Ustekinumab is a monoclonal antibody directed against the p40 subunit, which is part of interleukins IL-12 and IL-23. The efficacy of ustekinumab versus placebo in terms of clinical response and remission of induction has been shown in phase 3 clinical trials. When used as subcutaneous maintenance therapy, the therapeutic benefit of ustekinumab over placebo has been confirmed in both clinical response and remission in patients who have responded clinically to induction therapy. In addition, ustekinumab has demonstrated an improvement in mucosal healing parameters. The safety profile of the drug has been good, with low infection rates (without reactivation of tuberculosis) and absence of tumour reporting. The development of drug immunogenicity appears to be rare. In summary, ustekinumab is a promising treatment option in patients with Crohn's disease, as an alternative to anti-TNFα drugs.
Ustekinumab es un anticuerpo monoclonal dirigido frente a la subunidad p40, que forma parte de las interleucinas IL-12 y IL-23. En los ensayos clínicos fase 3, ustekinumab ha demostrado su eficacia frente a placebo, en términos de respuesta clínica y remisión de la inducción. Cuando se ha empleado como terapia de mantenimiento por vía subcutánea, ustekinumab ha confirmado su beneficio terapéutico (sobre placebo) tanto en la respuesta clínica como en la remisión, en pacientes que habían respondido clínicamente a la terapia de inducción. Adicionalmente, ustekinumab ha demostrado una mejoría de los parámetros de curación mucosa. El perfil de seguridad del fármaco ha sido bueno, con tasas de infecciones infrecuentes (sin reactivación de tuberculosis) y ausencia de descripción de tumores. El desarrollo de inmunogenicidad frente al fármaco parece ser infrecuente. En resumen, ustekinumab representa una prometedora opción de tratamiento en pacientes con enfermedad de Crohn, como alternativa a los fármacos anti-TNFα.
Crohn's disease, a chronic inflammatory disease mediated by gastrointestinal tract immunity, is characterised by periods of activity and remission.1 Major breakthroughs have been made in the pharmacological treatment of this disease in recent years. However, treatments currently available are still ineffective in many patients.
Aminosalicylates have little effect (especially in ileal Crohn's disease), while corticosteroids are only effective in inducing, but not maintaining, remission. This is why it is often necessary (usually because of steroid dependence) to resort to immunosuppressants, particularly thiopurines, such as azathioprine or mercaptopurine, and methotrexate, which are associated with a relatively high incidence of adverse effects.2–6 Furthermore, these immunosuppressants have a slow onset of action (several months), and are therefore unsuitable for inducing remission.
Treatment often needs to be stepped up and tumour necrosis factor alpha (TNFα) inhibitors added. In Spain, these are limited to infliximab or adalimumab in the case of Crohn's disease.7–10 Approximately one third of patients fail to respond to TNFα inhibitors from the outset (primary failure), and a sizeable proportion develop loss of efficacy (secondary failure) or intolerance to these drugs.8,11–16 Moreover, TNF inhibitor-induced side effects, though rare, can be serious.17,18 Finally, response to re-treatment with these drugs is worse in prior null responders to TNF inhibitorsα (due either to lack or loss of efficacy) than in treatment-naïve patients.19
Recently, treatment with vedolizumab has been approved for adult patients with Crohn's disease with inadequate response, loss of response, contraindication or intolerance to conventional treatment or TNF inhibitorsα.20 Although this drug is a promising therapeutic alternative, it is only moderately effective in patients with Crohn's disease,21–23 and there is a pressing need for additional biological agents that act on other inflammatory pathways or therapeutic targets.24–27 Ustekinumab is a monoclonal antibody directed against the p40 subunit shared by the proinflammatory cytokines interleukin (IL) IL-12 and IL-23.
IL-12 and IL-23 play a key role in the inflammatory cascade in Crohn's disease.28,29 In the pathogenesis of this disease, bacterial antigen-mediated activation of toll-like receptors and nucleotide-binding oligomerization domain (NOD)-like receptors stimulate antigen-presenting cells to secrete IL-12 and IL-23.28–30 IL-12, a heterodimer composed of a p35 and p40 subunit, is mainly instrumental in activating the maturation of undifferentiated T lymphocytes into Th1 lymphocytes.30 Patients with Crohn's disease show a Th1-type response that is similar in many respects to that seen in acute infection.28,29 This Th1 response is associated with the expression of proinflammatory cytokines, including interferon gamma (IFNγ) and TNFα.28,29,31 IL-12 mediated production of IFNγ amplifies the inflammatory cascade and produces cytokines, including IL-7, IL-15, IL-18 and IL-21, which further stimulate the Th1 lymphocyte response.31
IL-23, meanwhile, is a heterodimeric protein that shares a common subunit, p40, with IL-12. In IL-12, the p40 subunit is coupled with p1928,29 and acts on Th17 lymphocytes by stimulating the production of a series of effector cytokines. These trigger the inflammatory response by inducing neutrophilic infiltration and the expression of other proinflammatory cytokines, such as TNFα, IL-1β and IL-6.28,29 In this way, the accumulation of cytokine-mediated effects produced by Th17 lymphocytes contributes to the chronic inflammation characteristic of Crohn's disease.29 Ustekinumab prevents the interaction of these cytokines with the T-cell surface IL-12Rβ1 receptor,32–34 thus interrupting the activation of these cell lines involved in the aforementioned inflammation described in Crohn's disease.29,35,36
This article aims to review the role of ustekinumab in Crohn's disease, particularly its pharmacokinetic and pharmacodynamic properties, dosage, indications, efficacy, safety profile and, finally, the role of this drug in the treatment of Crohn's disease. For this purpose, we searched the Medline database and the most relevant international conferences (European Crohn's and Colitis Organisation [ECCO], United European Gastroenterology [UEG] and Digestive Disease Week [DDW]) for relevant articles using the keywords “Crohn's disease” and “ustekinumab”.
