal lymphadenopathies, with no other relevant findings (Fig. 2B). The CE confirmed the radiological findings and also revealed a de novo mucosal stenosis that was impassable by the capsule (Fig. 2C). After two weeks of treatment with budesonide (9mg/24h), the device was expelled naturally without incident. The biopsies obtained previously were restudied, this time observing cell clusters with an immunophenotype consistent with primary GI-FL of the ileum. CHOP chemotherapy+rituximab was initiated, with radiological improvement observed on the control CT scan after two treatment cycles.
Primary GI-FL is a variant of nodal follicular lymphoma.1 It is a rare lymphoma, accounting for between 1% and 3.6% of all primary lymphomas of the gastrointestinal tract.2 Prevalence tends to be higher among middle-aged women. Although it is often asymptomatic (43%), the most common symptoms to manifest are abdominal pain (28%), nausea-vomiting (8%) and gastrointestinal bleeding (6%).3 They tend to be unifocal tumours and most commonly arise in the second part of the duodenum close to the ampulla of Vater. Nevertheless, as was the case with our first patient, multifocal cases of primary GI-FL have been reported. In such cases, capsule endoscopy, which is a harmless and well-tolerated procedure, may be used extremely effectively to detect and locate all synchronous lesions. It can also serve as a guide for biopsy when the conventional endoscopy is negative.4 Primary GI-FL often presents endoscopically as small polypoid lesions coated in normal mucosa. However, on rare occasions it manifests as ulcers, which can lead to stenosis of the intestinal lumen.5,6 From a histological standpoint, they present a pattern of nodular growth comprising germinal centres made of centrocytes and centroblasts with an immunophenotype that is typically positive for the markers CD19, CD20, CD22 and CD79a and negative for CD43, CD5 and cyclin-D1.7 The extension study should include an endoscopic examination, a CT scan of the chest and abdomen and/or PET scan, as well as a bone marrow biopsy. Both the Ann-Arbour and Lugano classifications are useful for tumour staging, with the Lugano classification being particularly relevant for multifocal primary GI-FL.8 As an optimal treatment strategy has yet to be established, this must be personalised to suit the particular characteristics of each individual patient. In asymptomatic patients with localised cancer, expectant management may be appropriate. However, treatment should be started as soon as patients manifest symptoms and/or disease progression based on the histological and clinical grade. Radiotherapy tends to be the treatment of choice in patients with localised disease and no poor prognostic factors, reserving monotherapy with rituximab (anti-CD20 monoclonal antibody) for select cases where the adverse effects of radiotherapy are to be avoided. If, on the other hand, the disease has spread or the patient presents poor prognostic factors, systemic chemotherapy (CHOP, CVP) with or without rituximab is indicated.9
Please cite this article as: Juanmartiñena Fernández JF, Fernández-Urién I, Iglesias Picazo R, Aznárez Barrio MR, Montes Díaz M, Cebrian García A, et al. Linfoma folicular primario gastrointestinal: hallazgos endoscópicos y papel de la enteroscopia con cápsula. Gastroenterol Hepatol. 2017;40:621–623.