Sprue-like enteropathy associated with olmesartan, first identified by our group in 2012,1 is characterized by chronic diarrhea (often severe) and weight loss that is unresponsive to a gluten-free diet. Laboratory work-up commonly reveals non-specific anemia, hypoalbuminemia, electrolyte imbalance, and vitamin deficiencies, consistent with a severe malabsorption process. Histopathological findings include a combination of duodenal villous atrophy, increased intraepithelial lymphocytes, and a thickened subepithelial collagen layer (collagenous sprue). Histologic changes can be limited to the small bowel, or may include the entire gastrointestinal tract, with findings such as lymphocytic/collagenous gastritis and colitis. Individuals with sprue-like enteropathy associated with olmesartan have negative celiac serology. The majority may have either HLA-DQ2 or DQ8 haplotypes (61–81%).1 Diagnosis of olmesartan associated enteropathy should therefore be considered in cases of villous atrophy with negative celiac serology (so-called seronegative villous atrophy). Confirmation of diagnosis requires clinical resolution of symptoms after olmesartan withdrawal. Mucosal recovery is also expected within 3–6 months of olmesartan withdrawal and a follow-up duodenal biopsy is reasonable.
Severe sprue-like enteropathy associated with olmesartan appears to be rare although a spectrum of disease severity may be possible.2 The annual incidence rate of enteropathy in a French population-based study among patients treated with olmesartan for at least 6 months was calculated at 1.3 cases per 1000 individuals per year (95% confidence interval (CI) of 0.5–2.6).3 This rate is not significantly different from the rate of 0.63 cases of incident celiac disease per 1000 reported by the Mini-Sentinel (95% CI: .38–.99) (p=0.16).4 The Mini-Sentinel reported that rates of incident celiac disease were of similar magnitude for all angiotensin receptor blockers with, for instance, a rate of 0.43 cases per 1000 (95% CI: 0.33–0.55) for losartan. Mini-Sentinel data therefore suggest that enteropathy may be a class-related drug effect. Such a hypothesis is supported by sporadic case-reports of enteropathy possibly associated with irbesartan,5 losartan,6 and valsartan.7 However, a nation-wide case-control Swedish study failed to show any association between the use of either angiotensin converting enzyme blockers or non-olmesartan angiotensin receptor blockers and subsequent villous atrophy.8 Thus, there is clear predominance of published data relating olmesartan to enteropathy (Table 1).
