The management of inflammatory bowel disease (IBD) is currently based on the objective evaluation of intestinal lesions. It would therefore be interesting to have access to simple and non-invasive tools to monitor IBD activity and to identify the presence of lesions. Faecal calprotectin (FC) is the main cytosolic protein of neutrophils, it is resistant to bacterial degradation and it is stable at room temperature for several days, characteristics that make it suitable for use in clinical practice. It can be used to differentiate between inflammatory and functional processes, it correlates with endoscopic activity, it is associated with clinical and endoscopic response to treatment and it has short-term prognostic value. This paper offers an up-to-date perspective on the information that FC can provide clinicians to aid diagnosis, monitoring and management of IBD.
Actualmente, el manejo de la enfermedad inflamatoria intestinal (EII) se basa en la evaluación objetiva de las lesiones intestinales. Por ello, es de interés disponer de herramientas sencillas y no invasivas con las que monitorizar la actividad de la EII e identificar la presencia de lesiones. La calprotectina fecal (CF) constituye la principal proteína citosólica de los neutrófilos, es resistente a la degradación bacteriana y estable a temperatura ambiente durante días, características que la hacen adecuada para su uso en la práctica clínica. Es útil para diferenciar entre procesos inflamatorios y funcionales, se correlaciona con la actividad endoscópica, se asocia con la respuesta clínica y endoscópica al tratamiento y tiene valor pronóstico a corto plazo. El presente documento pretende ofrecer una visión actualizada sobre la información que la CF puede proporcionar al clínico en el diagnóstico, la monitorización y el manejo de la EII.
Crohn's disease (CD) and ulcerative colitis (UC) are chronic, progressive inflammatory diseases characterised by alternating periods of activity and remission of unpredictable duration. Management of inflammatory bowel disease (IBD) is currently based on the objective assessment of intestinal lesions and, in general, decision-making guided purely by clinical symptoms is not considered appropriate. There are two reasons for this. First of all, gastrointestinal symptoms do not accurately reflect the presence or severity of gastrointestinal lesions. More than a third of patients in clinical remission have endoscopic lesions and, in more than 10% of symptomatic patients, the endoscopy is normal.1,2 It is therefore easy to see how making therapeutic decisions based purely on the symptoms can lead to serious errors. Secondly, improvement or disappearance of intestinal lesions is known to be associated with a less severe disease course, with less likelihood of complications or the need for hospitalisation or surgery.3,4 All this has renewed interest in endoscopy and imaging techniques in the assessment of patients with IBD. These methods provide valuable information about the severity and extent of lesions and the development of complications. However, in view of their high cost, limited availability and invasive nature, they are not suitable for periodic monitoring of the disease.
It would therefore be of great benefit to clinicians to have simple and non-invasive tools with which to monitor IBD activity and identify the presence of lesions. A number of serum biomarkers have been proposed, with the most widely used being C-reactive protein (CRP). However, CRP is nonspecific and can be elevated in extraintestinal inflammatory processes.5 An ideal biomarker should accurately distinguish between the existence and absence of lesions, and be related to their severity and the response to treatment. It should also be widely accessible, easy to use and affordable. To a greater or lesser extent, faecal calprotectin (FC) meets these requirements and it is presently the best characterised commercially available biomarker in IBD.
FC is a calcium-binding protein with antimicrobial, antiproliferative and pro-inflammatory properties. It is derived predominantly from neutrophils, of which it is the main cytosolic protein and, to a lesser extent, from monocytes and activated macrophages. FC is released in very early stages of the inflammatory process and its concentration in the stool is directly proportional to the presence of neutrophils in the intestinal lumen.6 FC levels show good correlation with the excretion of indium-111-labelled leucocytes7 and with the permeability of the intestinal mucosa.8 It is resistant to bacterial degradation and stable at room temperature for days, with these characteristics making it suitable for use in clinical practice.
The aim of this document is to provide an update on the utility of FC in patients with IBD in clinical practice.
Available methods for measuring faecal calprotectinWhat methods are available for determining faecal calprotectin?The most commonly used methods are enzyme-linked immunosorbent assay (ELISA) and lateral-flow immunochromatography, which is used in so-called “rapid tests”. The antibodies used in both techniques can be polyclonal or monoclonal. The kits that use monoclonal antibodies are preferable as they have shown greater precision.9,10
The ELISA tests are the most validated, are cheaper and provide a quantitative result that usually covers a wider range of values. However, they require a specialised laboratory and several dozen samples have to be accumulated in order to make the cost of each determination affordable, with the consequent delay in obtaining the results. In contrast, immunochromatographic tests (rapid tests) have the advantages of not requiring a laboratory and each sample being analysed individually, with the result available in a few minutes. Using a reader with the appropriate software, some of immunochromatographic tests can provide a quantitative result that correlates very well with the result obtained by ELISA.11,12 Others provide a semiquantitative or qualitative result quickly, easily and cheaply, although less evidence is available on their diagnostic accuracy.
Kits have also recently been developed with a rapid faecal sample preparation device which combines the immunochromatographic technique with a smartphone-specific application, allowing reading from the phone itself and sending the result to a server to which the treating physician has access.13,14
In centres that process a large number of samples, the ELISA technique would be the best option, as it provides results with a wider range, is more economical and has shown greater accuracy. When an immediate result is required, or there are too few samples for the ELISA test to provide the result in a reasonable amount of time, quantitative rapid tests could be a good alternative. Although experience with this type of test is more limited, some of these kits have demonstrated a reliability similar to that of the ELISA tests in the prediction of endoscopic lesions.11,12
The semiquantitative or qualitative rapid tests are attractive for their simplicity and low cost. They have shown good accuracy for the differential diagnosis between IBD and noninflammatory pathology, so they could be used for this purpose. However, data are limited on their ability to identify endoscopic lesions in patients with IBD. An important drawback of this type of test is the loss of information that dichotomising a quantitative variable involves. There is a compromise between sensitivity and specificity along the continuum of FC values; different cut-off points may be considered optimal in different circumstances or indications, depending on whether we prefer to maximise sensitivity or specificity.
Are the results comparable with the different measurement techniques or commercial brands?Considerable variability has been demonstrated in the results obtained with different commercial kits, whether or not they use the same technique15–18 (ELISA, immunochromatography). This means that a sample may be above or below a certain cut-off point depending on the kit chosen, highlighting the urgent need to standardise the procedure. In the meantime, each manufacturer should determine their own reference limits or provide information about which kit their product has calibrated. For the same reason, it is not advisable to compare results obtained with different kits in the same patient.
In contrast, the results obtained with different techniques (ELISA vs quantitative rapid test) from the same manufacturer have shown good correlation.11–14,19
Basic rules for collecting samplesWhen and how should we take samples?The recommendation is to collect a small amount of stool (approximately 3–5g, equivalent to one coffee spoon) and place it in a collection bottle, usually dispensed by the requesting centre. These containers do not require any specific treatment. The sample can be taken from any part of the stool, as FC is known to be uniformly distributed.20
There is still no consensus on the ideal time of day for sample collection. It had been suggested that the samples obtained from the first stool of the day might be the most suitable.20 However, a rigorous study conducted in our area designed specifically to clarify this point showed the time of day to be irrelevant.21
A marked decrease in FC levels has been found during preparation for a colonoscopy.22 Therefore, if the patient has one scheduled, the sample should be collected before starting bowel cleansing or several days after the procedure.
