Self-expanding metal stents (SEMS) as a bridge to surgery have been used as an alternative for acute malignant left-sided colonic obstruction. However, the benefits are uncertain. The European Society of Gastrointestinal Endoscopy no longer recommends their use in patients with low surgical risk because of the risk of tumor recurrence.
MethodsPatients admitted for acute malignant left-sided colonic obstruction who underwent SEMS as a bridge to elective surgery or urgent surgery were retrospectively evaluated. Postoperative morbidity/mortality, stent complications and survival were recorded. Our aim was to compare the outcome between preoperative SEMS and direct emergent surgery in acute left-sided malignant colonic obstruction.
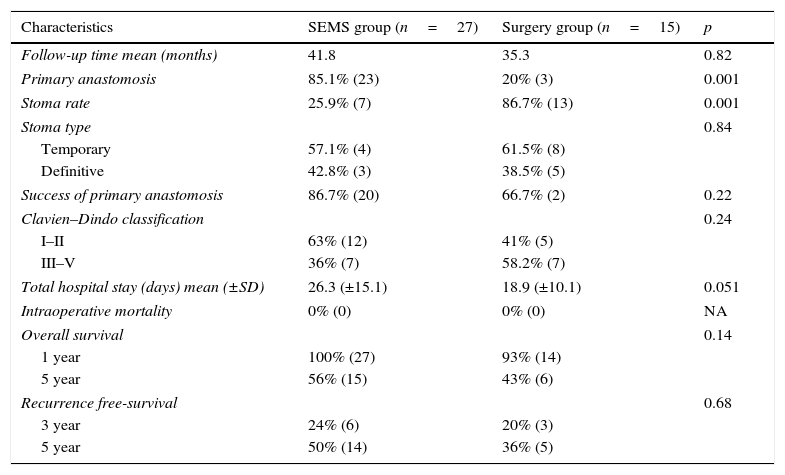
Results42 patients were included (SEMS group: 27 and surgery group: 15). There were no differences between groups in relation to age, ASA classification and tumor stage. The technical success of SEMS was 88.9% and the clinical success was 85.2%. There were three SEMS related perforations. In the surgery group, the stoma rate was higher (86.7% vs 25.9%, p<0.001) and there was a trend for a lower length of hospital stay (18.9 days vs 26.3 days, p=0.051).
SEMS verses surgery group: There were no differences in the rate of temporary stoma (57.1% vs 61.5%, p=0.84), definitive stoma (42.8% vs 38.5%, p=0.84), success of primary anastomosis (86.7% vs 66.7%, p=0.22) and Clavien–Dindo classification (≥III: 36% vs 58.2% p=0.24). Overall survival at 1/5 years was identical in the two groups 100%/56% in the SEMS group vs 93%/43% in the surgery group, p=0.14), as well as tumor recurrence at 3/5 years (24%/50% vs 20%/36% respectively, p=0.68).
ConclusionsSEMS are associated with a lower overall stoma rate and a higher primary anastomosis rate. However, there are no differences in complications, overall survival and recurrence between the groups.
As próteses metálicas auto-expansíveis (PMAE) como ponte para cirurgia são uma alternativa à cirurgia urgente nas neoplasias estenosantes do colon esquerdo. No entanto, os benefícios são controversos. A Sociedade Europeia de Endoscopia não as recomenda como ponte para cirurgia desde 2014, em doentes de baixo risco cirúrgico, pelo possível aumento de recidiva neoplásica.
MétodosAvaliação retrospetiva dos doentes com neoplasia oclusiva do colon esquerdo candidatos a tratamento curativo, que colocaram PMAE como ponte para cirurgia ou que foram submetidos diretamente a tratamento cirúrgico urgente. Avaliada a morbilidade e mortalidade pós-operatória, complicações relacionadas com as PMAE e sobrevivência. O nosso objetivo foi comparar os resultados das PMAE como ponte para cirurgia com o tratamento cirúrgico urgente nas neoplasias oclusivas do colon esquerdo.
ResultadosAvaliados 42 doentes (grupo submetido a PMAE: 27; grupo submetido a cirurgia:15). Não existem diferenças entre os dois grupos no que diz respeito à idade classificação ASA e o estadio da neoplasia. O sucesso técnico das PMAE foi de 88,9% e o sucesso clínico da prótese foi de 85,2%. Ocorreram 3 perfurações após colocação das PMAE.
No grupo submetido a cirurgia, a realização de estoma foi superior (86,7% vs 25,9%), p<0,001), e verificou-se um menor número de dias de internamento hospitalar total, embora sem resultado estatisticamente significativo (18,9 vs 26,3 dias, p=0,051).