Pharmacokinetic propertiesMedian volume of distribution during the terminal phase (Vz) following a single intravenous administration to patients with psoriasis ranged from 57 to 83ml/kg. Median systemic clearance (CL) following a single intravenous administration to patients with psoriasis ranged from 1.99 to 2.34ml/day/kg.37
Median half-life (t1/2) of ustekinumab was approximately 3 weeks in patients with psoriasis, psoriatic arthritis or Crohn's disease, ranging from 15 to 32 days across all psoriasis and psoriatic arthritis studies.37
In a population pharmacokinetic analysis, the apparent clearance (CL/F) and apparent volume of distribution (V/F) were 0.465l/day and 15.7l, respectively, in patients with psoriasis. The CL/F of ustekinumab was not impacted by gender. The population pharmacokinetic analysis showed that there was a trend towards higher ustekinumab clearance in patients who tested positive for antibodies to ustekinumab.37
Weight appears to affect the pharmacokinetic parameters. When given the same dose, patients with psoriasis or psoriatic arthritis weighing >100kg had lower median plasma ustekinumab concentrations compared to those subjects weighing ≤100kg.34
There are currently no pharmacokinetic data on the use of ustekinumab in patients with kidney or hepatic impairment.
Pharmacodynamic propertiesUstekinumab is a fully human monoclonal antibody to interleukin (IL) that belongs to the pharmacotherapeutic group of immunosuppressants (ATC code: L04AC05).
As mentioned above, ustekinumab binds specifically to the p40 protein subunit shared by IL-12 and IL-23, thus preventing them from binding to the IL-12Rβ1 receptor expressed on the surface of T lymphocytes. However, ustekinumab cannot bind to cytokines that are already bound to the cell surface due to their interaction with these IL-12Rβ1 receptors. Because of this, ustekinumab is unlikely to activate the complement cascade that causes cell destruction (complement-mediated cytotoxicity).
Because it binds to the p40 subunit shared by IL-12 and IL-23, ustekinumab exerts its clinical effect in psoriasis,38,39 psoriatic arthritis39 and Crohn's disease28,31 by interrupting the Th1 and Th17 cytokine pathways that are key to the pathogenesis of these diseases.
Dosage and frequency of administration in Crohn's diseaseAccording to the summary of product characteristics, the first dose of ustekinumab in patients with Crohn's disease must be administered intravenously.37 This is a single, weight-based dose (approximately 6mg/kg). It is recommended that patients weighing ≤55kg receive 260mg, patients weighing >55 and ≤85kg receive 390mg, and patients weighing >85kg receive 520mg.37 Intravenous ustekinumab is presented in 130mg vials; therefore, patients will receive 2, 3 or 4 vials, based on their weight range.
The first subcutaneous dose of ustekinumab, 90mg, should be administered 8 weeks after the initial intravenous dose, and every 8 or 12 weeks thereafter, at the physician's discretion. Therefore, patients should be evaluated at 8 weeks of the first subcutaneous dose. At this point, suspension of treatment should be considered in patients who show no therapeutic benefit. Likewise, in the case of partial efficacy (clinical response), administration of a second dose at the time of evaluation can be considered; in the case of complete response (clinical remission), administration can be postponed to week 12.
During treatment, patients with inadequate response (or lost response) to the 90mg dose every 12 weeks can benefit from increasing administration frequency to every 8 weeks,40 at the physician's discretion.
No dose adjustment is needed for elderly patients, as pharmacokinetic studies have shown that age does not affect the efficacy of the drug. The safety and efficacy of ustekinumab for the treatment of Crohn's disease in children under 18 years have not yet been established.