Can we store the sample or do we have to process it immediately?Once the sample is collected, it can be kept at room temperature for three days; subsequently, the levels tend to decrease.20 If the sample is not going to be analysed immediately, it can be stored for up to one week at 2–8°C or up to 12 months at −20°C, according to most manufacturers.
Apart from inflammatory bowel disease, what other circumstances can elevate faecal calprotectin?There are several factors that can affect FC levels. Various studies have shown that non-steroidal anti-inflammatory drugs (NSAIDs) can elevate FC in asymptomatic patients, probably due to the gastrointestinal damage caused by these treatments.23–26 In healthy volunteers who received diclofenac for two weeks, FC increased in a quarter of the cases. However, in most the FC increase was modest (<100μg/g), many returned to normal during the treatment and all had returned to normal two weeks after it ended.26 Nevertheless, stopping NSAIDs two weeks prior to measuring FC is recommended. Otherwise, the possibility of a positive result being caused by NSAID treatment will have to be taken into account.
When aspirin is used as an antithrombotic drug at a dose of 100mg per day, it does not seem to have a clinically significant effect on FC levels.27 Although a significant increase in FC has been found in healthy volunteers who received 100mg of aspirin daily, the maximum levels reached were low (<60μg/g).28 With the information available to date, and taking into account the importance of antithrombotic treatment in at-risk patients, the withdrawal of aspirin treatment when it is deemed necessary to determine FC is not justified.
Proton pump inhibitors increase the risk of intestinal lesions in users of NSAIDs,29 but very little data is available on the impact these drugs can have on FC levels. In a study published in the form of a letter, treatment with proton pump inhibitors was associated with a rise in FC above normal levels.30 However, the evidence is insufficient to make a formal recommendation.
Age can affect FC levels. Healthy children under the age of 4 have higher FC concentrations than adults, often from 50 to 250μg/g.31,32 In contrast, in a healthy adult population the concentration of FC increases with age, although within levels considered as normal (<50μg/g).33 Obesity, sedentary lifestyle and a diet low in fibre have also been associated with higher levels of FC, but also still normal (<50μg/g), and are therefore factors with no clinical relevance which do not affect the accuracy of the test.33
Last of all, we have to keep in mind the fact that any inflammatory condition of the intestine, such as infections or diverticulitis, can elevate FC.34–36 It has been suggested that measuring FC could be useful in the assessment of acute diarrhoea to differentiate between bacterial or viral origin. Markedly high values would point to bacterial aetiology and help select the patients who would most benefit from having a rectal swab culture.37,38 More studies would be necessary to identify the optimal cut-off points for this purpose.
Faecal calprotectin in the diagnosis of inflammatory bowel diseaseCan faecal calprotectin help us in the differential diagnosis of a patient with gastrointestinal symptoms?Gastrointestinal symptoms are common in the general population and not very specific to organic disease. Therefore, basing the decision of whether or not to perform endoscopic examinations to rule out organic disease only on the patient's symptoms is not an efficient method. The value of FC for distinguishing between functional and organic gastrointestinal symptoms has been analysed in numerous studies. A meta-analysis that included 2475 patients found the sensitivity and specificity for differentiating organic from functional disease to be 83% and 84%, respectively.39 The main drawback of FC in this context is its low accuracy for detecting colorectal cancer (CRC).40 For that reason, in a study population at risk of CRC (e.g., patient aged >50 or with a family history of CRC) FC will not be the most appropriate test. However, in a context of low CRC risk (e.g., population aged below 50) FC can be a very valuable tool for distinguishing between IBD and irritable bowel syndrome. The two disorders have similar symptoms and performing endoscopic examinations to differentiate them can be costly, invasive and inefficient. A meta-analysis which included 13 studies with 1041 patients (670 adults and 371 children) demonstrated a sensitivity of 93% and specificity of 96% for the identification of IBD in adults. In the paediatric population, the sensitivity was similar, but there was lower specificity (76%).41
A recent study measured FC levels in 895 patients aged from 18 to 50 with gastrointestinal symptoms42; 10% were diagnosed with IBD. The area under the ROC curve of faecal calprotectin concentrations to distinguish between IBD and functional disease was 0.97. In order to maximise sensitivity, by combining FC levels and five alarm symptoms (rectal bleeding, bloody diarrhoea, nocturnal symptoms, weight loss and anaemia) a sensitivity of 100% and a specificity of 55% were obtained.
FC can therefore be considered as an adequate test for identifying symptomatic patients with a high likelihood of organic disease who will then need additional investigations, especially in a population at low risk of CRC. This could be particularly useful in primary care as a screening method to decide on colonoscopy or specialist referral.
The most accepted FC cut-off point in this clinical context is 50μg/g. A systematic review which included 28 studies evaluated the diagnostic accuracy of FC for distinguishing between IBD and irritable bowel syndrome. Using this FC threshold, an overall sensitivity of 93% (range 83–100%) and a specificity of 94% (range 60–100%) were obtained. In the paediatric population, with this same cut-off point, the sensitivity ranged from 95% to 100% and the specificity from 44% to 93%.43
However, in the study by Kennedy et al.,42 with an FC cut-off point of 100μg/g, the sensitivity was 96% and the negative predictive value 99%, very similar to that obtained with a cut-off point of 50μg/g (97 and 99%, respectively), significantly improving the positive predictive value (from 37% to 54%) and the specificity (from 74% to 87%). Along the same lines, in a recent study carried out in primary care in 789 young patients, an FC ≥100μg/g differentiated between functional disease and IBD with positive and negative predictive values of 49% and 99% respectively.44 It should be noted that this study included 311 patients with clinical signs of alarm in which FC maintained a high negative predictive value (98%).
Therefore, in the differential diagnosis of young patients with gastrointestinal symptoms, it seems reasonable not to indicate invasive tests if the FC is less than 100μg/g. With values from 100 to 150μg/g, a repeat FC should be considered within a few weeks. Lastly, with values above 150μg/g it would be prudent to indicate additional tests.
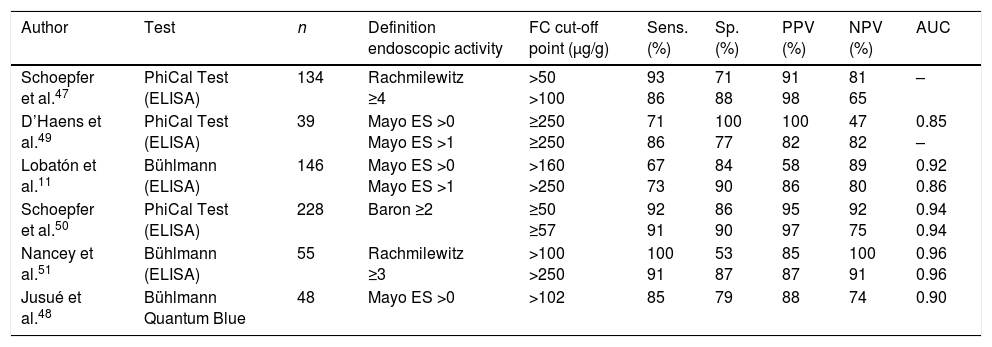
Faecal calprotectin as biomarker in ulcerative colitisIs faecal calprotectin a reliable endoscopic activity marker in ulcerative colitis?Numerous studies have shown that FC is a reliable marker of endoscopic activity in UC, and this has been confirmed by two recent meta-analyses.45,46 For this particular purpose, FC is superior to CRP and other faecal biomarkers.11,47,48 Overall, the sensitivity and specificity obtained in these studies were 80–90% and 70–80%, respectively, depending on the cut-off point used (Table 1).11,45–50 In the majority of studies, FC has been shown to correlate not only with the presence or absence of endoscopic activity, but also with the degree of activity assessed by different endoscopic indices,11,47–51 although the optimal cut-off point for identifying serious lesions has not been defined.