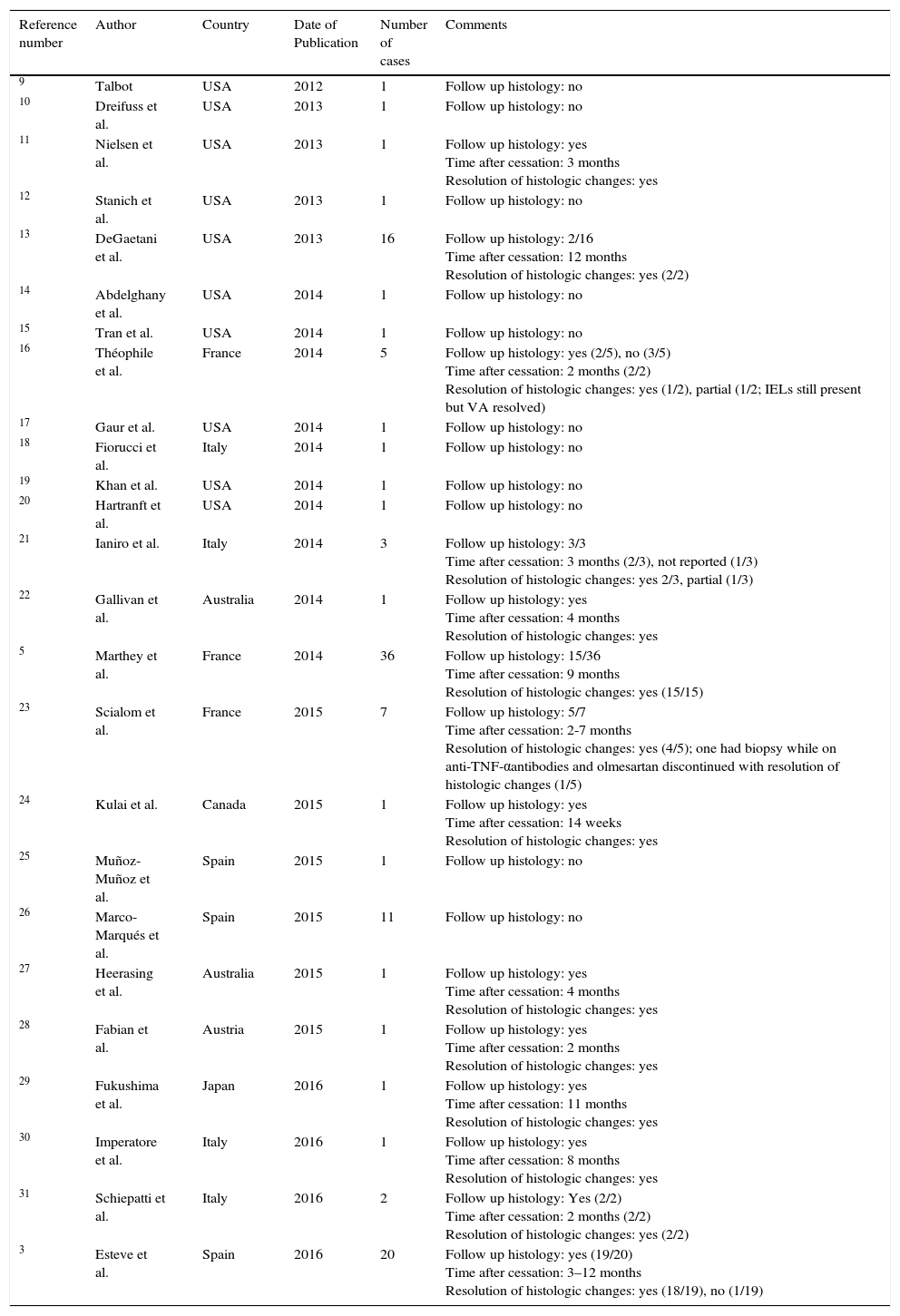
Case-reports and case-series of sprue-like enteropathy associated with olmesartan published after the Mayo Clinic 2012 original case-series.
| Reference number | Author | Country | Date of Publication | Number of cases | Comments |
|---|---|---|---|---|---|
| 9 | Talbot | USA | 2012 | 1 | Follow up histology: no |
| 10 | Dreifuss et al. | USA | 2013 | 1 | Follow up histology: no |
| 11 | Nielsen et al. | USA | 2013 | 1 | Follow up histology: yes Time after cessation: 3 months Resolution of histologic changes: yes |
| 12 | Stanich et al. | USA | 2013 | 1 | Follow up histology: no |
| 13 | DeGaetani et al. | USA | 2013 | 16 | Follow up histology: 2/16 Time after cessation: 12 months Resolution of histologic changes: yes (2/2) |
| 14 | Abdelghany et al. | USA | 2014 | 1 | Follow up histology: no |
| 15 | Tran et al. | USA | 2014 | 1 | Follow up histology: no |
| 16 | Théophile et al. | France | 2014 | 5 | Follow up histology: yes (2/5), no (3/5) Time after cessation: 2 months (2/2) Resolution of histologic changes: yes (1/2), partial (1/2; IELs still present but VA resolved) |
| 17 | Gaur et al. | USA | 2014 | 1 | Follow up histology: no |
| 18 | Fiorucci et al. | Italy | 2014 | 1 | Follow up histology: no |
| 19 | Khan et al. | USA | 2014 | 1 | Follow up histology: no |
| 20 | Hartranft et al. | USA | 2014 | 1 | Follow up histology: no |
| 21 | Ianiro et al. | Italy | 2014 | 3 | Follow up histology: 3/3 Time after cessation: 3 months (2/3), not reported (1/3) Resolution of histologic changes: yes 2/3, partial (1/3) |
| 22 | Gallivan et al. | Australia | 2014 | 1 | Follow up histology: yes Time after cessation: 4 months Resolution of histologic changes: yes |
| 5 | Marthey et al. | France | 2014 | 36 | Follow up histology: 15/36 Time after cessation: 9 months Resolution of histologic changes: yes (15/15) |
| 23 | Scialom et al. | France | 2015 | 7 | Follow up histology: 5/7 Time after cessation: 2-7 months Resolution of histologic changes: yes (4/5); one had biopsy while on anti-TNF-αantibodies and olmesartan discontinued with resolution of histologic changes (1/5) |
| 24 | Kulai et al. | Canada | 2015 | 1 | Follow up histology: yes Time after cessation: 14 weeks Resolution of histologic changes: yes |
| 25 | Muñoz- Muñoz et al. | Spain | 2015 | 1 | Follow up histology: no |
| 26 | Marco-Marqués et al. | Spain | 2015 | 11 | Follow up histology: no |
| 27 | Heerasing et al. | Australia | 2015 | 1 | Follow up histology: yes Time after cessation: 4 months Resolution of histologic changes: yes |
