Colorectal cancer presents itself as acute bowel occlusion in 10–40% of patients. There are two main therapeutic approaches: urgent surgery and endoluminal placement self-expandable metallic stents (SEMS).
Aims and MethodsThis study intended to better clarify the risk/benefit ratio of the above-mentioned approaches. We conducted a retrospective longitudinal multicenter study, including 189 patients with acute malignant colorectal occlusion, diagnosed between January 2005 and March 2013.
ResultsGlobally (85 patients – 35 bridge-to-surgery and 50 palliative), SEMS's technical success was of 94%. Palliative SEMS had limited clinical success (60%) and were associated with 40% of complications. SEMS occlusion (19%) was the most frequent complication, followed by migration (9%) and bowel perforation (7%). Elective surgery after stenting was associated with a higher frequency of primary anastomosis (94% vs. 76%; p=0.038), and a lower rate of colostomy (26% vs. 55%; p=0.004) and overall mortality (31% vs. 57%; p=0.02). However, no significant differences were identified concerning postoperative complications. Regarding palliative treatment, no difference was found in the complications rate and overall mortality between SEMS and decompressive colostomy/ileostomy. In this SEMS subgroup, we found a higher rate of reinterventions (40% vs. 5%; p=0.004) and a longer hospital stay (14, nine vs. seven, three days; p=0.004).
ConclusionSEMS placement as a bridge-to-surgery should be considered in the acute treatment of colorectal malignant occlusion, since it displays advantages regarding primary anastomosis, colostomy rate and overall mortality. In contrast, in this study, palliative SEMS did not appear to present significant advantages when compared to decompressive colostomy.
O cancro colorrectal manifesta-se como oclusão intestinal aguda em 10–40% dos doentes. Existem duas abordagens terapêuticas principais: cirurgia de urgência e prótese endoluminal.
Objectivo e MétodosEste estudo teve como objetivo clarificar o risco/benefício das abordagens mencionadas. Foi realizado um estudo multicêntrico, retrospetivo longitudinal, que incluiu 189 doentes com oclusão colorrectal maligna aguda, diagnosticados entre janeiro de 2005 e março de 2013.
ResultadosGlobalmente (85 pacientes – 35 como ponte para cirurgia e 50 como paliação) a colocação de prótese teve sucesso técnico de 94%. As próteses paliativas apresentaram sucesso clínico limitado (60%) e associaram-se a 40% de complicações. A oclusão tumoral da prótese (19%) foi a complicação mais frequente, seguindo-se a migração (9%) e a perfuração intestinal (7%). A cirurgia eletiva após colocação de prótese associou-se a maior frequência de anastomoses primárias (94% vs 76%; p=0.038) e a menores taxas de colostomia (26% vs 55%; p=0.004) e mortalidade (31% vs 57%; p=0.02). Contudo, não houve diferenças significativas nas complicações pós-cirúrgicas. No tratamento paliativo, a prótese e a colostomia/ileostomia descompressiva não apresentaram diferenças significativas nas complicações ou mortalidade. Neste subgrupo de próteses, observou-se elevada taxa de reintervenção (40% vs 5%; p=0.004) e de tempo de internamento (14,9 vs 7,3 dias; p=0.004).
ConclusãoA colocação de prótese como ponte para a cirurgia deve ser considerada no tratamento agudo da oclusão maligna colorrectal, pois apresenta vantagens nas taxas de anastomoses primárias, colostomias e mortalidade. Em contraste, neste estudo as próteses paliativas não apresentaram vantagem clínica significativa em comparação à colostomia descompressiva.
Colorectal cancer (CRC) is the fourth most common tumor worldwide1 and one of the cancers with the highest incidence and mortality in Portugal.2
CRC presents itself as acute intestinal occlusion in 10–40% of patients.1,3–9 This is more common when the tumor is located in the left colon4 and results in a higher postoperative mortality (12%) when compared to non-occlusive tumors (3.5%).10
Currently, there are two main approaches for acute decompression: the traditional urgent surgery and the endoluminal placement of self-expandable metallic stents (SEMS).3,4,11–13 Urgent surgical resection usually involves a defunctioning stoma with or without primary resection of the obstructing tumor. Additionally, it is associated with a high mortality (15–34%) and morbidity (15–64%) when compared to elective surgery (0.9–6%).1,3,11,14–16 Therefore, non-surgical approaches have been proposed as an alternative. Among them, SEMS placement has been increasingly used for the relief of colonic occlusion symptoms, since its first application in the early 1990s.
The endoluminal endoscopic approach is theoretically advantageous, allowing the circumvention of an emergency surgical intervention which, besides the already mentioned implications, results in a permanent colostomy in 50–66% of cases.1,3,4,11,14,16 Additionally, it allows palliative treatment without any surgical intervention.3,4,12,15,17 Thus, SEMS placement can be used as a bridge to elective surgery in patients with potential for curative resection or palliative treatment in patients who cannot undergo surgery or with advanced disease. However, SEMS placement also has complications (21.0–34.4%),5,8,16 namely bowel perforation (1.2–13%), migration (1.2–11.8%) and reocclusion by tumor ingrowth (1.8–9%).3,7,8,16,18 Moreover, SEMS does not appear to provide a significant improvement in the overall survival or in the long term prognosis.13,14,19
Many investigations have tried to compare SEMS vs. surgery. However, the reported results are diverse, the randomized studies are scarce and globally the population samples per study are small and have varying criteria for outcomes measurement. With this study, we aim to clarify the risk/benefit ratio of these approaches in real clinical practice, comparing: (a) SEMS placement with curative and palliative purposes; (b) SEMS placement as bridge-to-surgery vs. urgent surgery (curative purpose); (c) SEMS placement vs. urgent surgery (palliative purpose).