Diagnostic accuracy of faecal calprotectin in the identification of endoscopic activity in ulcerative colitis.
| Author | Test | n | Definition endoscopic activity | FC cut-off point (μg/g) | Sens. (%) | Sp. (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|---|---|---|
| Schoepfer et al.47 | PhiCal Test (ELISA) | 134 | Rachmilewitz ≥4 | >50 >100 | 93 86 | 71 88 | 91 98 | 81 65 | – |
| D’Haens et al.49 | PhiCal Test (ELISA) | 39 | Mayo ES >0 Mayo ES >1 | ≥250 ≥250 | 71 86 | 100 77 | 100 82 | 47 82 | 0.85 – |
| Lobatón et al.11 | Bühlmann (ELISA) | 146 | Mayo ES >0 Mayo ES >1 | >160 >250 | 67 73 | 84 90 | 58 86 | 89 80 | 0.92 0.86 |
| Schoepfer et al.50 | PhiCal Test (ELISA) | 228 | Baron ≥2 | ≥50 ≥57 | 92 91 | 86 90 | 95 97 | 92 75 | 0.94 0.94 |
| Nancey et al.51 | Bühlmann (ELISA) | 55 | Rachmilewitz ≥3 | >100 >250 | 100 91 | 53 87 | 85 87 | 100 91 | 0.96 0.96 |
| Jusué et al.48 | Bühlmann Quantum Blue | 48 | Mayo ES >0 | >102 | 85 | 79 | 88 | 74 | 0.90 |
AUC: area under the ROC curve; ES: endoscopic subscore; FC: faecal calprotectin; NPV: negative predictive value; PPV: positive predictive value; Sens.: sensitivity; Sp.: specificity.
Sensitivity, specificity and predictive values were calculated considering as “true positive” the patient with activity and FC>the cut-off point.
The extent of the disease seems to have little influence on FC levels, less than the severity of endoscopic lesions.49,52,53 In two studies, one Spanish and the other Belgian, the extension of colitis was significantly related to FC concentration in the univariate analysis. However, in the multivariate analysis, after adjusting for extension and severity of endoscopic activity, only the severity continued to be statistically significant.11,54
FC is therefore a highly reliable biomarker for detecting endoscopic activity in UC.
What cut-off points are indicative of endoscopic remission in ulcerative colitis?The cut-off point will depend on the definition of endoscopic remission and what compromise between sensitivity and specificity is decided on (Table 1). In general, endoscopic remission is usually accepted, in addition to completely normal mucosa or the presence of mild changes without erosions or spontaneous bleeding (Mayo endoscopic subscore 0 or 1). With this definition of endoscopic remission, the most appropriate cut-off point would be 250μg/g.11,49,51
However, if a stricter definition of remission is considered, such as completely normal mucosa (Mayo endoscopic subscore 0), the cut-off point will be lower. Although there is less evidence on this aspect, a cut-off point between 100 and 150μg/g has shown very good diagnostic accuracy.11,48
Can faecal calprotectin predict histological activity in ulcerative colitis?One of the most interesting characteristics of FC is its ability to detect intestinal inflammation early, even before endoscopic changes have occurred.6
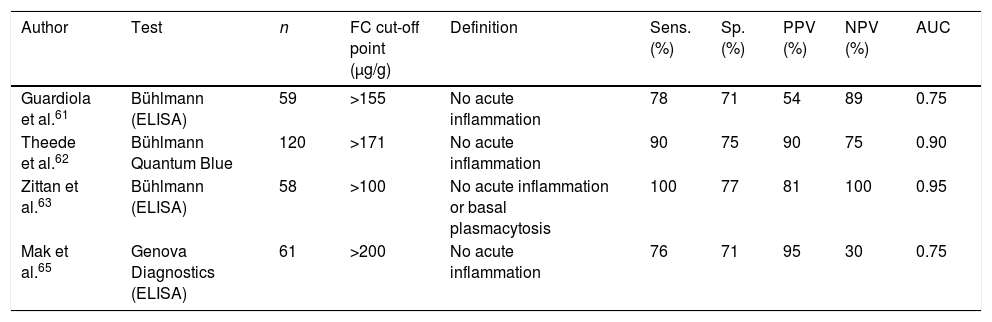
In UC, the mucosa often does not completely return to normal histologically, even in patients who achieve clinical and endoscopic remission. There is growing evidence that persistent microscopic inflammation, even in the absence of endoscopic lesions, is associated with an increased risk of recurrence.55–58 Added to that, the severity of the inflammation is an important determinant of the risk of colorectal cancer.59,60 For these reasons, it could be useful to have a non-invasive method for estimating histological activity. Several studies have shown that FC can identify histological remission in patients with UC to an acceptably accurate degree, with a sensitivity of 76–100% and specificity of 71–77%, respectively61–65 (Table 2). The cut-off points proposed for this purpose are between 100 and 170μg/g.
Diagnostic accuracy of faecal calprotectin in the identification of histological remission in UC.
| Author | Test | n | FC cut-off point (μg/g) | Definition | Sens. (%) | Sp. (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|---|---|---|
| Guardiola et al.61 | Bühlmann (ELISA) | 59 | >155 | No acute inflammation | 78 | 71 | 54 | 89 | 0.75 |
| Theede et al.62 | Bühlmann Quantum Blue | 120 | >171 | No acute inflammation | 90 | 75 | 90 | 75 | 0.90 |
| Zittan et al.63 | Bühlmann (ELISA) | 58 | >100 | No acute inflammation or basal plasmacytosis | 100 | 77 | 81 | 100 | 0.95 |
| Mak et al.65 | Genova Diagnostics (ELISA) | 61 | >200 | No acute inflammation | 76 | 71 | 95 | 30 | 0.75 |
AUC: area under the ROC curve; FC: faecal calprotectin; NPV: negative predictive value; PPV: positive predictive value; Sens.: sensitivity; Sp.: specificity.
Sensitivity, specificity and predictive values were calculated considering as “true positive” the patient with activity and FC>the cut-off point.