Therapeutic indication in Crohn's diseaseAccording to the summary of product characteristics, ustekinumab is indicated for the treatment of adult patients with moderately to severely active Crohn's disease who have had an inadequate response with, lost response to, or were intolerant to either conventional therapy or to anti-TNFα, or have medical contraindications to these therapies.37
Clinical efficacy in Crohn's diseaseThe efficacy and safety of ustekinumab in patients with Crohn's disease has been evaluated in two phase 2 clinical trials and in three phase 3 trials. Two additional clinical trials are under way to evaluate efficacy in paediatric patients (12–17 years)41 and to investigate the benefit of different therapeutic strategies, based on the hypothesis that a strategy of maintenance treatment with ustekinumab together with early endoscopic evaluation would be associated with greater efficacy (in terms of mucosal healing).42
The phase 2b CERTIFI trial evaluated the efficacy of ustekinumab in patients with Crohn's disease who failed to respond to anti-TNFα drugs. Ustekinumab was administered intravenously at doses of 1, 3 and 6mg/kg weight. Clinical response at week 6 was significantly greater than that of placebo in all therapeutic groups.43
Subsequently, two phase 3 clinical trials investigating ustekinumab induction therapy in Crohn's disease (UNITI-1 and UNITI-2) and one maintenance trial (IM-UNITI) were conducted. All the trials were randomised, double-blind and placebo-controlled. The outcomes are summarised below.44
Efficacy as induction therapy (UNITI-1 and UNITI-2)Both trials included patients with moderate to severe Crohn's disease, i.e., with scores ranging from 220 to 450 on the Crohn's Disease Activity Index (CDAI). Patients were required to show no response or to be intolerant to anti-TNFα drugs (UNITI-1) or to conventional treatment (UNITI-2), including immunosuppressants (azathioprine, mercaptopurine, methotrexate) or corticosteroids. Patients in UNITI-2 could have previously received anti-TNFα therapy, provided they had not shown no response or developed intolerance to this treatment. Induction was studied in both trials, analysing the efficacy of a fixed dose of 130mg or a variable, weight-adjusted dose (6mg/kg).
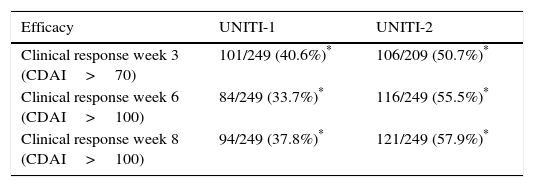
The primary objective of the study (clinical response at week 6) was achieved with both doses studied in the UNITI-1 and UNITI-2 trials. The main efficacy outcomes of both studies for the dose ultimately approved in the summary of product characteristics (6mg/kg) are shown in Table 1.
Efficacy results of ustekinumab in Crohn's disease in the UNITI-1 and UNITI-2 trials.
| Efficacy | UNITI-1 | UNITI-2 |
|---|---|---|
| Clinical response week 3 (CDAI>70) | 101/249 (40.6%)* | 106/209 (50.7%)* |
| Clinical response week 6 (CDAI>100) | 84/249 (33.7%)* | 116/249 (55.5%)* |
| Clinical response week 8 (CDAI>100) | 94/249 (37.8%)* | 121/249 (57.9%)* |
CDAI: Crohn's Disease Activity Index.
The first efficacy data from these studies were analysed at week 3 of induction, where a significant difference between ustekinumab and placebo was already observed, suggesting that the treatment would be faster-acting than other biologic drugs.22 This difference increased progressively over the course of the trial, and by week 6 (primary endpoint of the study), 34% of patients in UNITI-1 and 55% in UNITI-2 met clinical response criteria (CDAI<100), and 38% and 58%, respectively, by week 8. As with other biological treatments, efficacy was greater in patients naïve to anti-TNFα drugs (UNITI-2) than in previous null responders to such drugs (UNITI-1).45
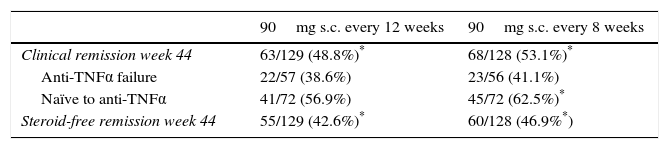
Efficacy as maintenance therapy (IM-UNITI)The IM-UNITI trial showed that maintenance therapy with ustekinumab (administered subcutaneously) was effective in patients that had responded to intravenous induction therapy in the UNITI-1 or UNITI-2 trials.44 Although the percentage of remission was higher in the group receiving 90mg of ustekinumab every 8 weeks than in the group receiving the same dose every 12 weeks, the difference was not statistically significant.44 With both administration regimens, the clinical remission rate (CDAI<150) at week 44 (primary endpoint) was significantly higher versus placebo. Table 2 shows the efficacy results of the IM-UNITI trial with the two foregoing dosage regimens.
