Chronic diarrhoea is a common clinical problem in gastroenterology practice and often it is difficult to diagnose the cause. Villous atrophy is not specific and the rarer possibility of drug-induced enteritis should always be considered. Olmesartan has recently been described as a cause of drug-induced enteropathy characterized by chronic diarrhoea and varying degrees of duodenal mucosa atrophy resembling celiac disease.
We describe two cases of sprue-like enteropathy in patients treated with olmesartan for arterial hypertension several years before the onset of symptoms. Patients presented severe diarrhoea and significant weight loss, and both had histological evidence of intestinal villous atrophy. The clinical signs completely resolved after drug withdrawal.
Olmesartan-induced enteropathy is a new clinical entity that must be included in the differential diagnosis of villous atrophy with negative celiac serology. The clinical and histological alterations easily and completely resolve after drug discontinuation, restoring quality of life to patients and avoiding unnecessary investigation.
A diarreia crónica é um problema clínico comum na prática de gastroenterologia e, muitas vezes, o diagnóstico da causa é difícil. A atrofia das vilosidades intestinais não é específica e a rara possibilidade de enterite induzida por fármacos deve ser considerada. O olmesartan foi recentemente descrito como uma causa de enteropatia induzida por fármacos caracterizada por diarreia crónica e graus variáveis de atrofia da mucosa duodenal semelhante á doença celíaca.
Os autores descrevem dois casos de enteropatia tipo celíaca em doentes com história de hipertensão arterial medicada com olmesartan vários anos antes do início dos sintomas. Ambos os doentes apresentaram diarreia grave, perda ponderal significativa e evidência histológica de atrofia vilositária. Os sinais clínicos resolveram completamente após a interrupção do fármaco.
A enteropatia induzida por olmesartan constitui uma nova entidade clínica que deve ser incluída no diagnóstico diferencial de atrofia vilositária seronegativa. As alterações clínicas e histológicas resolvem rápida e completamente após a suspensão do fármaco restaurando a qualidade de vida aos doentes e evitando, muitas vezes, investigações invasivas desnecessárias.
Intestinal villous atrophy with negative celiac serology may be a diagnostic challenge. Apart from celiac disease, a variable degree of villous atrophy can be found in other conditions as autoimmune enteropathy, common variable immune deficiency, small bowel bacterial overgrowth, parasitic infection such as giardiasis, intestinal lymphoma, human immunodeficiency virus infection-related enteropathy, Whipple's disease, and tropical sprue. Villous atrophy can also occur with prolonged use of some medications.
An association between olmesartan and enteropathy development, which is histologically indistinguishable from celiac disease, has been described recently.1–4 Olmesartan-induced enteropathy can cause severe chronic diarrhoea with substantial weight loss, even months or years after drug initiation. The Food and Drug Administration issued a statement on olmesartan labelling in July 2013 after a case series with 22 patients was reported by Mayo Clinic.1 The physiopathogenic mechanism of olmesartan-induced enteropathy remains unknown. One proposed mechanism is related to a cell-mediated immune response that damages the small intestinal brush border.1–5 Additionally, a predisposition in patients with an autoimmune background has been suggested.5,6
We report two cases of severe olmesartan-induced enteropathy and discuss the natural history of this condition
2Clinical cases2.1Case 1A 60 year-old Caucasian male with arterial hypertension, treated with olmesartan and hydrochlorothiazide (20+12.5mg/day) for the last 4 years, was admitted to our hospital with a 3-month clinical history of abdominal pain, diarrhoea and marked weight loss (16% body weight). He reported between 5 and 7 daily episodes of watery and nonbloody diarrhoea. He denied any other symptoms suggestive of local or systemic infections, recent travel, consumption of contaminated food or water, animal contact, or changes in diet or medications within the past few years.
Because the patient was dehydrated and had tachycardia with orthostatic hypotension, antihypertensive drugs were discontinued. Abdominal palpation was diffusely painful but there were no masses or organ enlargement. On physical examination, there was also diffuse skin thickening without sclerodactyly or telangiectasias.
Blood tests showed hypokalaemia (serum potassium 2.0mmol/L; N: 3.6–5.1), hypophosphataemia (phosphorus 1.8mg/dL, N: 2.3–4.7), and hypoalbuminaemia (albumin 2.8g/dL; N: 3.4–4.8). The patient's leucocyte count, C-reactive protein level, and liver chemistries were all normal. The remaining initial workup including immunoglobulin levels, serum thyroid stimulating hormone, stool cultures, Clostridium difficile toxin assay, stool ova and parasites, and stool osmolality and electrolytes was unremarkable. Conventional serologic tests (tissue transglutaminase, endomysial, and antigliadin antibodies) and a lack of clinical response to a gluten-free diet ruled out celiac disease.