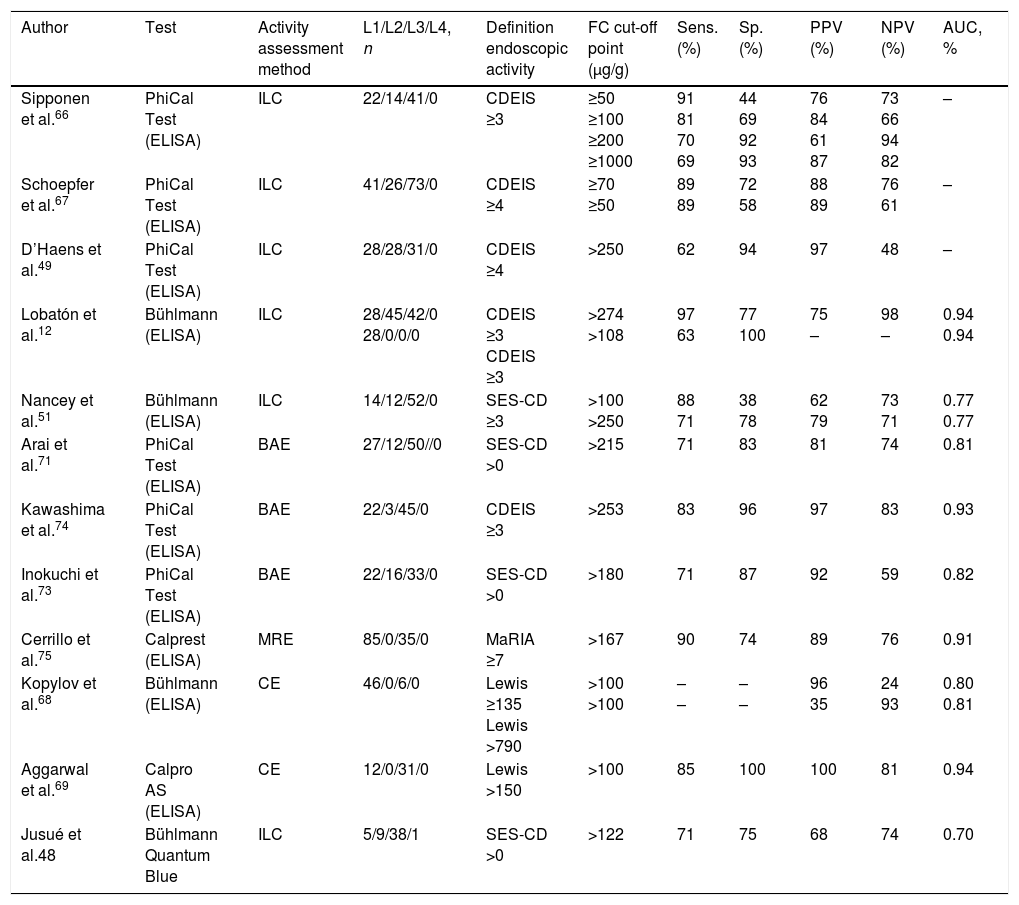
The studies carried out to date and two recent meta-analyses show that there is good correlation between the FC concentration and the endoscopic activity of CD assessed by different endoscopic indices, such as the Crohn's Disease Endoscopic Index of Severity (CDEIS), the Simple Endoscopic Score for Crohn's Disease (SES-CD) or the Lewis score (Table 3).12,45,46,49,51,66–69 The correlation is much higher than that between the clinical activity scores and CRP.12,48,67 According to a recent meta-analysis, the area under the ROC curve for the prediction of endoscopic activity is around 0.85.46 FC is also the only marker to have shown to discriminate between remission and mild, moderate and severe activity.12,67
Diagnostic accuracy of faecal calprotectin in the identification of endoscopic activity in Crohn's disease.
| Author | Test | Activity assessment method | L1/L2/L3/L4, n | Definition endoscopic activity | FC cut-off point (μg/g) | Sens. (%) | Sp. (%) | PPV (%) | NPV (%) | AUC, % |
|---|---|---|---|---|---|---|---|---|---|---|
| Sipponen et al.66 | PhiCal Test (ELISA) | ILC | 22/14/41/0 | CDEIS ≥3 | ≥50 ≥100 ≥200 ≥1000 | 91 81 70 69 | 44 69 92 93 | 76 84 61 87 | 73 66 94 82 | – |
| Schoepfer et al.67 | PhiCal Test (ELISA) | ILC | 41/26/73/0 | CDEIS ≥4 | ≥70 ≥50 | 89 89 | 72 58 | 88 89 | 76 61 | – |
| D’Haens et al.49 | PhiCal Test (ELISA) | ILC | 28/28/31/0 | CDEIS ≥4 | >250 | 62 | 94 | 97 | 48 | – |
| Lobatón et al.12 | Bühlmann (ELISA) | ILC | 28/45/42/0 28/0/0/0 | CDEIS ≥3 CDEIS ≥3 | >274 >108 | 97 63 | 77 100 | 75 – | 98 – | 0.94 0.94 |
| Nancey et al.51 | Bühlmann (ELISA) | ILC | 14/12/52/0 | SES-CD ≥3 | >100 >250 | 88 71 | 38 78 | 62 79 | 73 71 | 0.77 0.77 |
| Arai et al.71 | PhiCal Test (ELISA) | BAE | 27/12/50//0 | SES-CD >0 | >215 | 71 | 83 | 81 | 74 | 0.81 |
| Kawashima et al.74 | PhiCal Test (ELISA) | BAE | 22/3/45/0 | CDEIS ≥3 | >253 | 83 | 96 | 97 | 83 | 0.93 |
| Inokuchi et al.73 | PhiCal Test (ELISA) | BAE | 22/16/33/0 | SES-CD >0 | >180 | 71 | 87 | 92 | 59 | 0.82 |
| Cerrillo et al.75 | Calprest (ELISA) | MRE | 85/0/35/0 | MaRIA ≥7 | >167 | 90 | 74 | 89 | 76 | 0.91 |
| Kopylov et al.68 | Bühlmann (ELISA) | CE | 46/0/6/0 | Lewis ≥135 Lewis >790 | >100 >100 | – – | – – | 96 35 | 24 93 | 0.80 0.81 |
| Aggarwal et al.69 | Calpro AS (ELISA) | CE | 12/0/31/0 | Lewis >150 | >100 | 85 | 100 | 100 | 81 | 0.94 |
| Jusué et al.48 | Bühlmann Quantum Blue | ILC | 5/9/38/1 | SES-CD >0 | >122 | 71 | 75 | 68 | 74 | 0.70 |
AUC: area under the ROC curve; BAE: balloon-assisted enteroscopy; CDEIS: Crohn's Disease Endoscopic Index of Severity; CE: capsule endoscopy; FC: faecal calprotectin; ILC: ileocolonoscopy; L1-L4: location of the disease according to the Montreal classification; MaRIA: Magnetic Resonance Index of Activity; MRE: magnetic resonance enterography; NPV: negative predictive value; PPV: positive predictive value; Sens.: sensitivity; SES-CD: Simple Endoscopic Score for Crohn's Disease; Sp.: specificity.
Sensitivity, specificity and predictive values were calculated considering as “true positive” the patient with activity and FC>the cut-off point.
In contrast to UC, there is no clearly established cut-off point in CD, partly because of the lack of a well-defined concept of remission for each of the endoscopic scores. The published studies propose cut-off points ranging from 70 to 270μg/g.12,48,49,51,66,67 In all cases there is a sensitivity of around 70–80% and a specificity of around 80–97%.