Efficacy results of ustekinumab in Crohn's disease in the IM-UNITI trial.
| 90mg s.c. every 12 weeks | 90mg s.c. every 8 weeks | |
|---|---|---|
| Clinical remission week 44 | 63/129 (48.8%)* | 68/128 (53.1%)* |
| Anti-TNFα failure | 22/57 (38.6%) | 23/56 (41.1%) |
| Naïve to anti-TNFα | 41/72 (56.9%) | 45/72 (62.5%)* |
| Steroid-free remission week 44 | 55/129 (42.6%)* | 60/128 (46.9%*) |
Administration of ustekinumab in the maintenance phase starts with a subcutaneous dose at week 8, after which, according to clinical criteria, it can be administered every 8 or 12 weeks,40 as described above. The possibility of dose adjustment arose from a sub-analysis of the IM-UNITI trial, in which, after failing to respond to the 12-week regimen, patients were switched to an 8-week schedule and clinical remission rates of 41% were achieved 16 weeks after modifying the regimen.46
As in the induction phase of the trial, efficacy was greater in patients naïve to biologics than in null responders to anti-TNFα therapy (Table 2). Thus, patients from the UNITI-2 trial (naïve to biologics) achieved higher efficacy rates than those in UNITI-1 (failure of anti-TNFα therapy). This difference is in line with efficacy outcomes described in other biological drugs, such as vedolizumab.21
Based on all these results, the report prepared by the committee for medicinal products for human use of the EMA considered that “ustekinumab brings significant clinical benefit in comparison with existing therapies”.47
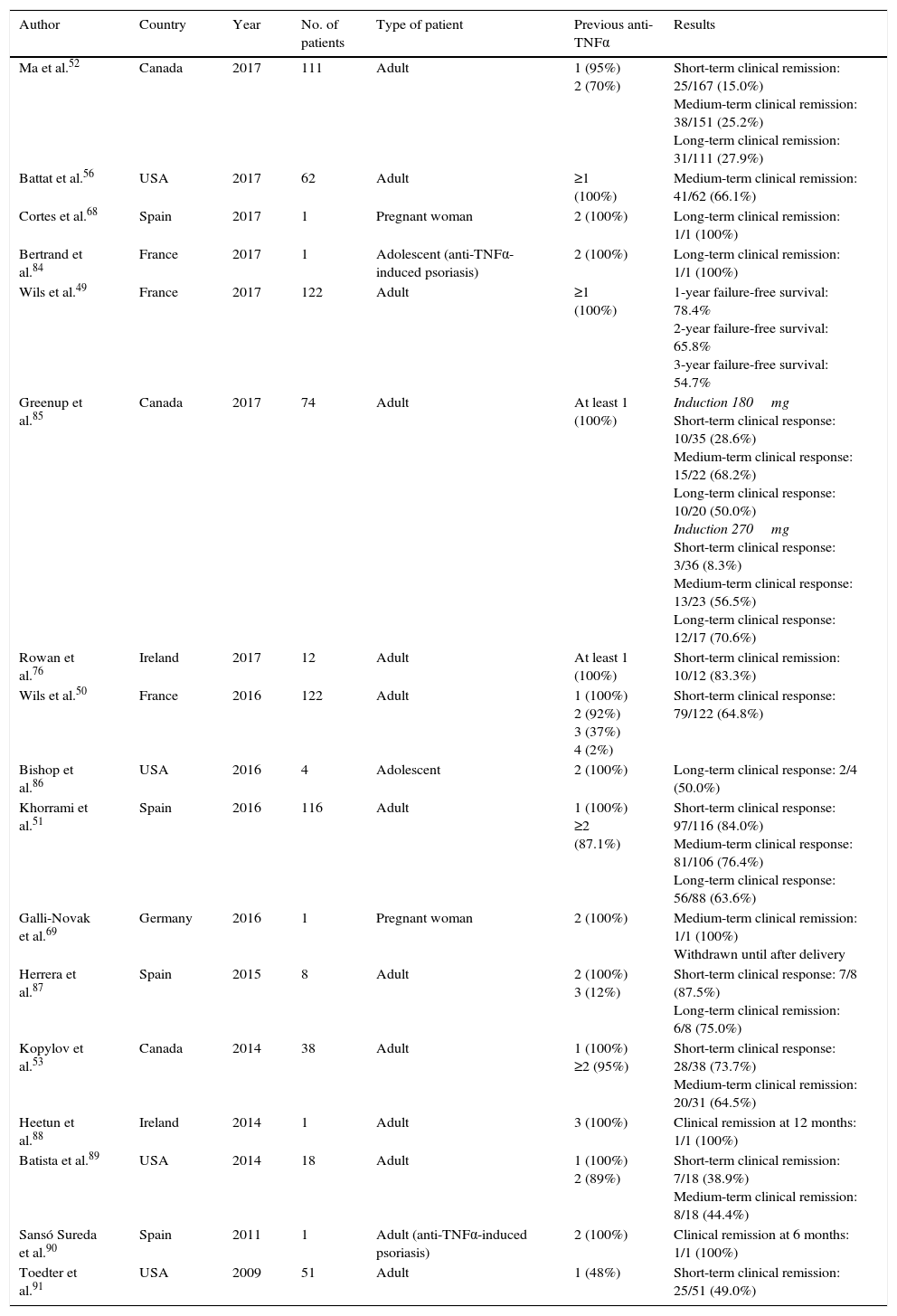
Outcomes in clinical practiceThe series of patients treated with ustekinumab enrolled in clinical practice studies which have been published to date, together with their main efficacy outcomes, are summarised in Table 3. It should be noted that all patients included in these series had previously failed to respond to at least one TNF inhibitor, and most had failed with 2 such drugs. In addition, most studies used exclusively subcutaneous doses, and the overall evaluation and interpretation of outcomes was greatly hampered by the highly variable frequency of administration (generally based on clinical criteria).