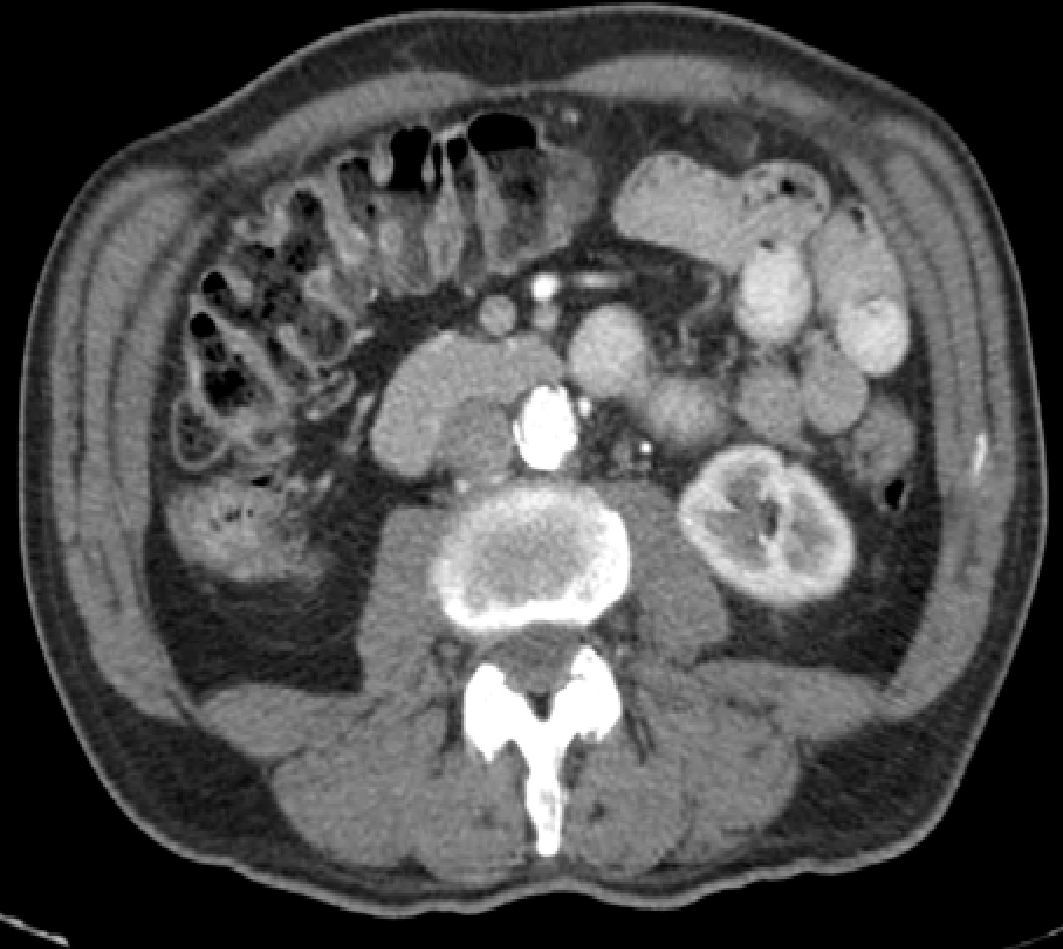
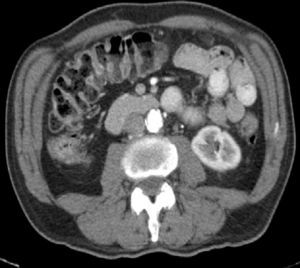
An abdominal CT scan showed diffuse small bowel wall thickening with surrounding mesenteric adenopathy (Fig. 1). Pancreatic abnormalities or potential malignancies were excluded. Colonoscopy and colonic biopsies were normal, and there was no evidence of microscopic colitis or inflammatory bowel disease. Immunological assays were positive for antinuclear antibody (titre, 1:1280) and Scl70 suggesting a diagnosis of systemic sclerosis that was confirmed by a skin biopsy.