2Aims and methods2.1Study designThis is a retrospective longitudinal nonrandomized multicenter study, involving two hospitals: Braga Hospital and Unidade Local de Saúde do Alto Minho (ULSAM). We studied all patients with acute malignant colorectal occlusion that were treated by stenting or surgery between January 2005 and March 2013. Patients’ data were collected by analyzing all clinical, endoscopic, radiologic and surgical reports (manual and electronic records).
The inclusion criteria were: an acute colorectal occlusion caused by colorectal malignancy which underwent SEMS placement or urgent surgery, in the determined period. Patients with incomplete data or admitted with clinical symptoms of bowel perforation were excluded from the study.
2.2Colorectal occlusion diagnosis and managementDiagnosis of acute colorectal occlusion was based on classical clinical and imaging findings: abdominal distension and pain, nausea and vomiting without normal emission of stool or air and a dilated colon in the abdominal radiography. Malignant origin and tumor location were determined using flexible sigmoidoscopy or abdominal computerized tomography and confirmed by biopsy/specimen evaluation. Patients then underwent either endoscopic stenting or urgent surgery, according to the availability of resources at each moment and clinical particularities. Before acute therapeutic procedures, some patients already had a presumptive orientation – possible curative or palliative purpose.
2.3StentingSEMS placement was performed by experienced endoscopists (>20 procedures) in both hospitals, according to the generally accepted procedure – usually through-the-scope using a double channel therapeutic endoscope under fluoroscopic guidance or direct endoscopic vision if the stricture could be previously crossed with an ultra-slim endoscope. Five types of uncovered colonic stents were used, according to the hospital's availability, with no pre-dilation: Wallflex®, Wallstent® and Ultraflex® (Boston Scientific), Evolution® (Cook Medical) and Hanarostent® (MITec). After SEMS placement, immediate signs of decompression were once again evaluated and clinical examination and a plain abdominal radiography were usually repeated to assess the procedure's success and exclude complications.
2.4Urgent surgeryUrgent surgery was performed through laparotomy and the type of operation was determined based on tumor location, patient's general condition and the surgeon's experience. The surgical options included: one-stage procedure with primary anastomosis; two-stage procedure with segmentectomy and terminal colostomy (Hartmann's procedure) or segmentectomy with primary anastomosis and protecting ostomy; and three-stage procedure such as loop colostomy followed by segmentectomy maintaining the protective colostomy, to be closed at a later date.
After having acute treatment, patients received supportive care and adequate disease staging in order to decide the treatment purpose (curative vs. palliative). This decision was performed by an oncological decision group. According to their general condition, patients underwent other treatments such as elective surgery, chemotherapy and radiation therapy. However, most of these were not in the scope of this work.
2.5Study variables and outcomesPatients’ data were collected from the acute event until the last follow-up. Two main population groups were created, the “SEMS placement group” and the “Urgent surgery group”; then each one was divided into two subgroups according to the treatment purpose – “curative” and “palliative”. Baseline characteristics and outcomes of the procedure were documented and compared with the two major population groups studied.
Technical success in SEMS was defined as the successful placement of the stent across the malignant stricture allowing for patency. Clinical success was achieved when there was colonic decompression with obstructive symptom relief and without any complications requiring endoscopic or surgical reintervention. Patency (mainly for stent palliation) was defined as the period (in days) between SEMS placement and recurrence of obstructive symptoms or until a patient was lost in the follow-up or died. In SEMS bridge-to-surgery group, the time for surgery was defined as the number of days between SEMS placement and elective surgery.
Complications were defined as any adverse event related to SEMS placement or urgent surgery, leading to hospital readmission or prolonging the current hospital stay. Early and late complications were considered, as they occurred within 30 days or more after the time of the procedure, respectively. Concerning the overall complications, the elective surgery after stenting group's complications included those related to the SEMS placement, elective surgery and subsequent bowel reconstruction/recovery surgeries. Regarding the urgent surgery group, the overall complications included all those after urgent surgery and subsequent surgeries and ultimately bowel reconstruction/recovery.
Mortality was assessed as procedure-related death, occurring 30 days after intervention and as overall mortality during follow-up. Overall survival was calculated as the time interval from the date of the SEMS insertion or urgent surgery until the date of death or of the last follow-up. The overall length of hospital stay included, for each patient, the number of days as an inpatient during follow-up (acute procedures, surgeries, complications management).
As major outcome measurements for analytical comparison of colonic stenting vs. urgent surgery, we considered the overall rates of clinical success, primary anastomosis, colostomy rate, complications, hospital stay, mortality rate and overall survival.
2.6Statistical analysisStatistical analysis was performed using the Statistical Package for Social Sciences® (SPSS) software.
In order to compare SEMS as a bridge-to-surgery vs. Palliative SEMS and SEMS placement vs. urgent surgery, Student's t test (t) or Wilcoxon-Mann–Whitney test (Z) was used for quantitative variables (presented as mean±standard deviation (SD)) and chi-square test (X2) or Fisher's exact test were used for qualitative variables. The Kaplan–Meier method was performed to estimate median survival times of the SEMS and urgent surgery groups and the receiver operating characteristics (ROC) curve analysis was undertaken to identify a potential optimal threshold time to perform elective surgery after SEMS placement. The binary logistic regression model was applied to evaluate risk factors related to overall complications and mortality after treatment of acute malignant colorectal occlusion.
A p value less than or equal to 0.05 was considered to be statistically significant.