PMAE versus cirurgia: não existem diferenças no que diz respeito à constituição de estomas provisórios (57,1% vs 61,5%, p=0,84), estomas definitivos (42,8% vs 38,5%, p=0,84), sucesso de anastomose primária (86,7% vs 66,7%, p=0,22) e classificação de Clavien–Dindo (≥III:36% vs 58,2% p=0,24). A sobrevida aos 1 e 5 anos foi semelhante nos dois grupos (PMAE 1-5 anos vs cirurgia 1-5 anos: 100%-56% vs 93%-43%, p=0,14), bem com a recidiva aos 3 e 5 anos (PMAE 3-5 anos vs cirurgia 3-5 anos 24%-50% vs 20%-36%, p=0,68).
ConclusõesA realização de estoma foi superior nos doentes submetidos a tratamento cirúrgico, no entanto, não há diferenças entre os dois grupos relativamente às complicações pós-cirurgicas, sucesso de anastomose primária, recidiva e mortalidade.
Left-sided colon cancer can present with obstruction in 8% to 26% of cases.1,2 Large bowel obstruction results in massive colonic distension, bacterial translocation, electrolyte and fluid imbalance, and an increased risk of colonic necrosis and perforation.3 In this situation, urgent colonic decompression is necessary, either through surgery or by SEMS.
The general consensus for treatment of acute right-sided colonic obstruction is resection and primary anastomosis; SEMS placement is recommended as the preferred treatment for palliation of malignant colonic obstruction, except in patients treated or considered for treatment with antiangiogenic drugs.4 However, for patients with obstructive nonpalliative left-sided colonic cancer, the management remains controversial. In the last decade many studies have been published, including randomized controlled trials (RCTs) and systematic reviews, with conflicting results.
Some RCT showed that SEMS as a bridge to surgery with curative intent may be safer, with a trend toward lower stoma rate, post-surgical complications and mortality when compared with urgent surgery.5,6 However, another study failed to demonstrate that urgent preoperative SEMS could significantly decrease the need for stoma placement, with 53.3% of SEMS technical failure.7 The elevated rate of perforations lead to the premature closure of this study.7 A meta-analysis that included four RCT showed that SEMS are associated with a high incidence of clinical and silent perforations and that technical and clinical success rates for stenting were lower than expected.3
A recent meta-analysis with seven RCTs demonstrated that there was no statistically significant difference in the postoperative mortality comparing SEMS as bridge to surgery (10.7%) and urgent surgery (12.4%). The SEMS group had lower overall morbidity (33.1% vs 53.9%, p=0.03), a higher successful primary anastomosis rate (67.2% vs 55.1%, p<0.01) and lower permanent stoma rate (9% vs 27.4%, p<0.01).8 This study concluded that SEMSs are a safe and effective bridge to subsequent surgery in patients with obstructing left-sided colon cancer.
In the recent guidelines of the European Society of Gastrointestinal Endoscopy, SEMS placement as a bridge to elective surgery is no longer recommended as a standard treatment for symptomatic left-sided malignant colonic obstruction, except in patients with ≥70 years old and ASA≥III.4 Although some advantages of SEMSs as a bridge to surgery can be extracted from published studies, these recommendations state that this has to be balanced with the oncological outcomes in patients with a curable colonic cancer. Some data evaluated this issue, showing a higher oncologic recurrence in the SEMS group, which was even higher in patients with stent-related perforation.6,9,10 Because there is no reduction in postoperative mortality and stenting seems to have a negative impact on the oncological safety, the use of SEMSs as a bridge to elective surgery is no longer recommended by European Society of Gastrointestinal Endoscopy (ESGE) as a standard treatment for potentially curable patients with left-sided malignant colonic obstruction.4
Considering these new recommendations, our aim was to evaluate our experience with SEMS as a bridge to surgery in patients with obstructive left-sided colonic cancer compared to urgent surgery, regarding morbidity, mortality and oncological recurrence.
2Material and methods2.1Patient selection and data collectionPatients admitted between 2008 and 2014 in our hospital for acute malignant left-sided colonic obstruction who underwent urgent endoscopic stenting as a bridge to elective surgery (SEMS-group) or urgent surgery (surgery-group) were retrospectively evaluated. Left-sided colonic cancer was defined as being distal to and including the splenic flexure of the colon, up to and including the rectosigmoid colon.
Diagnosis of acute colonic obstruction was made clinically, based on symptoms of abdominal pain and distention, vomiting, and inability to pass stools, and confirmed radiologically on plain abdominal films demonstrating a dilated colon. The diagnosis of the colonic tumor causing the obstruction and the site of tumor was confirmed by flexible sigmoidoscopy or computer tomography scan of the abdomen and pelvis.
Patients with rectal cancer, with obstruction due to noncolonic malignancy, with clinical and radiologic signs of perforation or peritonitis and stage IV colonic tumors were excluded. At the time of presentation, patients were offered surgery or SEMS according to a multidisciplinary decision.
Medical records from these patients were analyzed to collect demographic data, ASA classification, tumor location, cancer stage, type of SEMS, Karnofsky index, surgical time, post-surgical complications (Clavien–Dindo classification), type of surgery, total hospital stay (including all hospitalizations), rate of primary anastomosis, stoma rate (including temporary and definitive stomas), intra-operative mortality, hospital mortality, overall survival to death and recurrence free survival. Overall survival to death was defined as the time from surgery or SEMS placement to death and recurrence free survival was defined as the time from surgery or SEMS placement to cancer recurrence.