Clinical studies evaluating ustekinumab in Crohn's disease in clinical practice.
| Author | Country | Year | No. of patients | Type of patient | Previous anti-TNFα | Results |
|---|---|---|---|---|---|---|
| Ma et al.52 | Canada | 2017 | 111 | Adult | 1 (95%) 2 (70%) | Short-term clinical remission: 25/167 (15.0%) Medium-term clinical remission: 38/151 (25.2%) Long-term clinical remission: 31/111 (27.9%) |
| Battat et al.56 | USA | 2017 | 62 | Adult | ≥1 (100%) | Medium-term clinical remission: 41/62 (66.1%) |
| Cortes et al.68 | Spain | 2017 | 1 | Pregnant woman | 2 (100%) | Long-term clinical remission: 1/1 (100%) |
| Bertrand et al.84 | France | 2017 | 1 | Adolescent (anti-TNFα-induced psoriasis) | 2 (100%) | Long-term clinical remission: 1/1 (100%) |
| Wils et al.49 | France | 2017 | 122 | Adult | ≥1 (100%) | 1-year failure-free survival: 78.4% 2-year failure-free survival: 65.8% 3-year failure-free survival: 54.7% |
| Greenup et al.85 | Canada | 2017 | 74 | Adult | At least 1 (100%) | Induction 180mg Short-term clinical response: 10/35 (28.6%) Medium-term clinical response: 15/22 (68.2%) Long-term clinical response: 10/20 (50.0%) Induction 270mg Short-term clinical response: 3/36 (8.3%) Medium-term clinical response: 13/23 (56.5%) Long-term clinical response: 12/17 (70.6%) |
| Rowan et al.76 | Ireland | 2017 | 12 | Adult | At least 1 (100%) | Short-term clinical remission: 10/12 (83.3%) |
| Wils et al.50 | France | 2016 | 122 | Adult | 1 (100%) 2 (92%) 3 (37%) 4 (2%) | Short-term clinical response: 79/122 (64.8%) |
| Bishop et al.86 | USA | 2016 | 4 | Adolescent | 2 (100%) | Long-term clinical response: 2/4 (50.0%) |
| Khorrami et al.51 | Spain | 2016 | 116 | Adult | 1 (100%) ≥2 (87.1%) | Short-term clinical response: 97/116 (84.0%) Medium-term clinical response: 81/106 (76.4%) Long-term clinical response: 56/88 (63.6%) |
| Galli-Novak et al.69 | Germany | 2016 | 1 | Pregnant woman | 2 (100%) | Medium-term clinical remission: 1/1 (100%) Withdrawn until after delivery |
| Herrera et al.87 | Spain | 2015 | 8 | Adult | 2 (100%) 3 (12%) | Short-term clinical response: 7/8 (87.5%) Long-term clinical remission: 6/8 (75.0%) |
| Kopylov et al.53 | Canada | 2014 | 38 | Adult | 1 (100%) ≥2 (95%) | Short-term clinical response: 28/38 (73.7%) Medium-term clinical remission: 20/31 (64.5%) |
| Heetun et al.88 | Ireland | 2014 | 1 | Adult | 3 (100%) | Clinical remission at 12 months: 1/1 (100%) |
| Batista et al.89 | USA | 2014 | 18 | Adult | 1 (100%) 2 (89%) | Short-term clinical remission: 7/18 (38.9%) Medium-term clinical remission: 8/18 (44.4%) |
| Sansó Sureda et al.90 | Spain | 2011 | 1 | Adult (anti-TNFα-induced psoriasis) | 2 (100%) | Clinical remission at 6 months: 1/1 (100%) |
| Toedter et al.91 | USA | 2009 | 51 | Adult | 1 (48%) | Short-term clinical remission: 25/51 (49.0%) |
Short-term: 2–3 months; medium term: ∼6 months; long term: ∼12 months.
The results of long-term follow-up (week 96) of the IM-UNITI study, including patients who continued with both the 12-week and 8-week regimens were published recently: 79% and 87% of patients, respectively, continued in clinical remission at the end of follow-up.48 Similarly, the results of the largest follow-up to the date of treatment with ustekinumab in patients with Crohn's disease were published recently, showing a failure-free survival rate of 78% at 1 year, 66% at 2 years, and 55% at 3 years.49 These long-term outcomes may be superior to those previously described with anti-TNF drugsα,15 although firm conclusions cannot be drawn due to the absence of direct comparative studies.