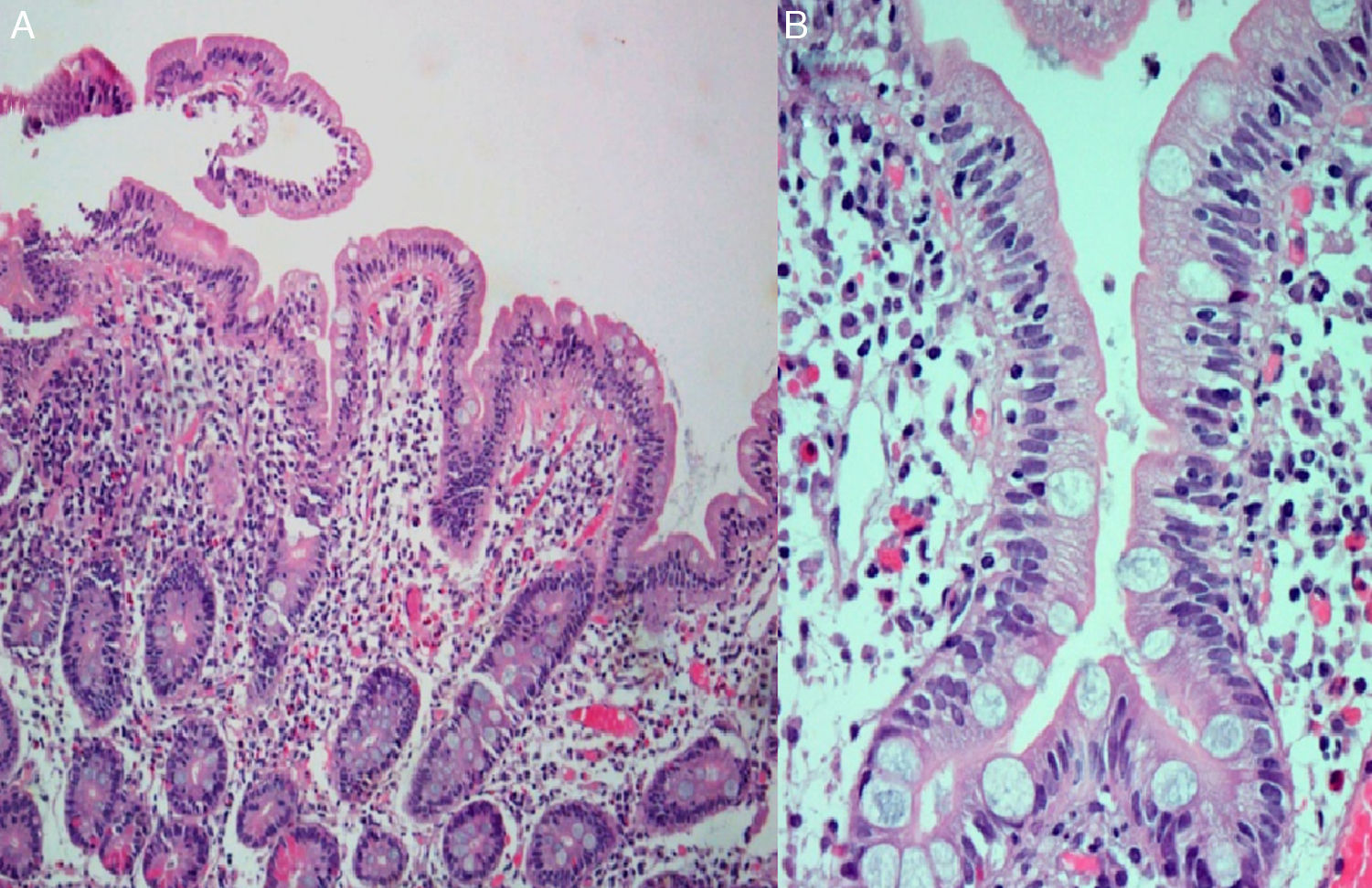
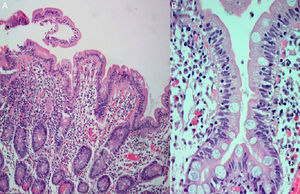
During hospitalization, the patient had progressive clinical and analytical improvements and was discharged with an indication for clinical and analytical reassessment. A month later, diarrhoea and weight loss recurred. Oral ciprofloxacin for possible small bowel bacterial overgrowth secondary to small intestinal dysmotility of systemic sclerosis was initiated, but he did not show any clinical improvement. An upper gastrointestinal endoscopy was performed and duodenal biopsies revealed moderate villous blunting and intraepithelial lymphocyte infiltration (Fig. 2). Because of clinical improvement during hospitalization (without antihypertensive drug) and intensification of complaints with the re-introduction of his usual medication, olmesartan-induced enteropathy was suspected. The drug was discontinued. Within 3 months, the patient gained weight and showed resolution of analytical changes.