We also evaluated the technical and clinical success of SEMS. Technical success was considered when the colonic stent was correctly deployed across the stricture without complications; and clinical success was defined as relief of obstructive symptoms within 72h after stent placement. All SEMS were placed under endoscopic and fluoroscopic guidance, by gastroenterologists with experience in colonic stent placement (more than twenty). Correct positioning of the stent was confirmed using both fluoroscopy and endoscopy. Balloon predilatation was not preformed. Patients in whom it was not possible to place SEMS were included in the surgery group.
In patients who underwent surgery for colonic decompression, the type of surgical procedure was determined by the surgeon according to tumor location, disease staging and the general condition of the patient. Urgent surgery was also indicated in cases of technical failure of stenting, stent perforation or clinical failure. Local oncologic recurrence and distant metastasis were identified by computed tomography (CT) or colonoscopy.
2.2Statistical analysisThe statistical analysis was performed using the Statistical Package for Social Sciences (SPSS) program version 19 (SPSS, Chicago, IL, USA). Quantitative variables were described using means and standard deviation. Categorical variables were described in absolute numbers and percentages. Chi-square test and Student's t-test, were used to compare non-continuous and continuous data, respectively. Oncologic recurrence during follow-up was analyzed with the Kaplan–Meier estimation method and compared between groups using the log-rank test. Significance was set at a p value ≤0.05.
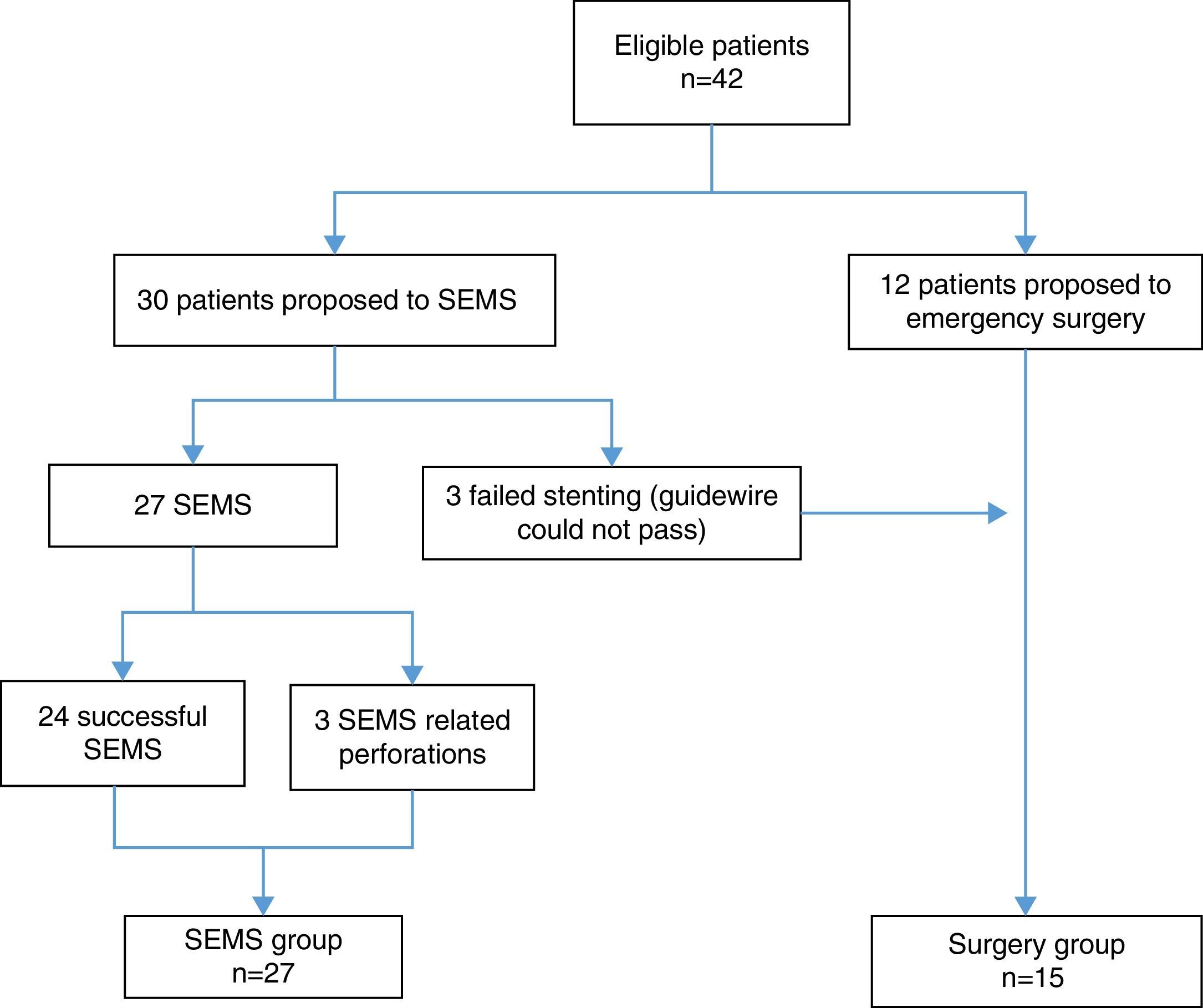
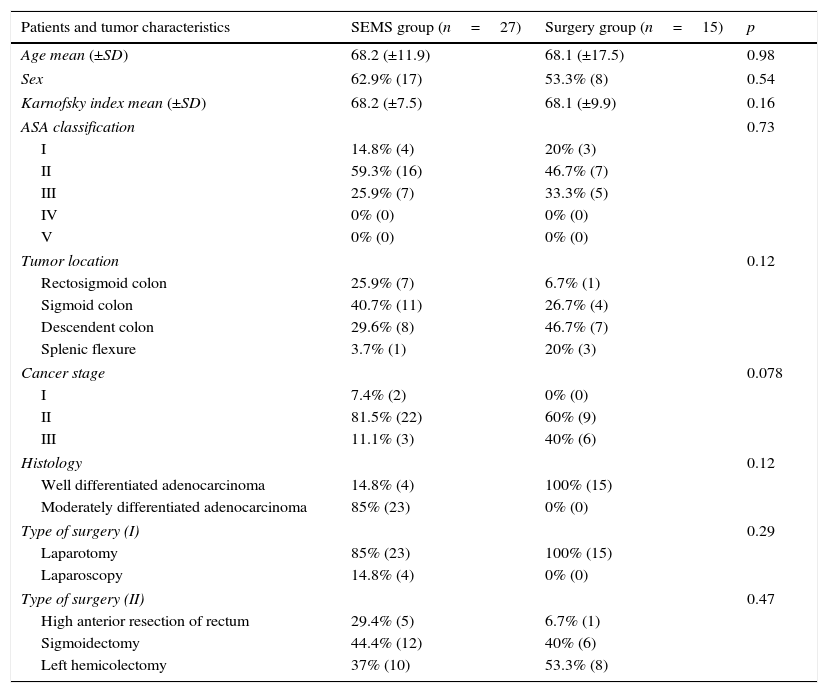
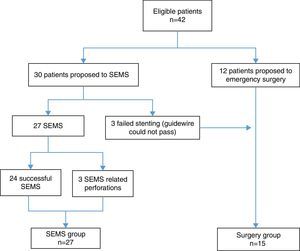
3ResultsForty-two patients were included. During the study period, 27 patients underwent SEMS placement and met the inclusion criteria (SEMS group). Emergency surgery was performed on 15 patients (surgery group) – Fig. 1. Stents were unable to be deployed in three patients because it was not possible to pass the guidewire; these patients underwent primary surgical decompression and were included in surgery group. The mean age was 68.1 years (±12.6) and most patients were male 59.5% (n=25). There were no significant differences between the two groups in relation to age, sex, ASA classification, Karnofsky index, tumor location, histologic features of the tumor, cancer stage and type of surgery (Table 1).
