Hepatitis C recurrence after liver transplantation is universal and increases morbidity and mortality in these patients. The development of new direct antiviral agents against the hepatitis C virus is a major treatment advance. Pre-transplant treatment avoids graft infection and sometimes improves liver function, allowing the patient to be withdrawn from the transplant waiting list. Delaying treatment until the postpostransplant period may be advisable in patients with advanced cirrhosis. Generally, antiviral therapy after liver transplantation is provided in patients with histological evidence of the disease. In these patients, treatment is more effective in the initial stages of the disease. The choice of antiviral therapy in these patients is based on the degree of liver function, the presence of renal failure, and potential drug–drug interactions.
La recidiva de la hepatitis C tras el trasplante hepático es universal y condiciona un aumento en la morbimortalidad del paciente. El desarrollo de nuevos agentes antivirales directos contra el virus C es un gran avance en el tratamiento de estos pacientes. El tratamiento antes del trasplante permite evitar la infección del injerto y, en algunos casos, obtener una mejoría de la función hepática que permita la retirada del paciente de la lista de espera. En pacientes con cirrosis avanzada, podría ser preferible diferir el tratamiento hasta el periodo postrasplante. Generalmente, el tratamiento antiviral tras el trasplante hepático se realiza en pacientes con evidencia de lesión histológica. En estos pacientes, la eficacia del tratamiento es mayor en estadios iniciales de la enfermedad. La elección del tratamiento antiviral en estos pacientes se basa en el grado de disfunción hepática, la presencia de fallo renal y las potenciales interacciones medicamentosas.
Recurrence of hepatitis C (HCV) after liver transplantation (LT) is widespread, and disease progression is more accelerated in comparison with immunocompetent patients. In fact, between 20% and 30% of patients develop cirrhosis within 5 years of LT.1 The risk of decompensation in cirrhotic patients is 40% within 1 year, and as high as 70% within 3 years following diagnosis.2 In these patients, decompensated cirrhosis is an indicator of early death, as 60% of decompensated patients die within 1 year once decompensation develops.3 A small proportion of patients (5–10%) develop a severe form of recurrent hepatitis C known as fibrosing cholestatic hepatitis. If not treated, this infection can cause graft loss and death.4
The introduction of direct-acting antivirals (DAA) in recent years has marked a major breakthrough in the treatment of chronic HCV. The combination of several such interferon (IFN) free drugs has shown high rates of sustained virologic response (SVR), an excellent safety profile and good tolerability, even in patients with advanced disease.5,6 In this paper, we review the latest developments in the treatment of HCV before and after LT.
Pre-transplant antiviral therapyThe main goal of antiviral treatment in patients awaiting LT is to prevent graft infection. Treatment can be administered in 2 regimens: (1) a short (or incomplete) course to achieve undetectable HCV-RNA levels prior to LT, or (2) a full course, to achieve SVR prior to LT.
In addition to preventing graft infection, pre-LT antiviral therapy can also improve liver function and allow some patients to be removed from the LT waiting list. However, the frequency of this improvement and the predictive factors that might identify which patients will present a significant improvement in liver function remain to be determined.7
Treatment of patients with compensated cirrhosisThe first study in patients on LT waiting lists evaluated administration of sofosbuvir (SOF) in combination with ribavirin (RBV) for 48 weeks in 61 patients with compensated cirrhosis of the liver (Child-Pugh A) and hepatocellular carcinoma (Milan criteria). Of the 43 patients with undetectable HCV-RNA levels at the time of transplantation, 70% achieved post-LT SVR. This study also found that the probability of achieving post-LT SVR was significantly higher in patients with undetectable HCV-RNA levels lasting more than 30 days prior to LT.8 Despite the significance of the findings, this drug combination is still considered suboptimal in patients infected with HCV genotype (G) 1, and the combination of at least 2 DAAs is still considered necessary. Two phase 3 studies evaluated the safety and efficacy of SOF and ledipasvir (LDV, either with or without RBV) for 12 or 24 weeks in cirrhotic patients infected with G1.9,10 The ION-1 study9 included 865 treatment-naive patients, 136 (16%) of whom were diagnosed with cirrhosis. In this cirrhotic subgroup, the authors observed SVR rates of 100% in patients treated with RBV, and 97% in patients who did not receive RBV. The ION-2 study10 evaluated 440 previously treated patients (including patients in whom triple therapy with first generation protease inhibitors had failed), 88 (20%) with cirrhosis. In this study, the authors observed SVR rates of 88% and 100% in patients treated for 12 and 24 weeks, respectively.