Perianal fistulising Crohn's diseaseThere is very limited, but promising, evidence for the efficacy of ustekinumab in the treatment of perianal fistulas. The French series published by Wils et al. (GETAID) included 9 patients with perianal involvement, of which 8 (89%) showed clinical benefit after 3 months of treatment with ustekinumab.50 Other series have reported similar results, such as a Spanish series (61% patients with improvement of perianal involvement),51 and 2 Canadian series that reported efficacy in perianal fistulas of 49%52 and 69%,53 respectively.
Co-treatment with immunosuppressantsThe benefit of adding immunosuppressive drugs to biologics to improve their efficacy is a controversial practice that is not supported by solid evidence.54,55 One third of the patients in the IM-UNITI trial received add-on immunosuppressants at the start of the trial, without this appearing to improve the efficacy of the treatment.43 Endoscopic studies have shown that the use of immunosuppressants does not modify response and remission rates.56 In summary, although there is no evidence that the efficacy of ustekinumab is increased with add-on immunosuppressants, more data are needed before a conclusive recommendation can be given in this respect.
Usefulness of biomarkers as predictors of treatment responsePhase 3 trials have evaluated the possible role of C-reactive protein (CRP) and faecal calprotectin levels as predictors of response to treatment with ustekinumab.57,58 In the UNITI-1 and UNITI-2 trials, plasma CRP levels at weeks 3, 6 and 8 were significantly more reduced compared to baseline with ustekinumab than with placebo.44 Similar results were observed for faecal calprotectin levels, which were already lower at week 6, in a statistically significant manner.44 In the IM-UNITI trial, the reductions achieved in CRP and faecal calprotectin levels during the induction phase were generally maintained until week 44 with both doses of ustekinumab.44 Finally, additional studies have identified other serum biomarkers as potential predictors of response to treatment, including reduced serum levels of IL-2 receptor, myeloperoxidase, creatine kinase MB and IL-8, as well as an increase in serum levels of amyloid protein A, IFNγ, IL-17A and TNFα.59,60
Efficacy on mucosal healingStudies dating from 2007 have proposed mucosal healing as a prognostic marker of the evolution of the disease, and therefore one of the main therapeutic objectives.61 In the UNITI and IM-UNITI clinical trials, an endoscopic sub-study was performed to analyse the efficacy of ustekinumab on mucosal healing. Patients underwent colonoscopy at baseline, and at weeks 8 and 44. A significantly greater reduction from baseline Simple Endoscopic Score for Crohn's Disease (SES-CD) scores was observed in patients treated with ustekinumab compared to those who received placebo. This therapeutic benefit of ustekinumab was confirmed in both induction studies (UNITI-1 and UNITI-2), and for both maintenance doses (every 8 and every 12 weeks).62 Finally, these findings have been confirmed in some non-randomised studies, such as that of Battat et al. (who reported an endoscopic response in 59% of patients),56 or that of Ma et al. (who confirmed an endoscopic response in half their series).52
Safety profileAlthough ustekinumab was only recently approved for Crohn's disease, it has been used extensively in dermatology and rheumatology. Because of this, the safety profile of the drug is based on a considerable number of patients treated in real clinical practice.
For example, findings from the PSOLAR study, a longitudinal assessment and registry of more than 12,000 patients with psoriasis treated with different biological drugs,63,64 suggest that treatment with infliximab and adalimumab was associated with an increased risk of serious infections compared to placebo; whereas patients receiving ustekinumab did not show a higher incidence of infectious complications.65 However, it must be borne in mind that safety outcomes in patients with psoriasis cannot necessarily be extrapolated to those with Crohn's disease, since psoriasis patients usually receive the drug as monotherapy and at lower doses.
On the other hand, it is worth noting that patients treated with ustekinumab have hitherto showed no sign of reactivation of latent tuberculosis. In this context, a recent meta-analysis compared rates of tuberculosis reactivation with ustekinumab and various anti-TNFα drugs in phase 2 and 3 clinical trials conducted in patients with rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, Crohn's disease and ulcerative colitis. The study found a significantly lower frequency of complications in patients receiving ustekinumab.66
Likewise, the PSOLAR registry suggests that ustekinumab is safe during pregnancy, insofar as no increase in abortion, premature birth or congenital anomalies were observed.67 Additional data on uneventful pregnancy with delivery at term have been published specifically in patients with Crohn's disease treated with ustekinumab.68,69 Despite this, and until further evidence emerges, it is advisable not to use ustekinumab during pregnancy, unless the benefits outweigh the risks. Similarly, it is unknown if the drug is excreted in breast milk, so patients treated with ustekinumab are currently advised to avoid breastfeeding.