A recent meta-analysis suggests that the best compromise between sensitivity and specificity for detecting clinically relevant endoscopic lesions is obtained with a cut-off point of 250μg/g, which has a sensitivity of 80% and specificity of 82% (area under the ROC curve 0.89).45
Is faecal calprotectin reliable as a marker of endoscopic activity in Crohn's disease only affecting the ileum?There is no unanimous agreement on whether or not the location of the CD affects the accuracy of FC to predict the presence of endoscopic lesions. While in some studies the accuracy is similar in the different locations,70,71 in most the correlation between FC and endoscopic activity is lower in ileal disease than in colonic or ileal-colonic disease.12,66,67,72 The validity of the above results is limited by the fact that the examination of the ileum in these studies was performed by ileocolonoscopy and was therefore incomplete (as it is not possible to visualise stretches of proximal small intestine).
Six recent studies specifically address this issue through a complete study of the ileum, three with balloon-assisted enteroscopy,71,73,74 one with magnetic resonance imaging75 and two with capsule endoscopy68,69 (Table 3). All six studies suggest that FC is a reliable marker of ileal endoscopic activity, although to a lesser extent than in colonic disease. However, the total number of patients included with disease localised purely in the small intestine is still small and does not allow definitive conclusions to be made.
What cut-off points are indicative of endoscopic remission in Crohn's disease only affecting the ileum?The value of FC in association with endoscopic remission in CD only affecting the ileum is lower than that associated with colonic or ileal-colonic disease.12,73,74 The level of 150μg/g has been proposed as a cut-off point with an optimal compromise to achieve a sensitivity of 85% and a specificity of 81%.70 This figure is consistent with the results of Cerrillo et al.,75 according to which a cut-off point of 167μg/g makes it possible to predict ileal activity assessed by magnetic resonance imaging with a sensitivity of 90% and a specificity of 74%. Other studies propose a slightly lower cut-off of 100μg/g.12,69 Further studies are needed to confirm these cut-off points.
Is faecal calprotectin reliable as a marker of post-surgical recurrence in Crohn's disease?Monitoring for postoperative recurrence is one of the most desirable situations for the use of biomarkers in CD. A simple, reliable and accurate non-invasive marker capable of detecting recurrent lesions could be an alternative to endoscopy in the follow-up of patients post-surgery.
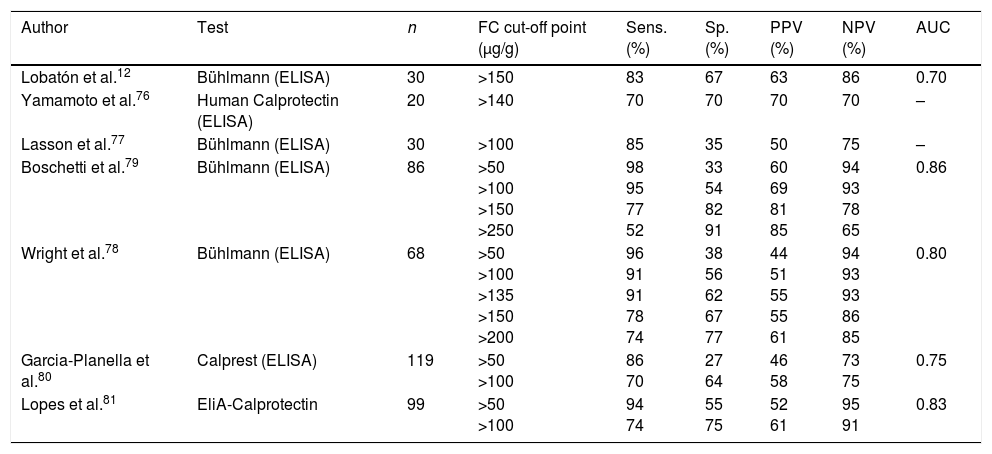
A number of prospective studies examined the role of FC in this scenario12,76–81 (Table 4). All reported that FC has a high sensitivity and negative predictive value in the identification of postoperative recurrence defined as the presence of lesions ≥Rutgeerts score i2. FC can therefore rule out recurrence with a high degree of reliability and could be a very useful marker in the monitoring of these patients after surgery. It has to be borne in mind that FC values can remain high in the first three months after surgery, so measuring levels during that period is not useful.82
Diagnostic accuracy of faecal calprotectin in the identification of post-surgical endoscopic recurrence in Crohn's disease (Rutgeerts Score ≥i2).
| Author | Test | n | FC cut-off point (μg/g) | Sens. (%) | Sp. (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|---|---|
| Lobatón et al.12 | Bühlmann (ELISA) | 30 | >150 | 83 | 67 | 63 | 86 | 0.70 |
| Yamamoto et al.76 | Human Calprotectin (ELISA) | 20 | >140 | 70 | 70 | 70 | 70 | – |
| Lasson et al.77 | Bühlmann (ELISA) | 30 | >100 | 85 | 35 | 50 | 75 | – |
| Boschetti et al.79 | Bühlmann (ELISA) | 86 | >50 >100 >150 >250 | 98 95 77 52 | 33 54 82 91 | 60 69 81 85 | 94 93 78 65 | 0.86 |
| Wright et al.78 | Bühlmann (ELISA) | 68 | >50 >100 >135 >150 >200 | 96 91 91 78 74 | 38 56 62 67 77 | 44 51 55 55 61 | 94 93 93 86 85 | 0.80 |
| Garcia-Planella et al.80 | Calprest (ELISA) | 119 | >50 >100 | 86 70 | 27 64 | 46 58 | 73 75 | 0.75 |
| Lopes et al.81 | EliA-Calprotectin | 99 | >50 >100 | 94 74 | 55 75 | 52 61 | 95 91 | 0.83 |
AUC: area under the ROC curve; FC: faecal calprotectin; NPV: negative predictive value; PPV: positive predictive value; Sens.: sensitivity; Sp.: specificity.
Sensitivity, specificity and predictive values were calculated considering as “true positive” the patient with activity and FC>the cut-off point.