Patients and tumor characteristics.
| Patients and tumor characteristics | SEMS group (n=27) | Surgery group (n=15) | p |
|---|---|---|---|
| Age mean (±SD) | 68.2 (±11.9) | 68.1 (±17.5) | 0.98 |
| Sex | 62.9% (17) | 53.3% (8) | 0.54 |
| Karnofsky index mean (±SD) | 68.2 (±7.5) | 68.1 (±9.9) | 0.16 |
| ASA classification | 0.73 | ||
| I | 14.8% (4) | 20% (3) | |
| II | 59.3% (16) | 46.7% (7) | |
| III | 25.9% (7) | 33.3% (5) | |
| IV | 0% (0) | 0% (0) | |
| V | 0% (0) | 0% (0) | |
| Tumor location | 0.12 | ||
| Rectosigmoid colon | 25.9% (7) | 6.7% (1) | |
| Sigmoid colon | 40.7% (11) | 26.7% (4) | |
| Descendent colon | 29.6% (8) | 46.7% (7) | |
| Splenic flexure | 3.7% (1) | 20% (3) | |
| Cancer stage | 0.078 | ||
| I | 7.4% (2) | 0% (0) | |
| II | 81.5% (22) | 60% (9) | |
| III | 11.1% (3) | 40% (6) | |
| Histology | 0.12 | ||
| Well differentiated adenocarcinoma | 14.8% (4) | 100% (15) | |
| Moderately differentiated adenocarcinoma | 85% (23) | 0% (0) | |
| Type of surgery (I) | 0.29 | ||
| Laparotomy | 85% (23) | 100% (15) | |
| Laparoscopy | 14.8% (4) | 0% (0) | |
| Type of surgery (II) | 0.47 | ||
| High anterior resection of rectum | 29.4% (5) | 6.7% (1) | |
| Sigmoidectomy | 44.4% (12) | 40% (6) | |
| Left hemicolectomy | 37% (10) | 53.3% (8) | |
In all patients, the type of SEMS used was non covered SEMS. The technical success was 88.9% (n=24) and the clinical success was 85.2% (n=23). There were three perforations related to stents, two of them detected during the procedure. The other patient developed peritonitis three days after the procedure from delayed colonic perforation. All these stent-related perforations required surgery with stoma (two temporary and one definitive). One patient had a stent successfully placed but failure to decompress the colon was observed; an emergency Hartmann procedure was performed, with posterior reconstruction of colonic transit. There were no deaths related to the procedure. All 23 patients who underwent surgery after a successful SEMS had a primary anastomosis, with a mean time between SEMS and surgery of 26.3 days (±15.1).