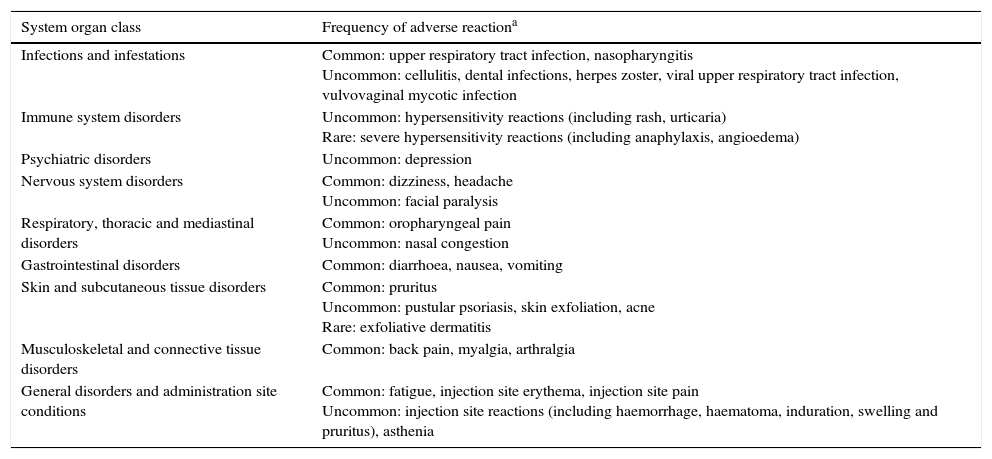
Table 4 summarises the adverse reactions reported during clinical trials conducted with ustekinumab in patients with psoriasis, psoriatic arthritis and Crohn's disease, as well as adverse reactions reported from post-marketing experience.37 Specifically, in clinical trials conducted in patients with Crohn's disease, the occurrence of adverse events was similar to that described with placebo.44 There were no deaths or instances of progressive multifocal leukoencephalopathy after one year of exposure to ustekinumab.44 These results have been corroborated in a recent safety meta-analysis that included 7 clinical trials evaluating ustekinumab in psoriasis, Crohn's disease, sarcoidosis and multiple sclerosis,70 and in several other series.50,52,71 Finally, paradoxical reactions similar to those described with anti-TNF drugs have been described with ustekinumab in exceptional casesα.72
Ustekinumab-induced adverse reactions reported in clinical trials and clinical practice studies.
| System organ class | Frequency of adverse reactiona |
|---|---|
| Infections and infestations | Common: upper respiratory tract infection, nasopharyngitis Uncommon: cellulitis, dental infections, herpes zoster, viral upper respiratory tract infection, vulvovaginal mycotic infection |
| Immune system disorders | Uncommon: hypersensitivity reactions (including rash, urticaria) Rare: severe hypersensitivity reactions (including anaphylaxis, angioedema) |
| Psychiatric disorders | Uncommon: depression |
| Nervous system disorders | Common: dizziness, headache Uncommon: facial paralysis |
| Respiratory, thoracic and mediastinal disorders | Common: oropharyngeal pain Uncommon: nasal congestion |
| Gastrointestinal disorders | Common: diarrhoea, nausea, vomiting |
| Skin and subcutaneous tissue disorders | Common: pruritus Uncommon: pustular psoriasis, skin exfoliation, acne Rare: exfoliative dermatitis |
| Musculoskeletal and connective tissue disorders | Common: back pain, myalgia, arthralgia |
| General disorders and administration site conditions | Common: fatigue, injection site erythema, injection site pain Uncommon: injection site reactions (including haemorrhage, haematoma, induration, swelling and pruritus), asthenia |
Various clinical trial sub-studies have shown that ustekinumab improves quality of life. The CERTIFI trial evaluated health-related quality of life using the Health-Related Quality of Life (HRQoL) questionnaire,73 and sleep impairment using the Jenkins sleep assessment questionnaire (JSEQ),74 and reported significant improvement in patients treated with ustekinumab compared to those who received placebo. More recently, health-related quality of life has been evaluated in phase 3 clinical trials using the generic SF-36 quality of life questionnaire and the specific inflammatory bowel disease questionnaire (IBDQ).75 At week 8 in both the UNITI-1 and UNITI-2 studies, patients treated with ustekinumab showed a statistically significant improvement in overall IBDQ score and in the physical and mental domains of the SF-36 compared to placebo.75 In general, the aforementioned improvement was maintained in patients treated with ustekinumab, and, in the IM-UNITI trial specifically, it continued until week 44.75
Serum levels of ustekinumabThe relationship between serum levels of ustekinumab and clinical efficacy parameters has been studied. In phase 3 clinical trials, the proportion of patients achieving clinical remission was evaluated on the basis of serum levels of the drug. In both the UNITI-2 and the IM-UNITI trials, the percentage of patients in remission was greater in groups with higher serum ustekinumab levels, although the differences did not reach statistical significance.44 This correlation, however, has been confirmed in other series in which higher serum levels of ustekinumab have been associated with greater clinical and endoscopic response and lower CRP levels.56,76 For example, serum levels of over 4.5μg/ml in the maintenance phase have been associated with greater endoscopic response.56
Another parameter of interest is the proportion of anti-drug antibodies generated after treatment with ustekinumab. In clinical trials in Crohn's disease, incidence of anti-drug antibodies was low (less than 3%),44 and levels of these immunoglobulins were not correlated with reduced response to treatment.77 These findings, associated with the promising safety profile, strongly suggest that ustekinumab is associated with relatively high survival rates in Crohn's disease. This has been confirmed in other diseases, such as psoriasis, in which survival is higher with ustekinumab compared to infliximab and adalimumab.78