The TURQUOISE-II study evaluated the efficacy and safety of paritaprevir/ritonavir/ombitasvir (PTV/r/OBV) therapy combined with dasabuvir (DVR) and RBV in a 12- or 24-week regimen in 380 patients with compensated cirrhosis. SVR rates of 92% and 96% were observed in patients treated for 12 and 24 weeks, respectively. Virologic response was lower in patients infected with G1a, in previously treated patients, and in patients with clinical signs of portal hypertension.11
In the case of patients infected with HCV G2 and G3, phase 3 studies evaluating treatment with SOF and RBV for 12 or 16 weeks showed that cirrhotic patients presented lower SVR rates than their non-cirrhotic counterparts (≈80%). Despite the progress made in antiviral therapy, the treatment of cirrhotic patients infected with G3, particularly those with previous treatment failure, is still challenging. The outcome of treatment with a combination of SOF+RBV (24 weeks), or SOF+daclatasvir (DCV, 12 weeks) is suboptimal in this patient population (61% and 59%, respectively).12,13
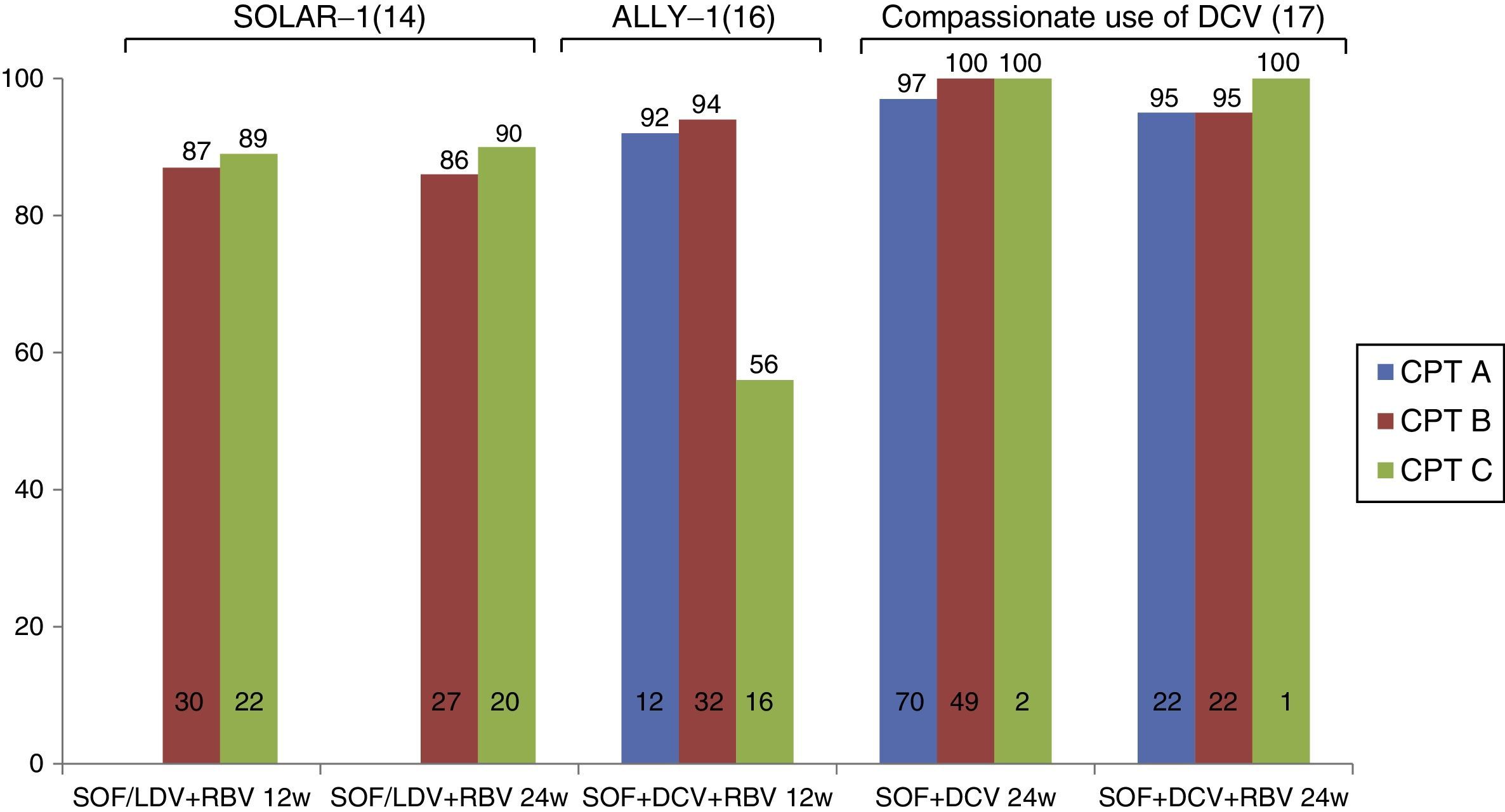
Treatment of patients with decompensated cirrhosisRecent studies have evaluated the efficacy of new antiviral drugs in patients with decompensated cirrhosis (Fig. 1). The SOLAR-1 study evaluated the efficacy and safety of SOF/LDV+RBV therapy for 12 or 24 weeks in 108 cirrhotic Child-Pugh B and C patients infected with HCV G1 and G4. As expected, most patients presented ascites or hepatic encephalopathy, with significantly impaired liver function. SVR rates were 87% and 89% in patients treated for 12 and 24 weeks, respectively, with no difference between Child-Pugh B and C patients. The safety profile was excellent, and only 3 patients discontinued the treatment due to adverse effects. Between 60% and 80% of patients in this study presented improved liver function (determined by Model for End-Stage Liver Disease [MELD] score).14 These results were recently confirmed in the SOLAR-2 study15 in 84 patients with decompensated cirrhosis. It is important to note that nearly all the patients included in these 2 studies had a MELD score of between 10 and 20; there is no evidence, therefore, to confirm the safety and efficacy of this combination (SOF+LDV) in patients with MELD>20.