| 28 | Fabian et al. | Austria | 2015 | 1 | Follow up histology: yes Time after cessation: 2 months Resolution of histologic changes: yes |
| 29 | Fukushima et al. | Japan | 2016 | 1 | Follow up histology: yes Time after cessation: 11 months Resolution of histologic changes: yes |
| 30 | Imperatore et al. | Italy | 2016 | 1 | Follow up histology: yes Time after cessation: 8 months Resolution of histologic changes: yes |
| 31 | Schiepatti et al. | Italy | 2016 | 2 | Follow up histology: Yes (2/2) Time after cessation: 2 months (2/2) Resolution of histologic changes: yes (2/2) |
| 3 | Esteve et al. | Spain | 2016 | 20 | Follow up histology: yes (19/20) Time after cessation: 3–12 months Resolution of histologic changes: yes (18/19), no (1/19) |
Sprue-like enteropathy associated with olmesartan should be ruled out early in the investigation of patients with seronegative villous atrophy. Indeed, a case-series on 72 patients with seronegative villous atrophy found the most frequent etiologies to be seronegative CD (28%), medication-related (26%), unclassified sprue (14%), autoimmune enteropathy (4%), and giardia (4%).13 Of the medication-related seronegative villous atrophy, roughly 84% were attributed to olmesartan.13 Early identification of individuals with sprue-like enteropathy associated with olmesartan is clinically relevant as symptoms can be severe and/or life-threatening with expected clinical response within days of olmesartan withdrawal.1
In this issue of GE Portuguese Journal of Gastroenterology, case reports by da Silva et al.32, Carneiro et al.33 and Eusébio et al.34, highlight the importance of considering sprue-like enteropathy associated with olmesartan when approaching a patient with seronegative villous atrophy and provide further information to aid in diagnosis of this emergent disease.
In their case-report, da Silva et al. outline a practical algorithm to approach diagnosis of seronegative villous atrophy that does not respond to a gluten free diet.32 The first proposed step following testing for celiac serology is a review of the patient's medication list. In the four cases reported in this issue, symptoms resolved within 48h to one week of discontinuation of olmesartan.32–34 Trialing a patient off of a medication early on in the evaluation of seronegative villous atrophy could therefore provide both a rapid and cost-effective diagnosis. It is our practice that following olmesartan withdrawal, we try to understand the primary indication for olmesartan therapy and reassess the need for alternative medications together with the patient's primary care physician. It has been our experience that a considerable number of patients do not need any medications after suspension of olmesartan.