Contraindications and interactionsTreatment with ustekinumab is contraindicated in the following situations: (a) hypersensitivity to the active substance or to any of the excipients, and (b) clinically important, active infections, such as tuberculosis.40 As discussed above, in patients with psoriatic arthritis and Crohn's disease, the pharmacokinetics of ustekinumab is not affected by the concomitant use of azathioprine, mercaptopurine, methotrexate, oral corticosteroids or non-steroidal anti-inflammatory drugs, or by prior exposure to anti-TNF drugsα.44,79 In vitro studies have suggested that dose adjustment is not necessary in patients concomitantly receiving drugs that are metabolised by CYP450.80 In addition, there is no evidence that concomitant use of immunosuppressants or corticosteroids affects the efficacy or safety of ustekinumab in patients with Crohn's disease.44
Role of ustekinumab in the current treatment of Crohn's diseaseIt is well known that approximately one third of patients do not respond to a first anti-TNFα drug, and that a certain, though hard to determine, percentage will present a partial response that may be insufficient; in these cases, a second anti-TNFα will have even less effect.19 Secondary loss of response to anti-TNFα drugs, meanwhile, is usually managed by optimising (intensifying) the treatment, a strategy that significantly increases costs.14 Switching to another anti-TNFα after loss of efficacy is also a very widespread strategy at present, but its effectiveness is discreet in the short term and poor in the medium term.19 Currently, the only approved therapeutic alternative is vedolizumab. The speed of action of this drug is modest, and it may be slower at inducing remission than anti-TNFα drugs, although its long-term efficacy appears to be similar to infliximab and adalimumab.
The approval of ustekinumab represents a necessary and long-awaited therapeutic alternative. It is currently the only treatment for Crohn's disease that targets IL-12/23 inflammatory pathways. This novel mechanism of action is a good alternative for patients who have failed or were intolerant to previous treatment with corticosteroids, immunotherapy, or anti-TNFα therapy.
So far, no clinical trials have directly compared ustekinumab to other approved agents for the treatment of Crohn's disease (i.e., infliximab, adalimumab or vedolizumab). Such studies would be very useful to clarify the role of ustekinumab in the therapeutic algorithm of Crohn's disease. It has, however, been indirectly compared in a systematic review of all induction and maintenance trials evaluating ustekinumab, infliximab, adalimumab and vedolizumab. The findings of this study suggest that the likelihood of achieving remission at one year with ustekinumab is greater compared to vedolizumab or adalimumab.81 However, this method of indirect comparison has limitations, and conclusions must be interpreted with caution.
In many patients, the route of administration (subcutaneous) and the regimen (every 8–12 weeks) of ustekinumab may be considered an advantage over intravenous therapies, such as infliximab and vedolizumab, in which dose frequency is higher during the induction phase.82,83 Ustekinumab has a very favourable safety profile, with no evidence to date of the development of tumours or re-activation of tuberculosis. In addition, few patients appear to develop antibodies to ustekinumab, and the risk of immunogenicity may be less than with infliximab and adalimumab, although this should be confirmed in future studies.
Anti-TNFα drugs are currently widely used in clinical practice, and, at least for the time being, are likely to continue to be first-choice therapy, with ustekinumab being reserved for patients with no response, intolerance, or contraindication for anti-TNFα. Nevertheless, in the opinion of international regulatory agencies – such as the EMA and the FDA – ustekinumab is also a first-line biological therapy for patients with Crohn's disease.
In conclusion, ustekinumab is a promising alternative for the treatment of active, moderate to severe Crohn's disease in patients who do not respond or are intolerant to treatment with conventional or anti-TNFα therapies. Its position with respect to other drugs in the therapeutic arsenal, particularly anti-TNFα, has yet to be clearly defined. This will depend on the experience accumulated over the next few years in terms of efficacy (including a greater number of patients and a longer follow-up), safety (if the favourable profile described so far is confirmed) and, obviously, cost of treatment.
Conflicts of interestJavier P. Gisbert: scientific consultancy, research support and/or training activities: MSD, Abbvie, Hospira, Pfizer, Kern Pharma, Biogen, Takeda, Janssen, Roche, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Vifor Pharma.
María Chaparro: scientific consultancy, research support and/or training activities: MSD, Abbvie, Hospira, Pfizer, Takeda, Janssen, Ferring, Shire Pharmaceuticals, Dr. Falk Pharma, Tillotts Pharma.
Please cite this article as: Gisbert JP, Chaparro M. Ustekinumab en el tratamiento de la enfermedad de Crohn. Gastroenterol Hepatol. 2017;40:688–698.