Similarly, the ALLY-1 study16 evaluated combined SOF+DCV+RBV therapy for 12 weeks in patients with advanced cirrhosis (MELD 8–40). Thirty-two patients were Child-Pugh B (MELD 10–20), and 20 were Child-Pugh C (MELD 16–25). SVR rates were 94% and 56% in Child-Pugh B and C patients, respectively. Treatment was less effective in patients with ascites, hepatic encephalopathy or albumin levels below 28g/l.16 In clinical practice, the compassionate use of SOF+DCV (with or without RBV) across Europe has been associated with response rates of over 90% in patients with decompensated cirrhosis receiving treatment for 24 weeks. However, no conclusions can be drawn from this study, as very few patients with Child-Pugh C cirrhosis (n=2) were included.17
Data from decompensated patients (MELD>10) from the TARGET cohort, treated with SOF and simeprevir (SMV) with or without RBV, were recently published. In the treatment-naive group, 78% and 69% of patients receiving SOF+SMV or SOF+SMV+RBV, respectively, achieved SVR. Similar results were observed in treatment-experienced patients (72% and 69%).18
Treatment of patients on liver transplant waiting listsPatients on LT waiting lists have certain characteristics that could influence the decision to administer antiviral therapy.
- (a)
In these patients, antiviral therapy is primarily given to prevent graft infection. As transplantation effectively removes the main source of virions (the liver), it is logical to assume that a short course of antiviral therapy will suffice to achieve undetectable viral levels.19 However, it is difficult to establish when antiviral treatment should be started to allow time to eliminate the viral load. To prevent post-LT recurrence in patients with compensated cirrhosis, the patient must present undetectable levels of HCV-RNA for at least 30 days prior to LT.8 However, it is unclear whether 30 days with undetectable HCV-RNA levels is sufficient in patients with decompensated cirrhosis, as studies suggest that viral decline is slower in decompensated compared with compensated patients.20 Despite the lack of data, the same criteria used for compensated cirrhosis should be used in decompensated patients, i.e., undetectable HCV-RNA for at least 30 days before LT.