In patients with successful SEMS placement followed by elective surgery, three require a protective stoma (two temporary and one definitive).
In total, the stoma rate was 26% (n=7): four temporary and three definitive. Definitive stoma resulted from anastomotic leakage, oncologic recurrence before reconstruction of colonic transit and multiple post-surgical complications (respiratory and cardiac) in a patient with several comorbidities.
3.2Surgery groupSurgery was successful in relieving obstruction in all 15 patients. The types of surgical procedures are summarized in Table 1. A laparotomic approach was used in all patients submitted to urgent surgery. Three patients had a primary anastomosis, one with a protective temporary stoma. All other patients required surgery with the construction of a stoma. In the majority of patients (n=8, 61.5%) it was possible to reconstruct colonic transit. Cancer recurrence (n=2), a death not related to cancer (n=1), abdominal abscess (n=1) and anastomotic dehiscence (n=1) in patients with comorbidities were the causes of definitive stomas. The mean hospital stay was 18.9 days (±10.1).
3.3SEMS vs surgery group – short and long term outcomesPrimary anastomosis was higher in SEMS group (85.1% vs 20%, p=0.001) and the stoma rate was superior in surgery group (86.7% vs 25.9%, p=0.001). However, there was no difference between the temporary and definitive stoma rate, in SEMS and surgery group, respectively (temporary stoma: 57.1% vs 61.5%; definitive stoma: 42.8% vs 38%, p=0.84).
Although postoperative complications were higher in the surgery group (Clavien–Dindo score ≥III: 36% vs 58.3%, p=0.24) and the total hospital stay (26.3 vs 18.9 days, p=0.051) and success of primary anastomosis (86.7% vs 66.7%, p=0.22) were higher in SEMS group, the differences were not statistically significant. Comparison of patients with SEMS or urgent surgery is demonstrated in Table 2. There was no intra-operative or hospital mortality at 30 days in both groups.