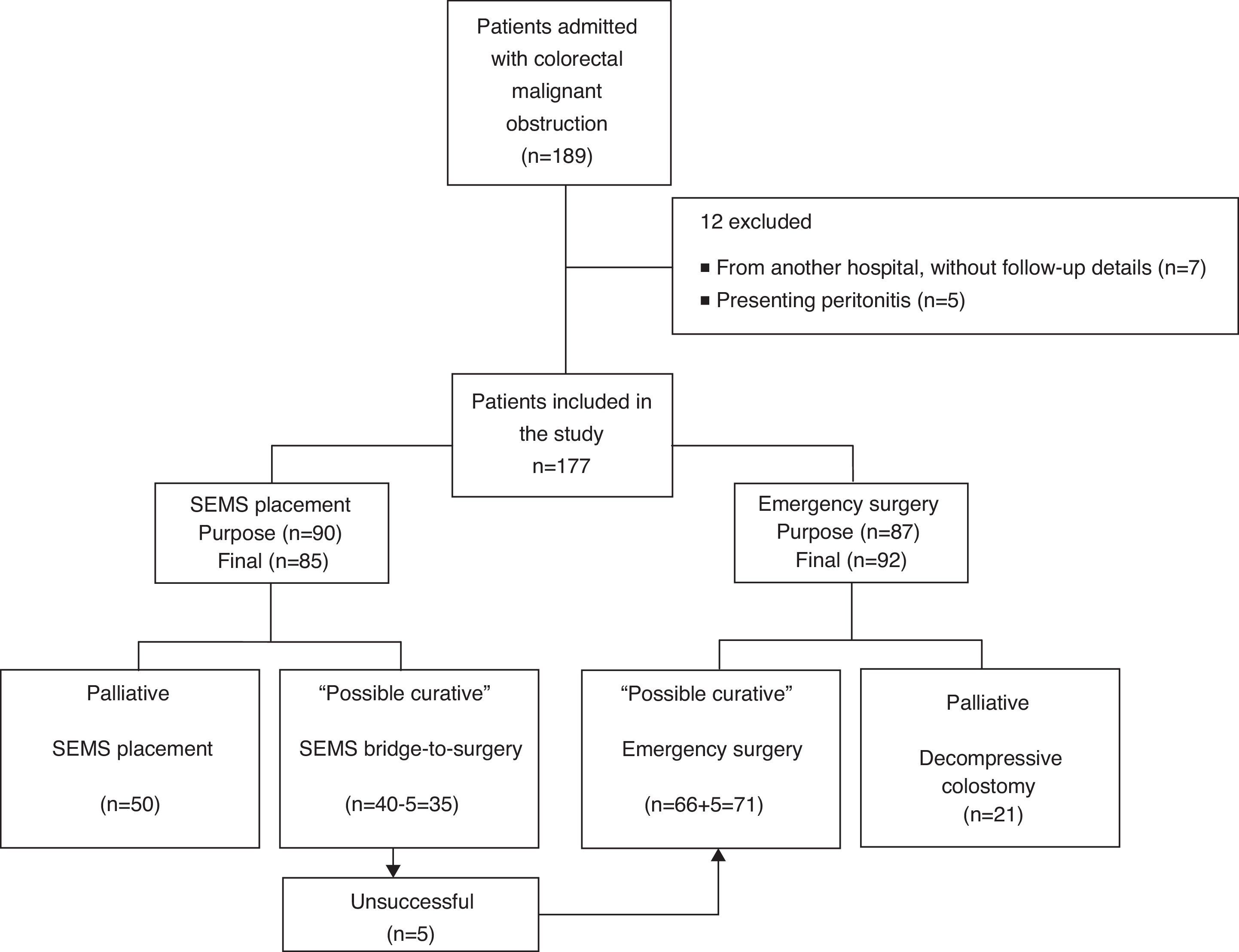
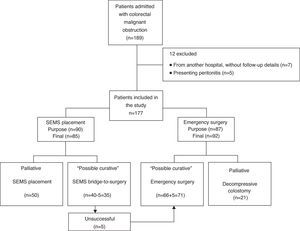
3ResultsBetween January 2005 and March 2013, 189 patients were diagnosed with malignant colorectal occlusion of which 12 were immediately excluded (seven from another hospital, without follow-up details and five with specific exclusion criteria). Thus, the population of this study included 177 patients, 85 of which underwent SEMS placement and 92 urgent surgery. In five patients proposed for SEMS as a bridge-to-surgery, stenting was not possible due to technical reasons; this was considered in the SEMS technical success calculations. However, in the general analysis these patients ended up in the urgent surgery group. Therefore, on the SEMS group, 35 were performed as a bridge-to-surgery and 50 as a definitive treatment (palliation). In the group of patients who underwent urgent surgery, the tumor was resected in 71 by colectomy with primary anastomosis or Hartmann's procedure, while 21 underwent only decompressive colostomy/ileostomy (palliation). Fig. 1 presents the flowchart of the patients’ management.
3.1Demographic, clinical characteristics and tumor featuresThe descriptive and global comparative analysis of the population studied (two main groups) is presented in Table 1. There were no noteworthy differences in patient characteristics or outcome between malignant occlusion treated in Braga Hospital and ULSAM.