- (b)
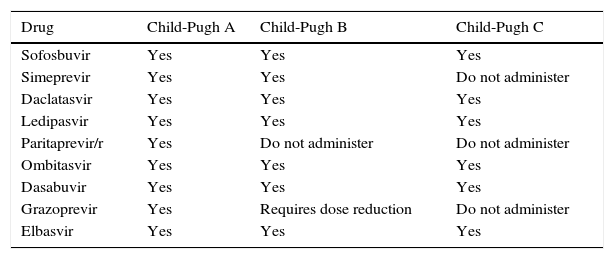
The pharmacokinetics of some DAAs is altered in patients with advanced liver disease, mainly those with decompensated cirrhosis (Table 1). Some drugs, such as SMV and PTV/r, should not be used in patients with Child-Pugh B or C cirrhosis.21–23
Table 1.DAA in patients with advanced cirrhosis.
Drug Child-Pugh A Child-Pugh B Child-Pugh C Sofosbuvir Yes Yes Yes Simeprevir Yes Yes Do not administer Daclatasvir Yes Yes Yes Ledipasvir Yes Yes Yes Paritaprevir/r Yes Do not administer Do not administer Ombitasvir Yes Yes Yes Dasabuvir Yes Yes Yes Grazoprevir Yes Requires dose reduction Do not administer Elbasvir Yes Yes Yes - (c)
Virological failure (recurrence of infection) after withdrawal of antiviral therapy seems to be more common in patients with advanced cirrhosis, which could encourage the development of drug-resistant variants. Generally speaking, drug-resistant strains, being less robust, are usually replaced by native variants. The exception to this rule appears to be strains selected by NS5A inhibitors, which persist for long periods of time.24,25 It is unclear whether the appearance of these drug-resistant variants is associated with severe forms of HCV recurrence (fibrosing cholestatic hepatitis). Therefore, the evolution of patients receiving pre-LT therapy with new generation DAAs without achieving SVR should be monitored and studied to determine the effect of these drug-resistant variants on clinical evolution.
The primary indication for LT in developed countries is liver cirrhosis due to HCV, with or without hepatocellular carcinoma. Recurrence of HCV infection in LT patients is widespread, and the disease shows a faster rate of progression in this population compared with immunocompetent patients.2,26,27 Approximately one third of post-LT patients develop cirrhosis of the graft within 5 years. Accordingly, both patient and graft survival rates in this population are lower when compared to patients transplanted for reasons other than HCV infection.27,28 There are 2 approaches to the treatment of post-LT HCV recurrence: (1) preventive treatment, administered before the onset of liver damage, or (2) treatment of the recurrence once the damage is already established.
Preventive treatmentPreventive treatment involves the administration of antiviral therapy before recurrence of HCV infection, thus preventing histological damage of the graft. As recurrence in the form of acute hepatitis usually occurs between 1 and 4 months after transplantation, preventive treatment should be started in the first weeks following transplantation.29 Studies carried out in the IFN era showed no benefits in terms of SVR or prevention of liver damage, but instead numerous adverse effects, including a high rate of graft rejection (3.8–23%).
Despite recent advances in antiviral treatment, no data have been published on the use of the latest DAAs for preventive treatment. Donato et al.30 described a case where treatment with SOF and RBV was started 1 week prior to LT, and maintained during and after surgery. The authors found no adverse effects and no need to suspend treatment, even though the patient ultimately required retransplant. A recent case report describes the treatment of 2 patients in whom antiviral therapy with IFN-free agents was started during the anhepatic phase.31 At the time of publication, RNA was still undetectable, and the treatment was well tolerated, with no evidence of toxicity or interactions with immunosuppressant drugs.
Treatment of recurrenceGenerally speaking, antiviral treatment is started once histological damage associated with HCV recurrence is observed. Traditionally, indications for treatment were the presence of fibrosing cholestatic hepatitis or recurrence of severe HCV infection (defined by fibrosis stage ≥2, portal hypertension, or severe inflammation within 1 year of transplantation). With the recent introduction of safer and more effective drugs, however, the indication for treatment has been extended to include all LT recipients with evidence of any level of HCV recurrence.
