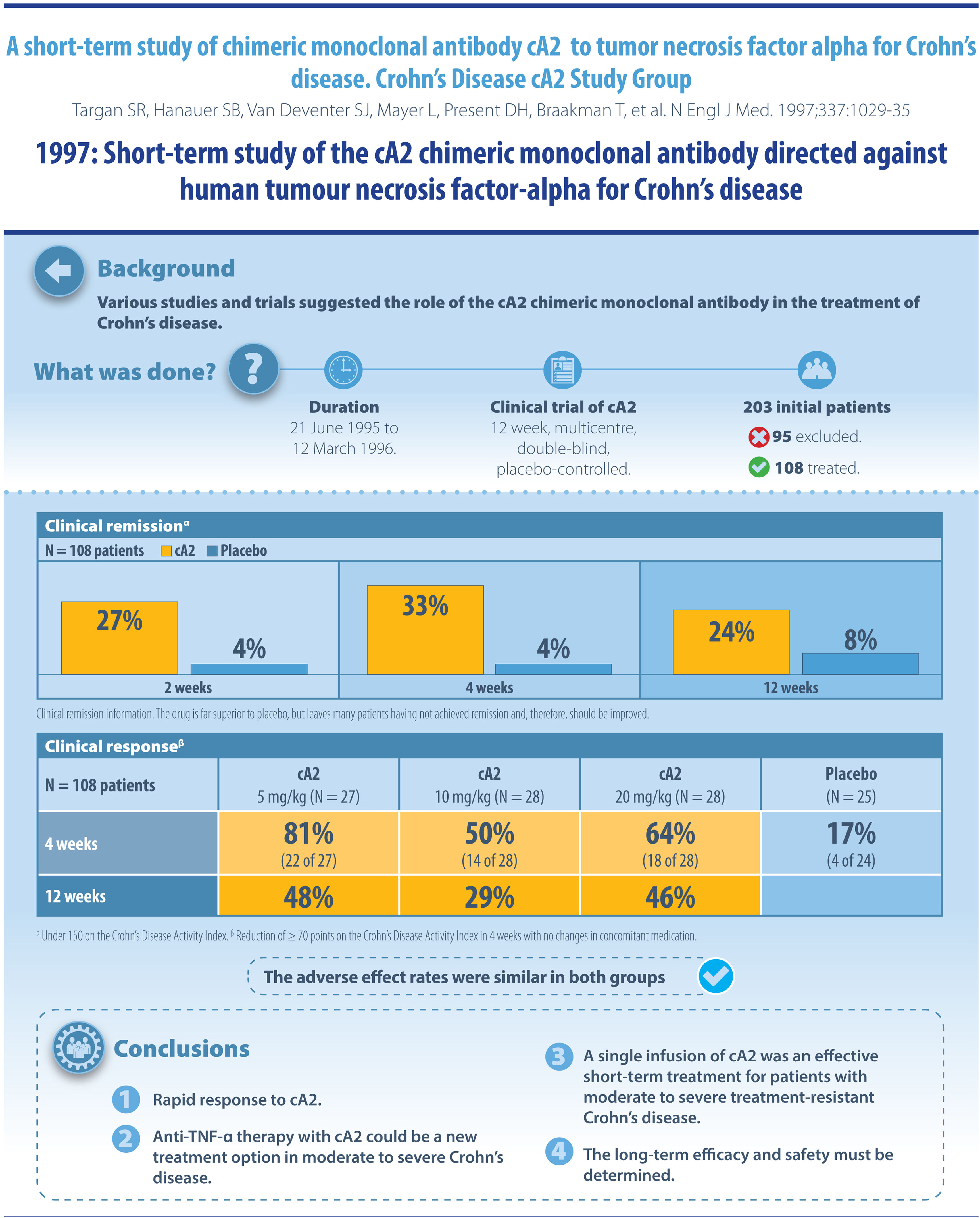
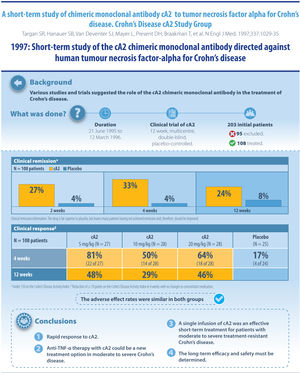
And with cA2 (infliximab), biologics have reached IBD. In this induction study with a single dose of infliximab, its unequivocal efficacy was demonstrated in a high percentage of patients with moderate–severe Crohn's disease who had not responded to conventional treatment. Today this study, which defined previous failure on mesalazine as an inclusion criterion, and wherein the only objective variable to measure inflammation was serum C-reactive protein, would have serious difficulties not being rejected by a multitude of prestigious medical journals. But it was another time (pre-mucosal healing, pre-histological healing, etc.) and the drug showed an efficacy that was unmatched in treatments previously approved for Crohn's disease. What's more, of the 3 doses assessed, it seemed that the 5mg/kg dose was even more effective than the 10 or 20mg/kg dose, with the former being the dose that we continue to use 23 years later in all our patients with Crohn's disease. Later came trials with maintenance doses, and those done in patients with perianal Crohn's disease. The magnificence of this drug, which has helped so many people over more than 20 years, is that it continues to be the drug of choice for our patients with IBD, and there are already a multitude of biosimilars on the market. Any new biologic that wants to become part of the therapeutic arsenal for managing patients with IBD must take into account that infliximab obtained results in trials that are hard to beat, and continues to show them in our daily use, at least as far as efficacy is concerned. This drug still has, and will have, a lot to say in the coming years for managing our patients’ conditions.
Please cite this article as: Gomollón F, Marín-Jiménez I. Año 1997: estudio a corto plazo del anticuerpo monoclonal quimérico cA2 contra el factor de necrosis tumoral alfa para la enfermedad de Crohn. Gastroenterol Hepatol. 2020;43:379–380.