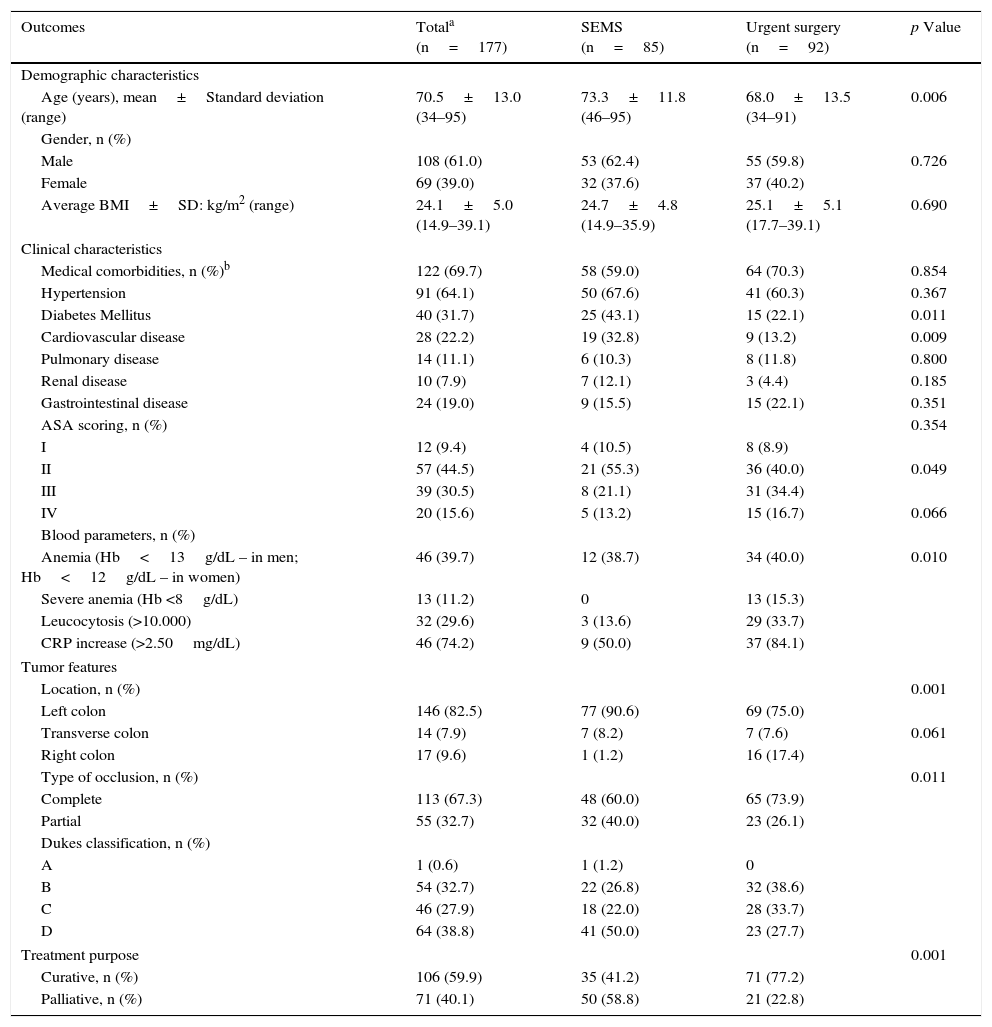
Demographic, clinical characteristics and tumor features of patients with malignant colorectal occlusion.
| Outcomes | Totala (n=177) | SEMS (n=85) | Urgent surgery (n=92) | p Value |
|---|---|---|---|---|
| Demographic characteristics | ||||
| Age (years), mean±Standard deviation (range) | 70.5±13.0 (34–95) | 73.3±11.8 (46–95) | 68.0±13.5 (34–91) | 0.006 |
| Gender, n (%) | ||||
| Male | 108 (61.0) | 53 (62.4) | 55 (59.8) | 0.726 |
| Female | 69 (39.0) | 32 (37.6) | 37 (40.2) | |
| Average BMI±SD: kg/m2 (range) | 24.1±5.0 (14.9–39.1) | 24.7±4.8 (14.9–35.9) | 25.1±5.1 (17.7–39.1) | 0.690 |
| Clinical characteristics | ||||
| Medical comorbidities, n (%)b | 122 (69.7) | 58 (59.0) | 64 (70.3) | 0.854 |
| Hypertension | 91 (64.1) | 50 (67.6) | 41 (60.3) | 0.367 |
| Diabetes Mellitus | 40 (31.7) | 25 (43.1) | 15 (22.1) | 0.011 |
| Cardiovascular disease | 28 (22.2) | 19 (32.8) | 9 (13.2) | 0.009 |
| Pulmonary disease | 14 (11.1) | 6 (10.3) | 8 (11.8) | 0.800 |
| Renal disease | 10 (7.9) | 7 (12.1) | 3 (4.4) | 0.185 |
| Gastrointestinal disease | 24 (19.0) | 9 (15.5) | 15 (22.1) | 0.351 |
| ASA scoring, n (%) | 0.354 | |||
| I | 12 (9.4) | 4 (10.5) | 8 (8.9) | |
| II | 57 (44.5) | 21 (55.3) | 36 (40.0) | 0.049 |
| III | 39 (30.5) | 8 (21.1) | 31 (34.4) | |
| IV | 20 (15.6) | 5 (13.2) | 15 (16.7) | 0.066 |
| Blood parameters, n (%) | ||||
| Anemia (Hb<13g/dL – in men; Hb<12g/dL – in women) | 46 (39.7) | 12 (38.7) | 34 (40.0) | 0.010 |
| Severe anemia (Hb <8g/dL) | 13 (11.2) | 0 | 13 (15.3) | |
| Leucocytosis (>10.000) | 32 (29.6) | 3 (13.6) | 29 (33.7) | |
| CRP increase (>2.50mg/dL) | 46 (74.2) | 9 (50.0) | 37 (84.1) | |
| Tumor features | ||||
| Location, n (%) | 0.001 | |||
| Left colon | 146 (82.5) | 77 (90.6) | 69 (75.0) | |
| Transverse colon | 14 (7.9) | 7 (8.2) | 7 (7.6) | 0.061 |
| Right colon | 17 (9.6) | 1 (1.2) | 16 (17.4) | |
| Type of occlusion, n (%) | 0.011 | |||
| Complete | 113 (67.3) | 48 (60.0) | 65 (73.9) | |
| Partial | 55 (32.7) | 32 (40.0) | 23 (26.1) | |
| Dukes classification, n (%) | ||||
| A | 1 (0.6) | 1 (1.2) | 0 | |
| B | 54 (32.7) | 22 (26.8) | 32 (38.6) | |
| C | 46 (27.9) | 18 (22.0) | 28 (33.7) | |
| D | 64 (38.8) | 41 (50.0) | 23 (27.7) | |
| Treatment purpose | 0.001 | |||
| Curative, n (%) | 106 (59.9) | 35 (41.2) | 71 (77.2) | |
| Palliative, n (%) | 71 (40.1) | 50 (58.8) | 21 (22.8) | |
Patients undergoing SEMS placement were older (73 vs. 68 years, p=0.006) and more likely to have Diabetes mellitus (43% vs 22%, p=0.011) or cardiovascular disease (33% vs. 13%, p=0.009). On the other hand, patients submitted to urgent surgery had more severe anemia (15% vs 0%, p=0.049) and higher CRP values (84% vs 50%, p=0.010). Most patients presented complete occlusion (67%) of the left colon (83%). However, the right colon was more frequently approached by surgery. The Dukes classification was also significantly different between the two groups: D stage was more frequent in the SEMS group (50%) and B stage in the urgent surgery group (39%). This was to be expected, considering the purpose of treatment: mainly palliative in patients undergoing SEMS placement (59%) and curative in patients submitted to urgent surgery (77%).