Treatment with Peg-IFN and RBV achieved extremely low rates of SVR and a high incidence of adverse events that required either 1 or both drugs to be down-titrated, or treatment to be suspended.4 The addition of a first generation protease inhibitor (telaprevir or boceprevir) to IFN and RBV significantly increased the likelihood of achieving SVR,32–35 but brought with it the risk of serious adverse effects and drug interactions.23 In one multicentre observational study performed in the USA, the authors described their experience with triple therapy (90% with teleprevir) in 81 patients infected with G1 following LT. Slightly over half (53%) of the cohort presented advanced fibrosis (F3–F4), and 57% did not respond to either Peg-IFN or RBV after transplantation; 63% achieved SVR at 12 weeks. The authors also observed that most patients presented anaemia: 81% required erythropoietin treatment, and 57% required blood transfusion. Other serious adverse effects were observed, with 27% requiring hospitalisation, and in 15% the treatment was suspended before completion.
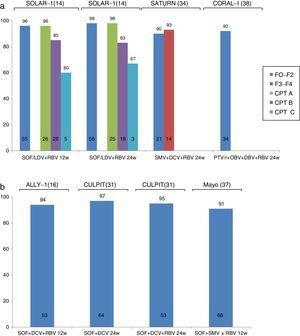
Recent studies have evaluated the efficacy and safety of new IFN-free regimens in the treatment of recurrent HCV infection. In these cases, depending on the regimen used and the severity of the recurrence, SVR was achieved in between 57% and 97% of patients (Fig. 2).15,16,36–41 Despite the small sample sizes in these studies, the findings suggest that new generation DAAs are a viable option in the treatment of recurrent HCV infection. Furthermore, programmes involving the compassionate use of these drugs have also reported SVR rates in excess of 70%, even in patients with severe or early recurrence, such as cholestatic hepatitis37,41.
The first study to evaluate the efficacy of IFN-free therapy in treating HCV recurrence following LT analysed the efficacy and safety of SOF in combination with RBV in 40 patients more than 12 months after transplantation. Despite the small sample size and suboptimal combination, 70% of patients achieved SVR, demonstrating the safety and efficacy of oral combinations in this patient population.42 Following publication of this study, significantly high rates of SVR have been reported in various clinical trials combining 2 or 3 DAAs.
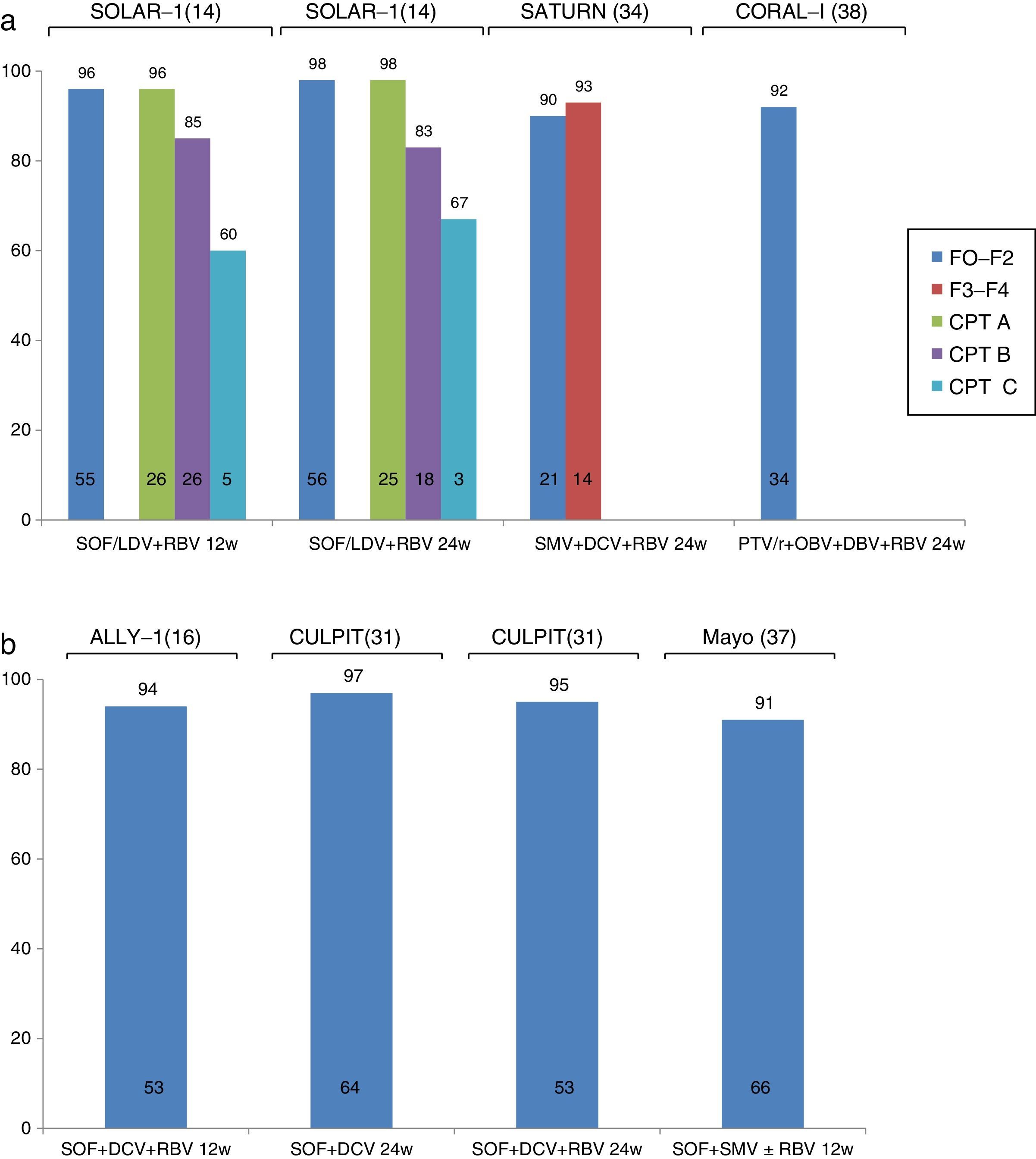