The larger studies carried out in this context agree that the optimal cut-off point for the prediction of endoscopic recurrence (Rutgeerts ≥i2) is 100μg/g.78–81 This value has been associated with a sensitivity and negative predictive value above 90%. In clinical practice, FC values below 100μg/g can be considered as meaning postsurgical recurrence is very unlikely and so avoid the need for colonoscopy. In the case of high values, however, endoscopic confirmation of recurrence would be advisable before making therapeutic decisions, as FC has limited specificity in this scenario. Repeated determinations might improve their accuracy, but the optimal frequency of testing has yet to be determined. A prospective study evaluated the determination of FC every two months for two years following an initial ileocolonoscopy without recurrent lesions.83 None of the patients with FC consistently below 140μg/g had advanced recurrence (i3-i4), and only 10% had mild recurrence (i2) at 24 months. As long as we have no objective evidence with which to establish the most cost-effective interval between FC determinations in CD follow-up after bowel resection, it is reasonable to measure FC every 4–6 months, as suggested by the Spanish Working Group on Crohn's Disease and Ulcerative Colitis (Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa; GETECCU).84
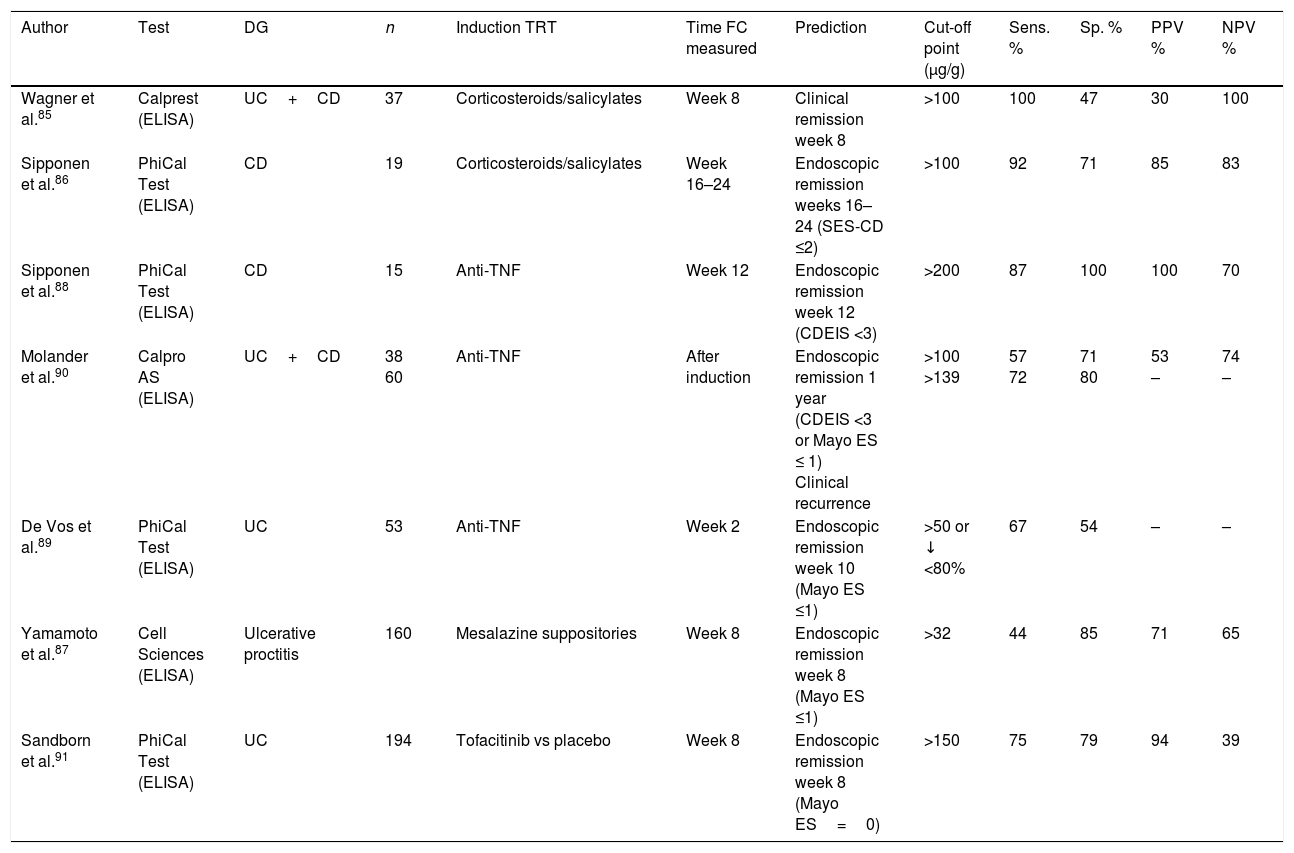
Faecal calprotectin in monitoring of response to treatmentAre faecal calprotectin levels associated with the success or failure of remission induction therapy in inflammatory bowel disease?In patients with active IBD and high levels of FC treated conventionally with corticosteroids or salicylates, the return to normal of FC (<100μg/g) is associated with a high probability of clinical and endoscopic remission.85,86 FC has also been shown to be effective as a marker of mucosal healing after treatment with mesalazine suppositories in patients with mild to moderate ulcerative proctitis.87 FC is also a good predictor of recurrence in these patients, as it increases about eight weeks before the onset of symptoms.87 The prognostic value of FC during hospital admission in patients with severe UC, when FC is extremely high, is limited by the great variability in FC over the course of the day.21 In patients with IBD treated with anti-TNF drugs, FC levels are significantly reduced and return to normal in the majority of those who achieve endoscopic remission, both during induction and maintenance,88–90 with FC being a better marker than the clinical scores. In a post hoc analysis of a clinical trial evaluating the efficacy of tofacitinib in UC, a close relationship was also found between FC levels and endoscopic remission.91
Consequently, FC levels seem to return to normal when treatment, whatever it is, achieves mucosal healing (Table 5).
Diagnostic accuracy of faecal calprotectin in monitoring treatment response.
| Author | Test | DG | n | Induction TRT | Time FC measured | Prediction | Cut-off point (μg/g) | Sens. % | Sp. % | PPV % | NPV % |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Wagner et al.85 | Calprest (ELISA) | UC+CD | 37 | Corticosteroids/salicylates | Week 8 | Clinical remission week 8 | >100 | 100 | 47 | 30 | 100 |
| Sipponen et al.86 | PhiCal Test (ELISA) | CD | 19 | Corticosteroids/salicylates | Week 16–24 | Endoscopic remission weeks 16–24 (SES-CD ≤2) | >100 | 92 | 71 | 85 | 83 |
| Sipponen et al.88 | PhiCal Test (ELISA) | CD | 15 | Anti-TNF | Week 12 | Endoscopic remission week 12 (CDEIS <3) | >200 | 87 | 100 | 100 | 70 |
| Molander et al.90 | Calpro AS (ELISA) | UC+CD | 38 60 | Anti-TNF | After induction | Endoscopic remission 1 year (CDEIS <3 or Mayo ES ≤ 1) Clinical recurrence | >100 >139 | 57 72 | 71 80 | 53 – | 74 – |
| De Vos et al.89 | PhiCal Test (ELISA) | UC | 53 | Anti-TNF | Week 2 | Endoscopic remission week 10 (Mayo ES ≤1) | >50 or ↓ <80% | 67 | 54 | – | – |
| Yamamoto et al.87 | Cell Sciences (ELISA) | Ulcerative proctitis | 160 | Mesalazine suppositories | Week 8 | Endoscopic remission week 8 (Mayo ES ≤1) | >32 | 44 | 85 | 71 | 65 |
| Sandborn et al.91 | PhiCal Test (ELISA) | UC | 194 | Tofacitinib vs placebo | Week 8 | Endoscopic remission week 8 (Mayo ES=0) | >150 | 75 | 79 | 94 | 39 |
AUC: area under the ROC curve; CD: Crohn's disease; CDEIS: Crohn's Disease Endoscopic Index of Severity; ES: endoscopic subscore; FC: faecal calprotectin; NPV: negative predictive value; PPV: positive predictive value; Sens.: sensitivity; SES-CD: Simple Endoscopic Score for Crohn's Disease; Sp.: specificity; UC: ulcerative colitis.
Sensitivity, specificity and predictive values were calculated considering as “true positive” the patient with activity and FC>the cut-off point.