3.2Procedure details and analysis3.2.1Global SEMS placement (bridge-to-surgery and palliative)Technical success was obtained in 94% (85/90) of patients in whom SEMS placement was attempted (Table 2). Technical failure occurred in five patients with complete occlusion and subsequent inability to pass the guide wire.
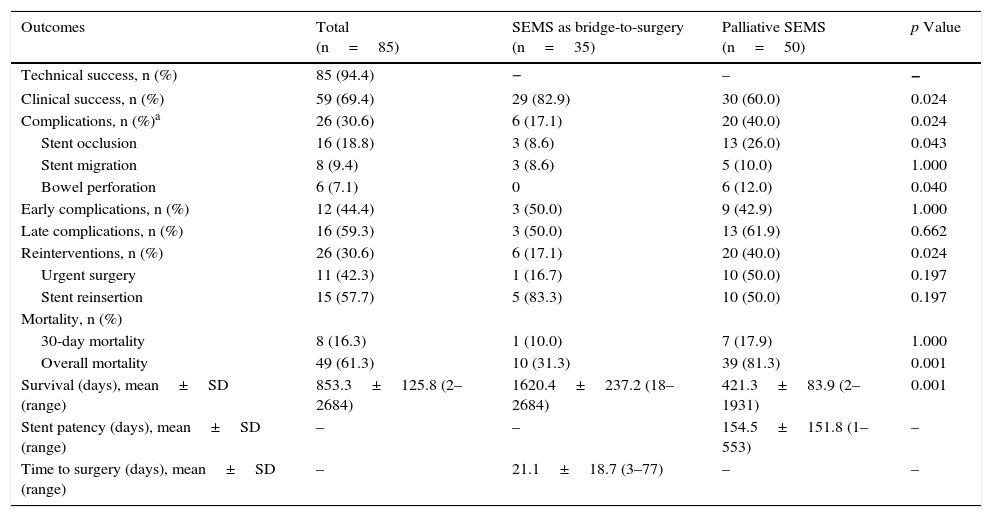
Comparison of the two main groups of SEMS placement (SEMS as bridge-to-surgery vs palliative SEMS).
| Outcomes | Total (n=85) | SEMS as bridge-to-surgery (n=35) | Palliative SEMS (n=50) | p Value |
|---|---|---|---|---|
| Technical success, n (%) | 85 (94.4) | − | – | − |
| Clinical success, n (%) | 59 (69.4) | 29 (82.9) | 30 (60.0) | 0.024 |
| Complications, n (%)a | 26 (30.6) | 6 (17.1) | 20 (40.0) | 0.024 |
| Stent occlusion | 16 (18.8) | 3 (8.6) | 13 (26.0) | 0.043 |
| Stent migration | 8 (9.4) | 3 (8.6) | 5 (10.0) | 1.000 |
| Bowel perforation | 6 (7.1) | 0 | 6 (12.0) | 0.040 |
| Early complications, n (%) | 12 (44.4) | 3 (50.0) | 9 (42.9) | 1.000 |
| Late complications, n (%) | 16 (59.3) | 3 (50.0) | 13 (61.9) | 0.662 |
| Reinterventions, n (%) | 26 (30.6) | 6 (17.1) | 20 (40.0) | 0.024 |
| Urgent surgery | 11 (42.3) | 1 (16.7) | 10 (50.0) | 0.197 |
| Stent reinsertion | 15 (57.7) | 5 (83.3) | 10 (50.0) | 0.197 |
| Mortality, n (%) | ||||
| 30-day mortality | 8 (16.3) | 1 (10.0) | 7 (17.9) | 1.000 |
| Overall mortality | 49 (61.3) | 10 (31.3) | 39 (81.3) | 0.001 |
| Survival (days), mean±SD (range) | 853.3±125.8 (2–2684) | 1620.4±237.2 (18–2684) | 421.3±83.9 (2–1931) | 0.001 |
| Stent patency (days), mean±SD (range) | – | – | 154.5±151.8 (1–553) | – |
| Time to surgery (days), mean±SD (range) | – | 21.1±18.7 (3–77) | – | – |
Colon decompression associated to symptom relief was maintained without any complication or need for reintervention in 70% of the patients until elective surgery in the SEMS as a bridge-to-surgery group or up to death in the palliative group. The clinical success was significantly higher in patients undergoing SEMS as a bridge-to-surgery (83% vs 60%, p=0.024). SEMS occlusion was the most frequent late complication (19%), followed by SEMS migration (9%). Bowel perforation was less frequent (7%), occurring shortly after the endoscopic procedure. The stents and respective numbers used were Wallflex® (45) Hanarostent® (32), Wallstent® (3), Ultraflex (3) and Evolution® (2). We found no differences in their use between bridge-to-surgery and palliative purposes and, in a general analysis, no significant differences related to clinical success or complication rate between them were found.