The CORAL-1 study, which evaluated the efficacy and safety of PTV/r/OBV in combination with DBV and RBV in 34 patients with mild recurrence of HCV G1 infection (F0–F2) following LT, reported an SVR rate of 97% and excellent tolerance. The main drawback of this combination in the context of LT is the risk of interactions between PTV/r and immunosuppressants. Therefore, the tacrolimus dose should be reduced to 0.5mg/week, and only a fifth of the usual dose of cyclosporines should be used. As a result, management of these patients is complicated by the potential risk of graft loss or toxicity due to under- or over-dosage of calcineurin inhibitors. The SOLAR-1 and SOLAR-2 studies14,15 analysed the efficacy and safety of LDV/SOF in combination with RBV in patients with recurrence of HCV infection at different stages of the disease: F0–F3, compensated cirrhosis (Child-Pugh A) and decompensated cirrhosis (Child-Pugh B and C). Patients were randomised to receive the drug combination for 12 or 24 weeks. The SOLAR-1 study showed that patients with mild fibrosis or compensated cirrhosis (96–98%) were more likely to achieve SVR than those with decompensated cirrhosis (83–85% in patients with Child-Pugh B and 60–67% in patients with Child-Pugh C). The SOLAR-2 study reported similar findings in European patients with the same characteristics.
More recently, the ALLY-1 study evaluated the efficacy of treatment with SOF+DCV+RBV in a cohort of 53 patients with recurrence of HCV following LT. Most (55%) presented advanced fibrosis (F3–F4). Nearly all (94%) study patients achieved SVR. Treatment was well tolerated, and only 1 patient discontinued therapy due to severe headaches.16 The same drug combination has been studied in various cohorts in clinical practice. Among these studies, the CULPIT series43 reported the results of administration of combined SOF+DCV with or without RBV for 12 or 24 weeks (at the discretion of the attending physician) in 130 patients with HCV infection and severe recurrence following LT. The SVR rate was 67–100% in patients receiving a 12-week course of treatment, and 96–100% in those on the 24-week regimen. It is important to note that very few patients (n=3) were included in the group with 67% SVR (12 weeks on RBV), and therefore no firm conclusions can be drawn from these findings. No significant differences were found in SVR rates according to response to previous treatment, virus genotype, or fibrosis stage. Although no interactions were reported between the immunosuppressants and antivirals administered, up to 56% of patients required immunosuppressant adjustment (probably due to improvement in liver function). These patients therefore require close monitoring.