Eusébio et al.34 identify a unique finding of elevated transaminases in a case of olmesartan associated enteropathy. They propose that this may be due to the same mechanism behind the hypertransaminasemia seen in CD.
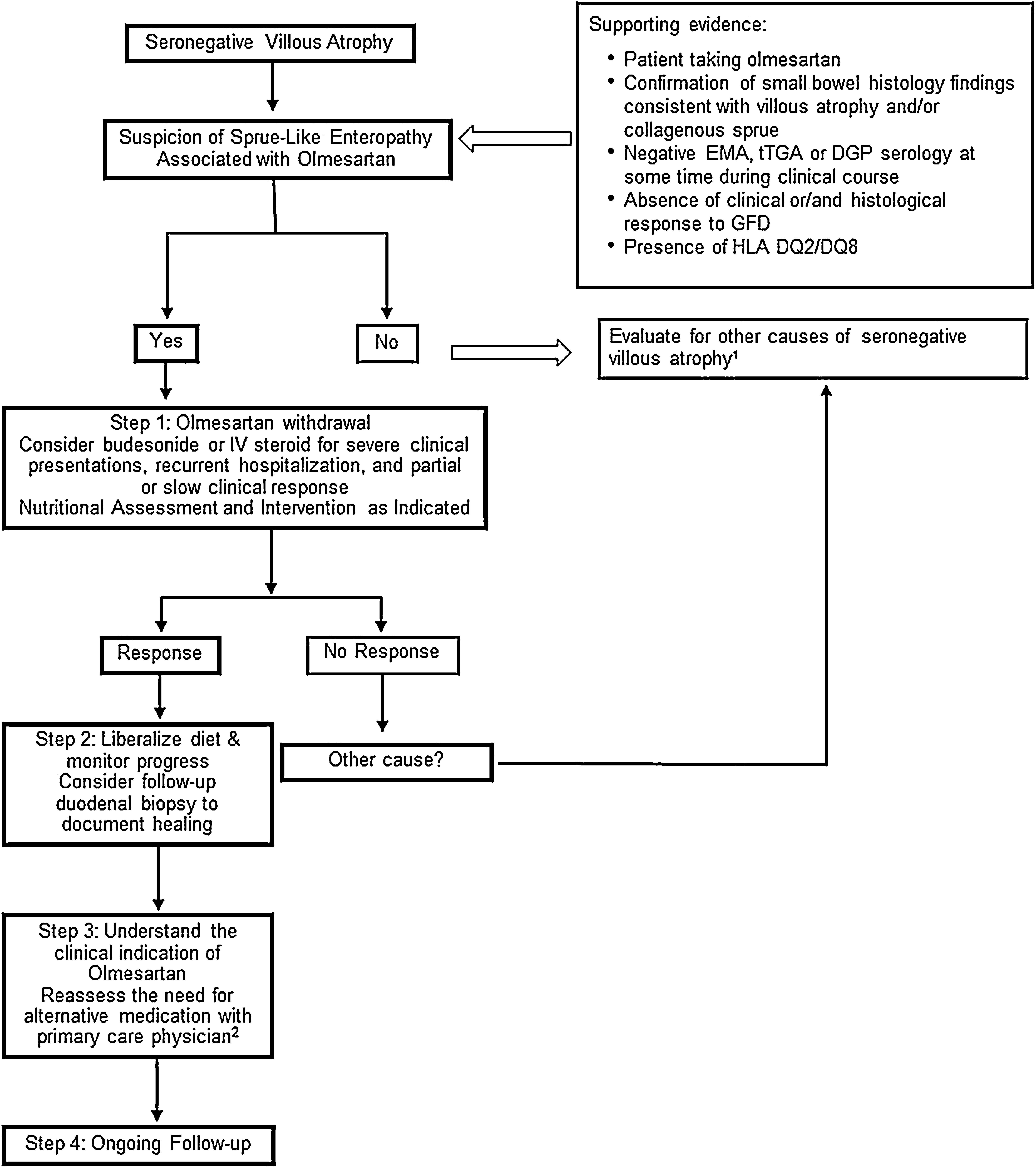
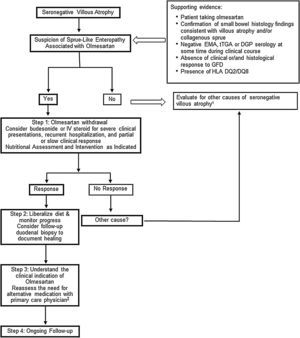
Carneiro et al.33 report on a case diagnosed with systemic sclerosis during the work-up for sprue-like enteropathy associated with olmesartan. The association with autoimmune diseases has been reported in several other studies.3,5,23 In line with this observation, there are reports of individuals with sprue-like enteropathy associated with olmesartan responding to immunosuppressive treatment.5,23 Our open-label experience suggests that some patients with severe symptoms, recurrent hospitalizations due to dehydration or both slow and/or partial response to olmesartan withdrawal may have some benefit from a short course of steroids such as budesonide (Fig. 1).
Proposed Management for Patients with Sprue-like Enteropathy associated with Olmesartan. 1. Differential diagnosis includes (but it is not limited to) seronegative celiac disease, other drug-related enteropathies, autoimmune enteropathy, tropical sprue, small-bowel bacterial overgrowth, hypogammaglobulinemic sprue, Giardiasis, refractory celiac disease, Whipple's disease, collagenous sprue, and unclassified sprue. 2. If there is a need for continuation of alternative therapy, we recommend to use a different class of medication whenever is clinically possible.
The association between autoimmune diseases and olmesartan associated enteropathy is consistent with the emerging evidence supporting an immune-based pathophysiology. One recent study by our group looking at duodenal biopsies of those taking olmesartan versus those who had discontinued the medication showed an increased CD8+ cells, FoxP3+ cells, and IL15R in biopsies of those taking olmesartan, similar to what is seen in CD.35 In addition, we demonstrated an increased IL15 expression and disruption of tight junction proteins (ZO-1) in olmesartan-treated Caco-2 cells.35 This suggests that olmesartan may trigger a similar change in intestinal epithelial cells as gluten does in those with CD although further study of the underlying mechanisms would be needed to fully understand the pathophysiology of sprue-like enteropathy associated with olmesartan, the new kid on the enteropathy block.
Conflict of interestThe authors declare no conflicts of interest.