SEMS and surgery groups complications and mortality.
| Characteristics | SEMS group (n=27) | Surgery group (n=15) | p |
|---|---|---|---|
| Follow-up time mean (months) | 41.8 | 35.3 | 0.82 |
| Primary anastomosis | 85.1% (23) | 20% (3) | 0.001 |
| Stoma rate | 25.9% (7) | 86.7% (13) | 0.001 |
| Stoma type | 0.84 | ||
| Temporary | 57.1% (4) | 61.5% (8) | |
| Definitive | 42.8% (3) | 38.5% (5) | |
| Success of primary anastomosis | 86.7% (20) | 66.7% (2) | 0.22 |
| Clavien–Dindo classification | 0.24 | ||
| I–II | 63% (12) | 41% (5) | |
| III–V | 36% (7) | 58.2% (7) | |
| Total hospital stay (days) mean (±SD) | 26.3 (±15.1) | 18.9 (±10.1) | 0.051 |
| Intraoperative mortality | 0% (0) | 0% (0) | NA |
| Overall survival | 0.14 | ||
| 1 year | 100% (27) | 93% (14) | |
| 5 year | 56% (15) | 43% (6) | |
| Recurrence free-survival | 0.68 | ||
| 3 year | 24% (6) | 20% (3) | |
| 5 year | 50% (14) | 36% (5) | |
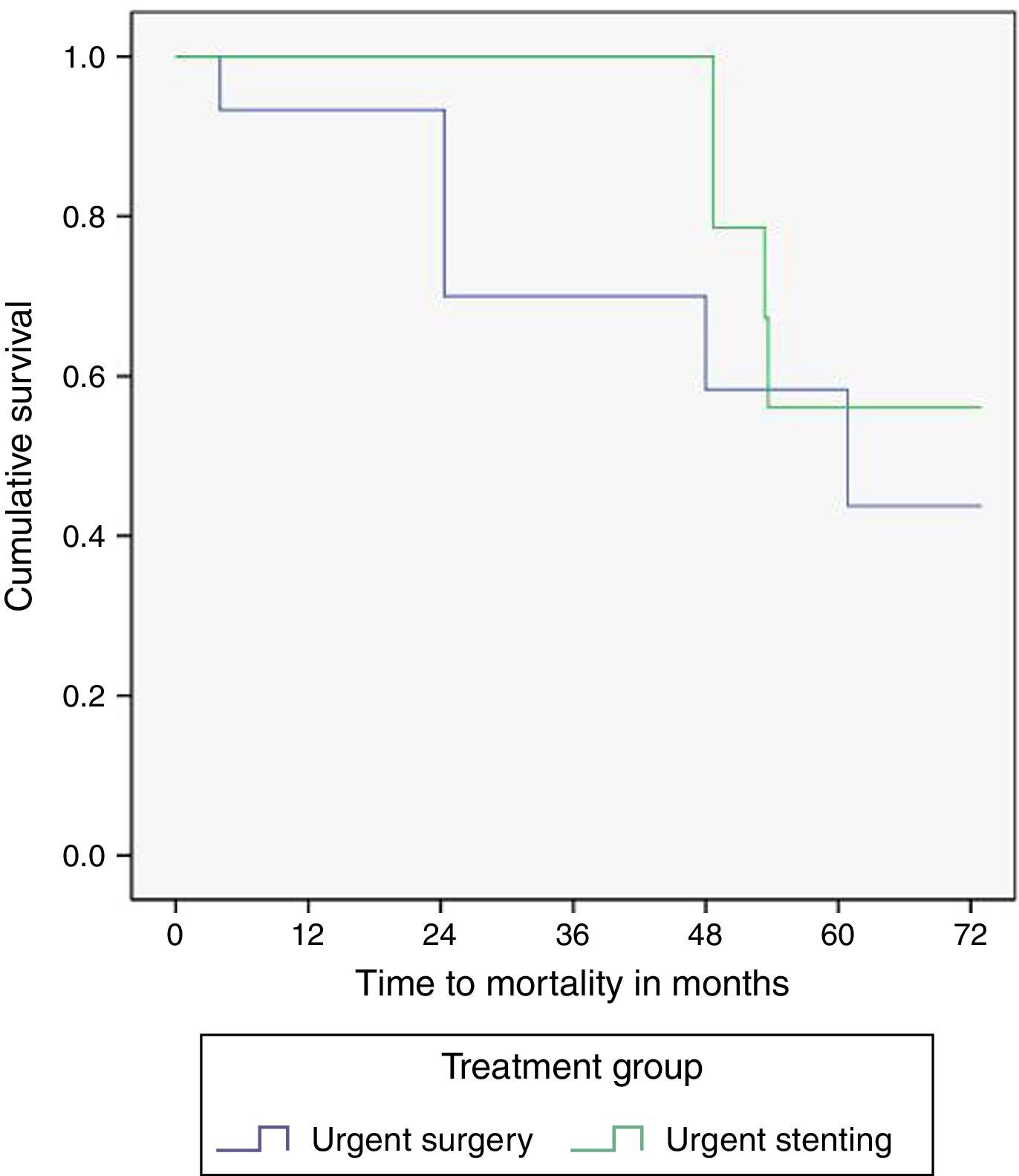
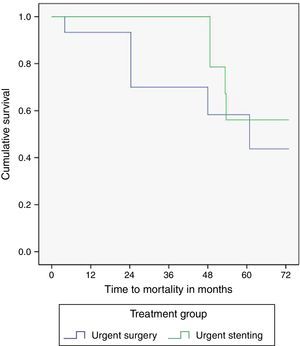
The median time of follow-up was 41.8 months in the SEMS group and 35.3 months in the surgery group (p=0.82). One and five year overall survival was identical in SEMS and surgery groups (100%/56% vs 93%/43%, p=0.14), although a trend toward a higher survival in the SEMS group was observed. The survival curves are illustrated in Fig. 2. Five patients (18.5%) in the SEMS group and six patients (40%) in the surgery group died during follow-up. Of these patients, four in the SEMS group (80%) and four in the surgery group (66.6%) died of cancer.