Based on the current evidence, monitoring FC can be useful in assessing the therapeutic response. A recent randomised clinical trial in patients with CD assessed the monitoring of FC and CRP every three months as indicators for adjusting anti-TNF therapy.92 The group in which these biomarkers were used in conjunction with the assessment of symptoms obtained better clinical results and higher rates of endoscopic remission than the group in which the treatment optimisation was based purely on symptoms. These findings highlight the role FC can play in identifying subclinical inflammation and the need to monitor patients with objective criteria.
Table 5 shows the diagnostic accuracy of FC in the prediction of therapeutic response in different scenarios. According to these studies, it can be useful to determine FC before the induction treatment and at 8–12 weeks, although earlier determinations could have short-term prognostic value. In general, values below 100–150μg/g are associated with a good response to treatment.
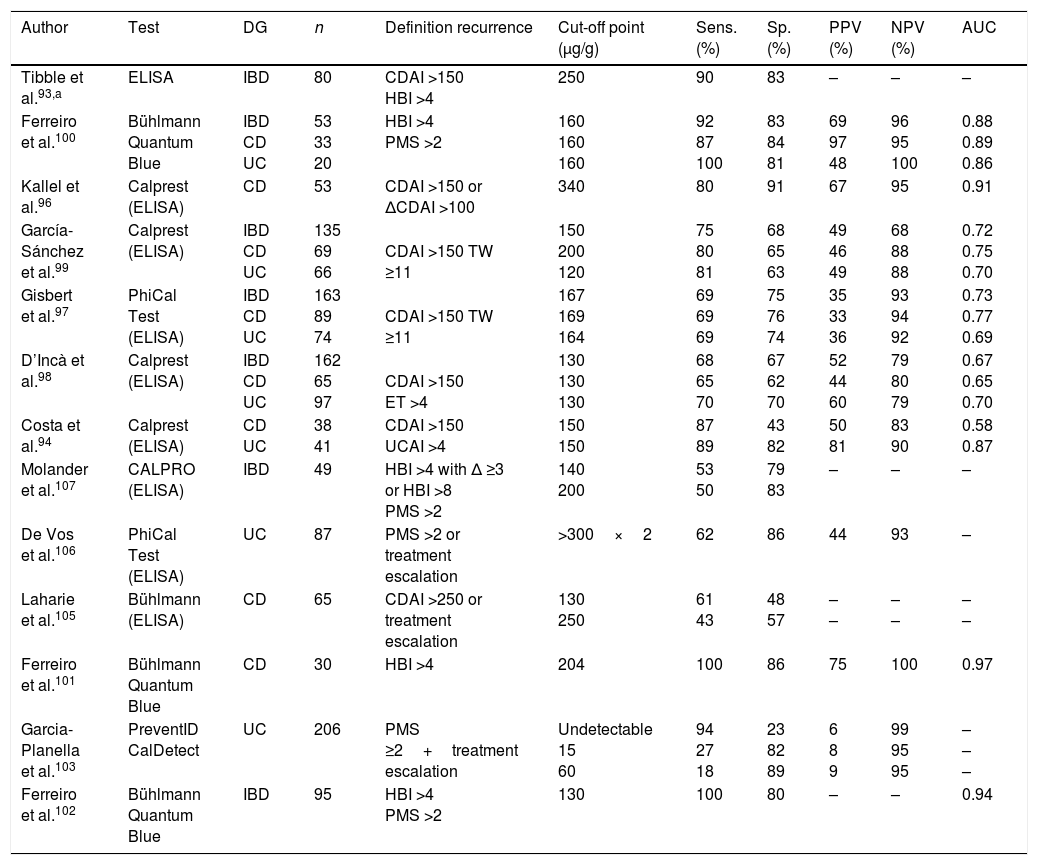
Faecal calprotectin as predictor of recurrenceCan measuring faecal calprotectin help predict recurrence of inflammatory bowel disease?Numerous studies, both in CD and UC, demonstrate the prognostic value of FC,93–107 particularly its high negative predictive power (Table 6). Six of these studies are included in the meta-analysis by Mao et al.,108 where the overall sensitivity and specificity for predicting recurrence were 78% and 73%, respectively. The prognostic capacity of FC is similar in CD and UC, although it seems lower in patients with CD with ileal involvement only. The best cut-off point has not been clearly defined and will depend on the desired compromise between sensitivity and specificity, but several studies coincide in placing it at around 150μg/g (Table 6). FC has also been shown to have an independent predictive value for recurrence after withdrawal of anti-TNF treatment, even in patients with endoscopic scarring.107,109–111
Diagnostic accuracy of faecal calprotectin in predicting recurrence.
| Author | Test | DG | n | Definition recurrence | Cut-off point (μg/g) | Sens. (%) | Sp. (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| Tibble et al.93,a | ELISA | IBD | 80 | CDAI >150 HBI >4 | 250 | 90 | 83 | – | – | – |
| Ferreiro et al.100 | Bühlmann Quantum Blue | IBD CD UC | 53 33 20 | HBI >4 PMS >2 | 160 160 160 | 92 87 100 | 83 84 81 | 69 97 48 | 96 95 100 | 0.88 0.89 0.86 |
| Kallel et al.96 | Calprest (ELISA) | CD | 53 | CDAI >150 or ΔCDAI >100 | 340 | 80 | 91 | 67 | 95 | 0.91 |
| García-Sánchez et al.99 | Calprest (ELISA) | IBD CD UC | 135 69 66 | CDAI >150 TW ≥11 | 150 200 120 | 75 80 81 | 68 65 63 | 49 46 49 | 68 88 88 | 0.72 0.75 0.70 |
| Gisbert et al.97 | PhiCal Test (ELISA) | IBD CD UC | 163 89 74 | CDAI >150 TW ≥11 | 167 169 164 | 69 69 69 | 75 76 74 | 35 33 36 | 93 94 92 | 0.73 0.77 0.69 |
| D’Incà et al.98 | Calprest (ELISA) | IBD CD UC | 162 65 97 | CDAI >150 ET >4 | 130 130 130 | 68 65 70 | 67 62 70 | 52 44 60 | 79 80 79 | 0.67 0.65 0.70 |
| Costa et al.94 | Calprest (ELISA) | CD UC | 38 41 | CDAI >150 UCAI >4 | 150 150 | 87 89 | 43 82 | 50 81 | 83 90 | 0.58 0.87 |
| Molander et al.107 | CALPRO (ELISA) | IBD | 49 | HBI >4 with Δ ≥3 or HBI >8 PMS >2 | 140 200 | 53 50 | 79 83 | – | – | – |
| De Vos et al.106 | PhiCal Test (ELISA) | UC | 87 | PMS >2 or treatment escalation | >300×2 | 62 | 86 | 44 | 93 | – |
| Laharie et al.105 | Bühlmann (ELISA) | CD | 65 | CDAI >250 or treatment escalation | 130 250 | 61 43 | 48 57 | – – | – – | – – |
| Ferreiro et al.101 | Bühlmann Quantum Blue | CD | 30 | HBI >4 | 204 | 100 | 86 | 75 | 100 | 0.97 |
| Garcia-Planella et al.103 | PreventID CalDetect | UC | 206 | PMS ≥2+treatment escalation | Undetectable 15 60 | 94 27 18 | 23 82 89 | 6 8 9 | 99 95 95 | – – – |
| Ferreiro et al.102 | Bühlmann Quantum Blue | IBD | 95 | HBI >4 PMS >2 | 130 | 100 | 80 | – | – | 0.94 |
AUC: area under the ROC curve; CD: Crohn's disease; CDAI: Crohn's Disease Activity Index; DG: diagnosis; ET: Edwards and Truelove score; HBI: Harvey-Bradshaw Index; IBD: inflammatory bowel disease; NPV: negative predictive value; PMS: Partial Mayo Score; PPV: positive predictive value; Sens.: sensitivity; Sp.: specificity; TW: modified Truelove Witts score; UC: ulcerative colitis; UCAI: Ulcerative Colitis Activity Index.