Another widely studied combination in clinical practice is SOF+SMV with or without RBV. The results of 2 large series have been published. The TARGET cohort included 227 LT recipients who received antiviral treatment with SOF. Most were infected with G1, and received SOF+SMV with or without RBV. The overall rate of SVR was 90%, with a slightly lower rate in patients with liver cirrhosis (86%), particularly those with MELD>10 (77%) or G1a infection (83%). The results of the study conducted by the Mayo Clinic in 123 LT recipients (30% with F3–F4 fibrosis, 11% with cholestatic hepatitis, and 82% with previous treatment) have also been published. Despite an overall SVR rate of 90%, incidence was significantly lower in patients infected with G1a and those with F3–F4 fibrosis (71% vs 91% in patients with F0–F2 fibrosis). Tolerance was good, although 72% of patients receiving RBV developed anaemia.
Finally, some studies have focussed on the efficacy of combined SMV and DCV in patients with recurrent HCV and G1b infection. The SATURN study44 included 35 patients with F1–F4 fibrosis, divided into 2 groups. The first group included patients with mild fibrosis treated with tacrolimus or cyclosporine, and the second included patients with advanced fibrosis treated with tacrolimus due to the risk of cyclosporine – SMV interaction detected in the first group. SVR rate at 4 weeks after completion of treatment was 90–93%, with good tolerance. All cases of virologic failure were due to viral breakthrough during treatment (1 in a patient with G1a who had been erroneously included in the study). The same combination was also evaluated in a clinical practice cohort study reporting compassionate use of SMV across Europe. Twenty-three patients with severe HCV recurrence received this drug combination, with an SVR rate of 72%. Four of the 5 cases of virologic failure were due to a new viral outbreak during treatment, and 1 to recurrence after completion of antiviral therapy. This combination seems to be slightly less effective than the foregoing therapies, and could be reserved for patients who fulfil the criteria for treatment but are contraindicated for other antiviral combinations.
The results of the foregoing studies allow us to draw some conclusions with regard to treatment of HCV in LT patients: (1) The ideal time for initiating antiviral treatment after LT is still unclear. Evidence strongly suggests that treatment is more effective in the early stages of the disease (better at F0–F1 than F3–F4, and in compensated rather than decompensated patients). Although there are no data on the use of preventive treatment (before the appearance of histological damage), treatment started in the immediate postoperative period could be hampered by the need for surgical reintervention, onset of renal failure, drug interactions, etc. For these reasons, once graft injury is confirmed, early initiation of antiviral treatment following LT is recommended, always at the very early stages of liver damage. (2) Aviremia is beneficial to liver function (Child-Pugh and MELD), and therefore patients with decompensated cirrhosis will also benefit from antiviral treatment. (3) IFN-free drug combinations can be safely administered after LT. (4) Treatment should be chosen taking into consideration the level of hepatic dysfunction, renal function, and possible drug interactions with immunosuppressants.
ConclusionsThe development of new direct-acting antiviral drugs has led to a breakthrough in the treatment of liver transplantation patients, and can be used to treat patients who were previously considered untreatable, either due to contraindications (risk of infection, death, or decompensation is patients with advanced cirrhosis), or due to the need to prematurely discontinue therapy due to adverse effects. Given the safety profile of direct-acting antivirals, SVR should ideally be achieved before LT in order to prevent graft infection. Some patients, however, could be removed from the LT waiting list due to an improved MELD score. For this reason, candidates for treatment with DAAs should be carefully selected. After LT, DAA therapy can have a considerable impact by improving liver function, and in some cases, achieving regression of liver fibrosis. Treatment should start in the early stages of the disease (before the development of cirrhosis or decompensation), and should be chosen taking into consideration the characteristics of the patients and the presence of potential drug interactions.
Conflict of interestsMaría-Carlota Londoño: Janssen, MSD, BMS, Abbvie, Gilead. Laura-Patricia Llovet and Sergio Rodríguez-Tajes have no conflicts of interest to declare.
Please cite this article as: Llovet LP, Rodríguez-Tajes S, Londoño MC. Tratamiento de la hepatitis C en el pre- y postrasplante hepático. Gastroenterol Hepatol. 2016;39:344–351.