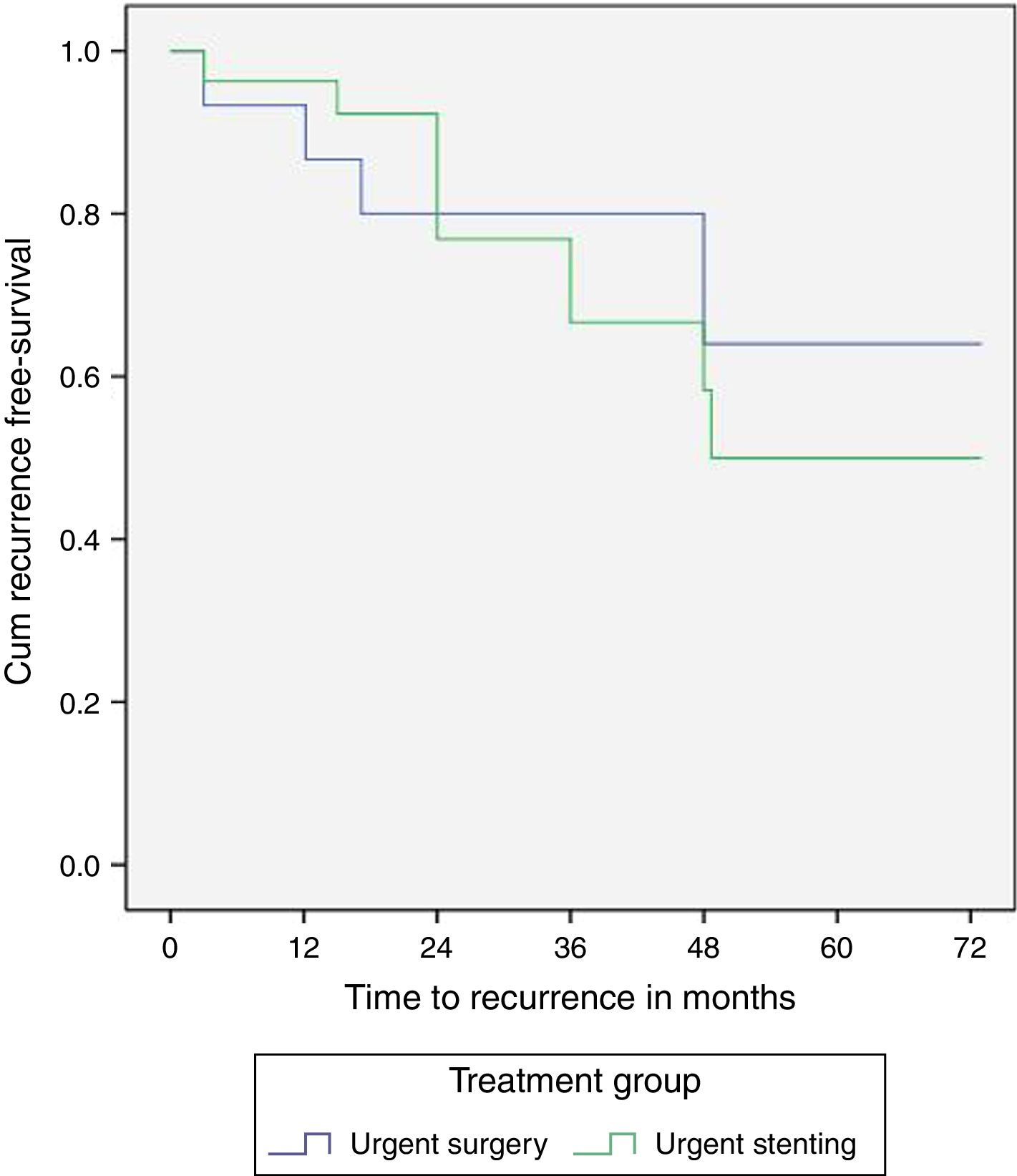
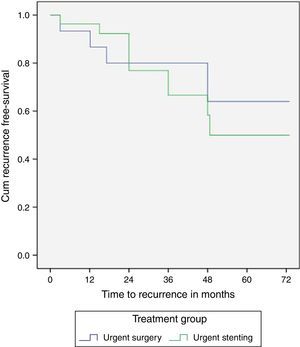
The three and five-year recurrence free survival was not significantly different between the SEMS and surgery groups (24%/50% vs 20%/36%, p=0.68), however with a trend toward a higher recurrence in the SEMS group (Fig. 3).
Thirteen patients, nine (33.3%) in the stent group and four (26.6%) in the surgery group developed cancer recurrence, all with distant metastasis. Among patients with recurrence, five patients had stage IIIB/C tumor (38.5%) and only one patient had a stent related perforation. In patients with oncologic recurrence, the time between SEMS placement and surgery was not statistically different from those with no oncologic recurrence (16.7 days vs 13.2 days, p=0.06). In the SEMS group, oncologic recurrence was not influenced by perforation (11.1% vs 11.1%, p=1) and tumor stage (stage III 11.1% vs 11.1%, p=1) did not influence oncologic recurrence.
4DiscussionColonic obstruction represents a surgical emergency associated with a high degree of morbidity and mortality because of the generally poor condition of these patients. Colonic stenting is an alternative method for treatment of obstructive left-sided colon cancer. However, its use as a preoperative bridge to surgery remains controversial, because of significant heterogeneity between studies in relation to tumor location, staging, study design, follow-up and overall morbidity.
In our study, the overall stoma rate was lower and the primary anastomosis rate was higher in the SEMS group, results also found in other data.3,8,11 These results support the hypothesis that SEMS placement as a bridge to surgery allows improvement of the patient's general condition creating the right conditions for a successful primary anastomosis. However, there was no significant difference in the rates of permanent stoma, a conclusion also supported by a meta-analysis published by Tan et al. These authors suggest that evaluation of the stoma rate is essential when comparing the two different interventions, because creation of a definitive stoma can have profound effects on the psychosocial well-being of patients and specialized counseling is needed to improve quality of life significantly.3 In our study, one permanent stoma in the SEMS group was performed after leakage of a primary anastomosis; probably, in this case, bowel decompression and improvement of the patients’ clinical condition were insignificant at the time of elective surgery. Intestinal continuity can be restored in only 60 percent of cases with up to 40 percent morbidity.12 The decision to create a stoma rather than to restore continuity is also related to the patient's overall condition and stability.