In five of six patients with SEMS as a bridge-to-surgery, the complication was solved by insertion of a new SEMS while in patients with palliative SEMS the complication was solved endoscopically in half of the cases and surgically in the other half. In SEMS as a bridge-to-surgery, the average time elapsed between SEMS placement and elective surgery was 21 days. The ROC curve analysis could not identify the most favorable time between SEMS placement and elective surgery (area under the ROC curve 0.465). The patency of SEMS in the palliation group was five months, which was significantly lower than the average time of survival of these patients (14 months).
3.2.2Elective surgery after SEMS placement vs. urgent surgeryThe comparative analysis between these two main groups is presented in Table 3. Concerning clinical success, no significant differences were observed between the groups, although it was slightly higher in the elective surgery group.
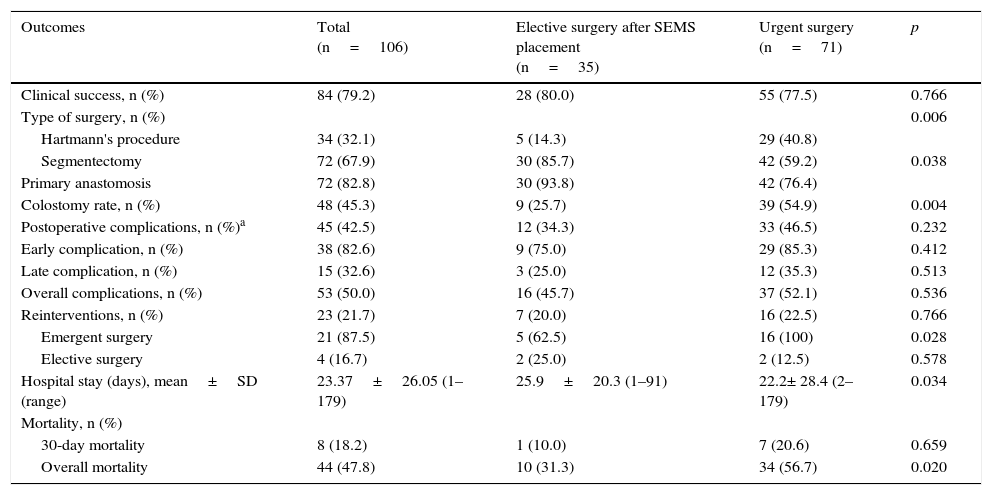
Comparison of outcomes obtained with elective surgery after SEMS placement vs. urgent surgery.
| Outcomes | Total (n=106) | Elective surgery after SEMS placement (n=35) | Urgent surgery (n=71) | p |
|---|---|---|---|---|
| Clinical success, n (%) | 84 (79.2) | 28 (80.0) | 55 (77.5) | 0.766 |
| Type of surgery, n (%) | 0.006 | |||
| Hartmann's procedure | 34 (32.1) | 5 (14.3) | 29 (40.8) | |
| Segmentectomy | 72 (67.9) | 30 (85.7) | 42 (59.2) | 0.038 |
| Primary anastomosis | 72 (82.8) | 30 (93.8) | 42 (76.4) | |
| Colostomy rate, n (%) | 48 (45.3) | 9 (25.7) | 39 (54.9) | 0.004 |
| Postoperative complications, n (%)a | 45 (42.5) | 12 (34.3) | 33 (46.5) | 0.232 |
| Early complication, n (%) | 38 (82.6) | 9 (75.0) | 29 (85.3) | 0.412 |
| Late complication, n (%) | 15 (32.6) | 3 (25.0) | 12 (35.3) | 0.513 |
| Overall complications, n (%) | 53 (50.0) | 16 (45.7) | 37 (52.1) | 0.536 |
| Reinterventions, n (%) | 23 (21.7) | 7 (20.0) | 16 (22.5) | 0.766 |
| Emergent surgery | 21 (87.5) | 5 (62.5) | 16 (100) | 0.028 |
| Elective surgery | 4 (16.7) | 2 (25.0) | 2 (12.5) | 0.578 |
| Hospital stay (days), mean±SD (range) | 23.37±26.05 (1–179) | 25.9±20.3 (1–91) | 22.2± 28.4 (2–179) | 0.034 |
| Mortality, n (%) | ||||
| 30-day mortality | 8 (18.2) | 1 (10.0) | 7 (20.6) | 0.659 |
| Overall mortality | 44 (47.8) | 10 (31.3) | 34 (56.7) | 0.020 |
The segmental colectomy was the most frequent approach, both in the elective surgery after SEMS group (86%) and in the urgent surgery group (59%). However, patients submitted to urgent surgery presented a higher number of Hartmann's (41% vs 14%, p=0.006) and thus a higher number of stoma (55% vs 26%, p=0.004), while most patients that underwent elective surgery after SEMS had a one-stage surgery and therefore having primary anastomosis in the majority of situations (94% vs. 76%, p=0.038).
Regarding postoperative complications, there was a trend, not statistically significant, for a higher occurrence in patients undergoing urgent surgery (peritonitis, with or without septic shock, intra-abdominal abscess and anastomotic leakage were the most common). Concerning overall complications (abdominal and extra-abdominal) we did not find significant differences. The urgent surgery carried more blood transfusions during surgery and the patients were also more frequently admitted in the intensive care unit (ICU), although this was not statistically significant.