In patients in remission, serial determinations of FC have a higher prognostic value than an isolated measurement. Studies which included periodic determinations show that FC elevation can be detected from three to six months before recurrence,103,106,107,111 and that repeatedly low values are highly predictive of sustained remission. De Vos et al.106 studied the predictive value of monthly measuring of FC in patients with UC in remission. Two consecutive measurements of FC >300μg/g predicted recurrence with a sensitivity of 61% and a specificity of 100%, both higher than those obtained with a single measurement. Also in that study, slight isolated elevations of FC were common, although without any clinical consequences.
Based on the available evidence, therefore, periodic determination of FC can be used to predict recurrence. As the elevation of FC usually precedes recurrence by about 12 weeks, testing on a three-monthly basis would seem reasonable, especially in situations requiring closer clinical monitoring, such as after induction therapy or treatment modifications. In patients with a low baseline risk of recurrence, such as those in long-term remission or with recent evidence of endoscopic cure, testing frequency may be extended to every six months. Being able to measure FC at home could make it much easier to monitor IBD in remission.13,14,18
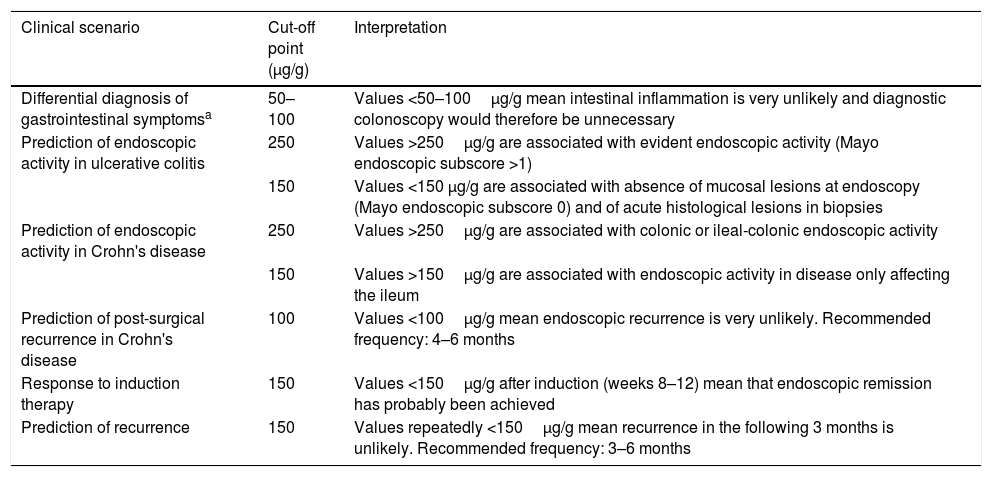
Final considerationsFC is the best biomarker of inflammation that we currently have in IBD. It is a valuable tool for differentiating between irritable bowel syndrome and inflammatory processes in patients with gastrointestinal symptoms. FC correlates with endoscopic activity in both UC and CD, is associated with clinical and endoscopic response to treatment and predicts short-term relapse, even in patients in endoscopic remission. It can therefore be a great help for clinicians in diagnosing and monitoring IBD, and adapting treatment. Table 7 shows suggested cut-off points for FC recommended for different clinical scenarios and explains how to interpret them.
Interpreting the determination of faecal calprotectin in different clinical scenarios.
| Clinical scenario | Cut-off point (μg/g) | Interpretation |
|---|---|---|
| Differential diagnosis of gastrointestinal symptomsa | 50–100 | Values <50–100μg/g mean intestinal inflammation is very unlikely and diagnostic colonoscopy would therefore be unnecessary |
| Prediction of endoscopic activity in ulcerative colitis | 250 | Values >250μg/g are associated with evident endoscopic activity (Mayo endoscopic subscore >1) |
| 150 | Values <150 μg/g are associated with absence of mucosal lesions at endoscopy (Mayo endoscopic subscore 0) and of acute histological lesions in biopsies | |
| Prediction of endoscopic activity in Crohn's disease | 250 | Values >250μg/g are associated with colonic or ileal-colonic endoscopic activity |
| 150 | Values >150μg/g are associated with endoscopic activity in disease only affecting the ileum | |
| Prediction of post-surgical recurrence in Crohn's disease | 100 | Values <100μg/g mean endoscopic recurrence is very unlikely. Recommended frequency: 4–6 months |
| Response to induction therapy | 150 | Values <150μg/g after induction (weeks 8–12) mean that endoscopic remission has probably been achieved |
| Prediction of recurrence | 150 | Values repeatedly <150μg/g mean recurrence in the following 3 months is unlikely. Recommended frequency: 3–6 months |
There are a number of considerations which should be taken into account for the appropriate use of FC in clinical practice. First of all, any decision based on the FC results must be from consecutive serial measurements (at least two), not one single test result. This increases the accuracy of the test and cancels out the effect of any isolated fluctuations. Secondly, FC measurements should not be interpreted in isolation from the clinical context in which they are performed. It is important to remember that the predictive value of a high or low FC depends on the pre-test likelihood of there being endoscopic activity. The higher the pre-test likelihood of activity, the greater the possibility that a high FC value is a true positive and a low value is a false negative, and vice versa. For example, in a patient who presents with diarrhoea and abdominal pain after stopping treatment, a situation with pre-test likelihood of a high degree of activity, high FC values mean we can virtually guarantee there will be activity. A low value is most likely to be a false negative. In a patient in long-term clinical remission with a pre-test likelihood of low-degree activity, low FC values make the absence of activity very likely and the result is therefore a true negative. Last of all, clinical decision-making guided by FC levels will depend not only on the predictive value of the test, but also on how important the decision is; in other words, it will depend on the possible consequences of a false positive or false negative result. For example, a clinician may feel comfortable deciding on the frequency and type of follow-up visit (face-to-face/email or telephone, medical/nursing, etc.), an increase in salicylate dose or the start of a topically-acting systemic steroid or topical treatment based purely on the FC result. However, in the case of very important clinical decisions, such as the possibility of a surgical intervention, it may be advisable to turn to endoscopic examinations or imaging tests in order to minimise uncertainty.
Nevertheless, to sum up, for clinicians aware of the benefits and limitations, FC can be a highly useful tool in the management of patients with IBD.
Conflicts of interestNone of the authors has declared a conflict of interest with respect to this study.
Please cite this article as: Guardiola J, Lobatón T, Cerrillo E, Ferreiro-Iglesias R, Gisbert JP, Domènech E, et al. Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) sobre la utilidad de la determinación de calprotectina fecal en la enfermedad inflamatoria intestinal. Gastroenterol Hepatol. 2018;41:514–529.