Although we did not find any statistically significant difference in relation to the total hospital stay, there was a trend to a longer hospital stay in the SEMS group. Eventually, the time between the SEMS placement and surgery could explain these results. SEMS perforations, which required emergency operation and stoma formation, could also result in a longer hospital stay.
In contrast to some RCT studies,5,6 we did not find any difference in post-operative complications using Clavien–Dindo classification. The therapy used to correct a specific complication is the basis of this classification in order to rank a complication in an objective and reproducible manner. A systematic review and meta-analysis published by Guang-Yao Ye et al. also did not find any differences between the two groups regarding anastomotic leakage and intra-abdominal infection.13 This may be related to the emergency nature of the surgery, the type of surgeon or the time after colonic stenting.8
A series of studies showed that SEMS have an overall rate for relief of obstruction of 84–94%, with complications such as perforation (4%), stent migration (10–12%) and re-obstruction (7–10%), causing a cumulative mortality of 1%.11,14 The technical and clinical success of SEMS varies in accordance to published studies. Sebastian et al.14 reported a technical success rate of 91.9% and a clinical success rate of 71.7% for SEMS placement as a bridge to elective surgery. Pirlet et al.7 had a 53% technical failure for stent insertion, two cases of stent perforation in the SEMS group and one perforation in a non-randomized patient leading to closure of the trial. Van Hooft et al.15 also stopped their trial because they found an unexpectedly increased absolute risk of 30-day morbidity in the SEMS group, with almost 20% of stent related perforations. A meta-analysis published by Tan et al. concluded that technical and clinical success rates for stenting were lower than expected (70% and 69%, respectively), with almost 20% of SEMS related perforations.3 The stent related technical failure could be related to the level of experience of the operator. In our study, we found 11% of stent related perforations, with a technical and clinical success of 88.9% and 85.2%, respectively. All stents were placed by gastroenterologists with experience in SEMS placement for the treatment of colonic obstruction. Operator experience and technical expertise in stent placement has been shown to reduce significantly the number of stent-related complications. Although Van Hooft et al. reported that stent placement was performed by experienced endoscopists, other RCT did not report the level of experience of stent placement required. Small et al. have also suggested that the degree of occlusion is another risk factor for SEMS complications as the completely occluded bowel may result in friable micro-perforated tissue and present as a very tight stricture that makes stent deployment technically difficult.16 In our study it was not possible to assess the type of obstruction but, eventually, the type of obstruction could influence the rate of perforations.
The oncological consequences of potential tumor dissemination caused by perforations are unclear, but the possibility of dissemination is worrisome. A recent retrospective study found that the 3-year overall survival (85.2 vs 82.8%; p=0.65) and recurrence free survival (80.7 vs 78.6%; p=0.916) were not significantly different between the stent and surgery groups; however, in the stent group, perforation was identified as an independent risk factor for cancer recurrence (odds ratio 22.0; 95% CI, 1.3–362.9; p=0.030) and seeded metastasis (odds ratio 46.0; 95% CI, 2.0–1047.8; p=0.016).11 Another retrospective study found that SEMS had an adverse effect on 5-year overall and disease-free survival rates.17 The poorer outcomes in this group could have been due to patients with more advanced disease presenting with emergency bowel obstruction. On the other hand, one prospective study reported longer survival in patients who underwent SEMS placement (hazard ratio 0.412; 95% CI, 0.217–0.789; p=0.007).18 Considering the differences in published studies regarding tumor recurrence and survival, there are no proven differences in long-term outcomes between the two treatment approaches. In our study, we did not find any statistically significant difference in one and five years overall survival and in three and five years recurrence free survival. Stent related perforation and tumor stage did not influence tumor recurrence in the SEMS group.
This study is limited by its retrospective design, and the small number of patients from a single institution. Small number of patients should be considered with caution when interpreting these results. Patients were not randomly assigned, and selection bias may have confounded our data. Some data could be missed, including post-operative complications and oncological recurrence. However, we analyzed a very important variable, not always explored in these types of studies: the oncologic recurrence. We think this is a strong point of our study. Also, to the best of our knowledge, this is the first Portuguese study comparing SEMS as a bridge to surgery with emergency surgery in patients with obstructive left-sided colon cancer. It shows that SEMS has some advantages because it is associated with a lower overall stoma rate and a higher primary anastomosis rate. However, there are no differences in complications, overall survival and recurrence free survival.
More studies to resolve these conflicting results regarding treatment of left-sided obstructive cancer are needed.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.