The hospital stay was longer in the elective surgery after SEMS group. However, total hospital stay and costs for reinterventions or late complications in the emergency surgery group were superior.
When analyzing mortality results, procedure-related mortality was not significantly different between the two groups, but overall mortality was significantly higher in the urgent surgery group (57% vs 31%, p=0.020).
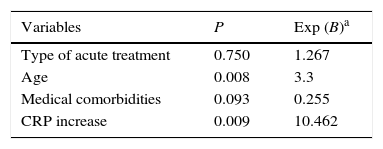
Concerning the overall complications, the logistic model (Table 4) identified patients’ age and CRP levels as predictive risk factors: the increase of five years of age was associated with a 3.3 higher risk of complications, and patients with increased CRP values were 10.5 times more prone to complications.
Predictors of overall complications in patients with curative purpose (patients who experienced elective surgery after SEMS placement and patients submitted to urgent surgery).
| Variables | P | Exp (B)a |
|---|---|---|
| Type of acute treatment | 0.750 | 1.267 |
| Age | 0.008 | 3.3 |
| Medical comorbidities | 0.093 | 0.255 |
| CRP increase | 0.009 | 10.462 |
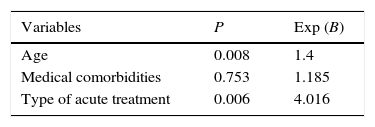
Concerning mortality, the logistic model (Table 5) identified patients’ age and the acute approach as predictive risk factors: the increase of five years of age and the urgent surgery were associated with a 1.4 and a four higher risk of death respectively, when compared with SEMS group. The medical comorbidities were not revealed to be a predictive risk factor.
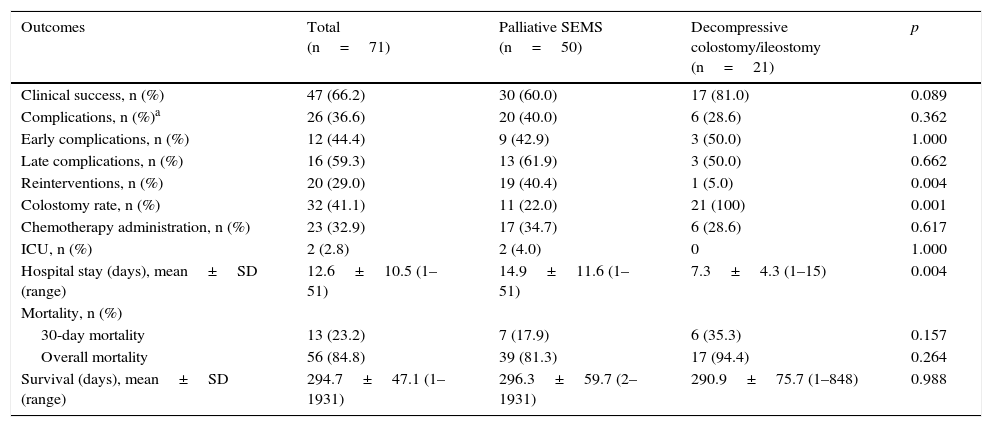
3.2.3Palliative SEMS vs. Urgent surgery (decompressive colostomy)In palliative treatment, urgent surgery obtained a higher clinical success than palliative SEMS, although these results were not statistically significant (81% vs 60%, p=0.089) (Table 6). Palliative SEMS was associated with more complications and therefore more reinterventions (out of 19 patients, 11 were submitted to surgery and eight to insertion of new SEMS) and a longer hospital stay. These two last outcomes were statistically significant. The percentage of patients treated with chemotherapy was not significantly different when comparing both groups (35% in the SEMS group and 29% in the urgent surgery group). Regarding mortality, no statistically significant differences were observed between the two groups, although, once again, the urgent surgery group presented a slightly higher mortality.
Comparison of outcomes obtained with palliative SEMS vs. decompressive colostomy.
| Outcomes | Total (n=71) | Palliative SEMS (n=50) | Decompressive colostomy/ileostomy (n=21) | p |
|---|---|---|---|---|
| Clinical success, n (%) | 47 (66.2) | 30 (60.0) | 17 (81.0) | 0.089 |
| Complications, n (%)a | 26 (36.6) | 20 (40.0) | 6 (28.6) | 0.362 |
| Early complications, n (%) | 12 (44.4) | 9 (42.9) | 3 (50.0) | 1.000 |
| Late complications, n (%) | 16 (59.3) | 13 (61.9) | 3 (50.0) | 0.662 |
| Reinterventions, n (%) | 20 (29.0) | 19 (40.4) | 1 (5.0) | 0.004 |
| Colostomy rate, n (%) | 32 (41.1) | 11 (22.0) | 21 (100) | 0.001 |
| Chemotherapy administration, n (%) | 23 (32.9) | 17 (34.7) | 6 (28.6) | 0.617 |
| ICU, n (%) | 2 (2.8) | 2 (4.0) | 0 | 1.000 |
| Hospital stay (days), mean±SD (range) | 12.6±10.5 (1–51) | 14.9±11.6 (1–51) | 7.3±4.3 (1–15) | 0.004 |
| Mortality, n (%) | ||||
| 30-day mortality | 13 (23.2) | 7 (17.9) | 6 (35.3) | 0.157 |
| Overall mortality | 56 (84.8) | 39 (81.3) | 17 (94.4) | 0.264 |
| Survival (days), mean±SD (range) | 294.7±47.1 (1–1931) | 296.3±59.7 (2–1931) | 290.9±75.7 (1–848) | 0.988 |
The aim of this study was to assess and compare the risk/benefit ratio of SEMS colorectal placement vs. urgent surgery in the treatment of acute colorectal occlusion, either with a curative or palliative purpose.
Regarding the SEMS group, the endoscopic procedure was performed in 41% of patients as a bridge-to-surgery and in 59% of patients as a palliative approach. We obtained a technical success of 94%, which is consistent with the literature.5,14,18,20 The clinical success in SEMS as bridge-to-surgery was significantly higher when compared to palliative SEMS (83% vs 60%). In published data, the clinical success in SEMS as a bridge-to-surgery presents similar values (69–94%),6,18,21 while in palliative SEMS the values reported are 76–93%.5,22. This difference may be explained by the variable definition of clinical success in different studies. We consider the same definition for both SEMS subgroups – obstructive symptom relief, without any complications requiring endoscopic or surgical reintervention – and, as would be expected, this has shown to be very restrictive in the case of palliative SEMS, with longer hospital stay and a higher probability of complications and need for reintervention.
After SEMS placement, 31% of the patients had complications, mainly later ones. These data are consistent with some studies reporting complication rates between 21 and 34%.5,8,16 However, the complication rate in the palliative patients was higher (40%), probably due to the previously mentioned reason. It is also suggested that the operator's technical experience8,23–25 and the endoscopic device characteristics26,27 can influence the procedure's safety and accuracy. In our study the endoscopist was not always the same but was always experienced and the different stents used did not present significant differences in the main outcome – clinical success.
In patients treated with a curative purpose, the clinical success obtained in SEMS as a bridge-to-surgery was slightly higher, compared to the urgent surgery group. This result is supported by several studies reporting higher clinical success rates after SEMS placement,6,8,21,28,29 although a recent meta-analysis revealed significantly higher clinical success rates in the urgent surgery.7 In addition, the SEMS group obtained a significantly higher number of primary anastomosis, associated to a significant reduction in colostomy rate, as reported by other authors.3,7,8,13,14,25,30 In the literature some advantages of primary anastomosis in one-stage are pointed out, specifically, the decrease of hospital costs and complications associated to colostomy and also avoiding the risks of a second intervention.9 Although few studies evaluated the impact that each procedure has in the patients’ quality of life, the colostomy has been associated with anxiety, depression, sleep alterations and social reclusion,6,31,32,34 features that can be overcome by an elective surgery preceded by stenting.
When analyzing postoperative complications, we did not identify significant differences between the two groups, even though elective surgery presented fewer complications. This is probably due to a lesser preoperative risk associated with the gap time between SEMS placement and surgery, allowing a proper clinical evaluation, better bowel conditions and nutritional optimization, as supported by other publications.1,7,8,21 Even though, there are some studies 8,21 suggesting an optimum time between SEMS placement and elective surgery around six and 14 days, we were not able to consistently define the appropriate gap time in this analysis.21 The elective surgery was performed, in average, 21 days after the stent placement, which may have contributed to an unexpected higher number of complications in this group.
When comparing other important outcomes, urgent surgery patients required more ICU admittance and more reinterventions due to postoperative complications. This group also presented a non-statistical trend toward a higher need of blood transfusion. The two single studies reporting these variables are in agreement with our results.34,35 After the acute procedure, the discharge occurred earlier in the SEMS group, but the global hospital stay was longer due to reinterventions. Despite the longer hospital stay of patients submitted to stenting as a bridge-to-surgery, this procedure seems to be more effective and associated with 12–20% less costs than the urgent surgery,36–38 which may be substantiated by the lower ICU admittance and reintervention rates.
To our knowledge, few studies compare the palliation approaches – acute palliative SEMS and decompressive colostomy/ileostomy – and have contradictory findings. Our results suggest that palliative SEMS does not have a significant advantage over palliative surgery for decompressing unresectable malignant colorectal occlusion because some patients had higher survival than expected and consequently had more complications and significantly more need for reinterventions and a hospital stay. This finding is consistent with a previous study also revealing higher complications rate in SEMS used in patients with advanced and metastatic colon cancer six months after SEMS placement. Nevertheless, the author added and we confirm, that part of these SEMS complications can be managed by stent replacement, without a significant increase in morbidity and mortality.39 On the other hand, a recent meta-analysis,40 demonstrates that SEMS is as effective as surgery, with shorter hospital stay, quicker recovery and comparable risk of short-term complications and mortality. However, these are mainly short-term outcomes and the study also showed a higher risk of long-term complications with SEMS. Liang et al.40 confirmed a general problem among the studies found in literature, remarking that the lack of data or the greater heterogeneity in primary studies precluded the analysis of other outcomes such as readmission and survival time.
Concerning the limitations of this study, the most important were the retrospective and therefore nonrandomized and uncontrolled design. It prevents us from having groups with similar population samples and characteristics, which can consequently bias the results. Additionally, we were not able to reliably evaluate the influence of the operator's experience in the obtained surgical outcomes.
5ConclusionSEMS placement as a bridge-to-surgery should be considered in the acute treatment of malignant colorectal occlusion, where it seems to be an effective and safer procedure, associated with a higher primary anastomosis rate and, consequently, a lower colostomy rate, number of complications and procedure-related mortality.
SEMS placement as palliative treatment does not seem to present significant clinical advantages comparatively to decompressive colostomy/ileostomy when treating an unresectable malignant occlusion and the longer the SEMS stays in place the higher is the risk of complications. So, unless the patient obstinately refuses the idea of having a stoma, patients with advanced local disease, but good general condition, in which a slightly longer survival is expected, surgery may be the best option. However, further studies comparing the two possible approaches of malignant colonic occlusion are needed, especially prospective and randomized ones. It will also be important to evaluate the impact of each procedure on the patients’ quality of life.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.