2.2Case 2A 62 year-old Caucasian male with arterial hypertension, who was administered olmesartan (20mg/day) for 5 years previously, was referred for a gastroenterology and internal medicine consultation because of chronic watery diarrhoea (7–10 stools/day) associated with pain in the lower abdomen quadrant and a 25kg weight loss (19% body weight). He has been admitted to the internal medicine department several times in the previous 3 years because of these complaints. He denied fever and any other relevant epidemiological context. A prior colonoscopy revealed a normal mucosa with intraepithelial lymphocyte infiltration, which was compatible with lymphocytic colitis detected by a histopathological examination. Based on the histological findings, he underwent treatment with multiple courses of oral steroids (budesonide 9mg/day) and antibiotics, but did not show clinical improvement.
Laboratory evaluations showed normocytic anaemia (11.9g/dL; N: 13–17), hypoalbuminaemia (20.9g/L; N: 3.4–4.8), and severe electrolyte abnormalities – potassium 2.3mEq/L (N: 3.6–5.1); calcium 0.48mmol/L (N: 1.15–1.35); and magnesium 0.77mg/dL (N: 1.6–2.6). Serology for celiac disease including thyroid hormone levels and autoantibodies, stool analysis for infectious agents (Clostridium, virology, bacteriology, mycobacteriology, and parasitology), and an investigation for a possible neuroendocrine neoplasm were all negative.
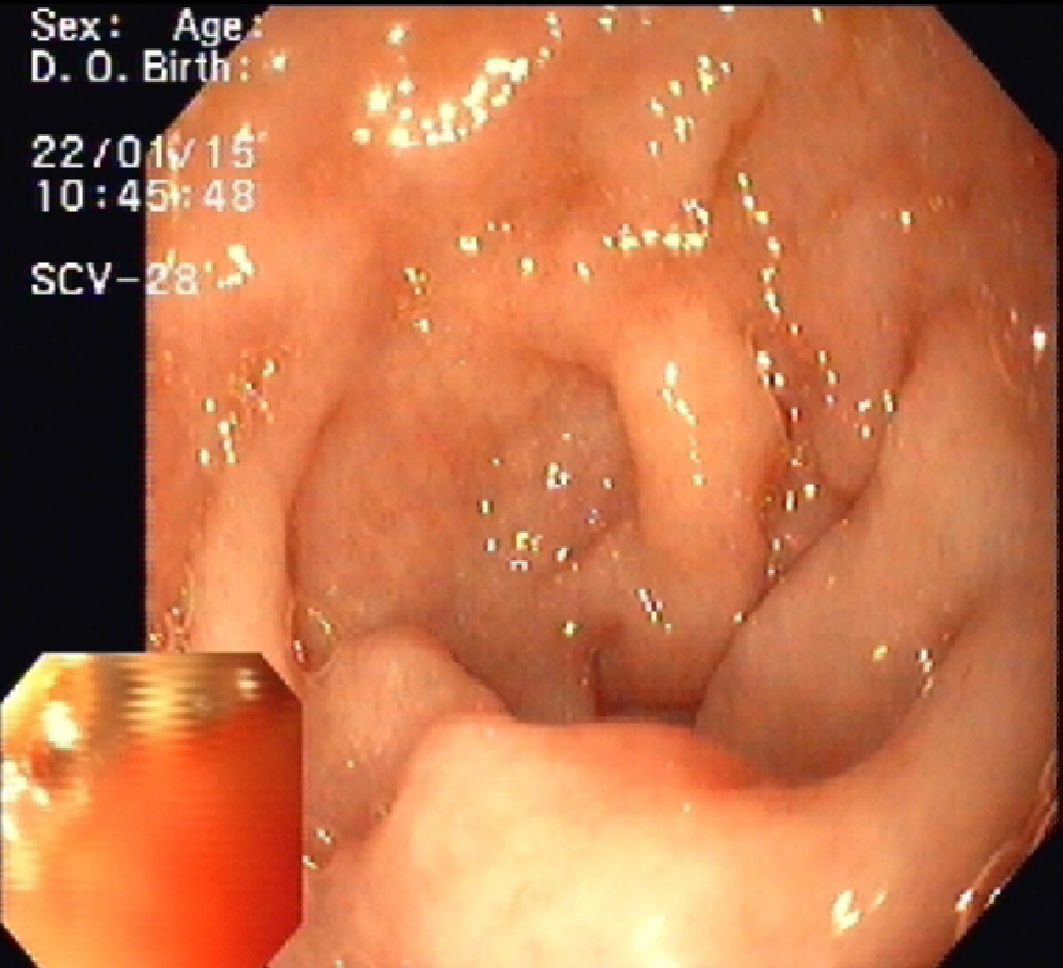
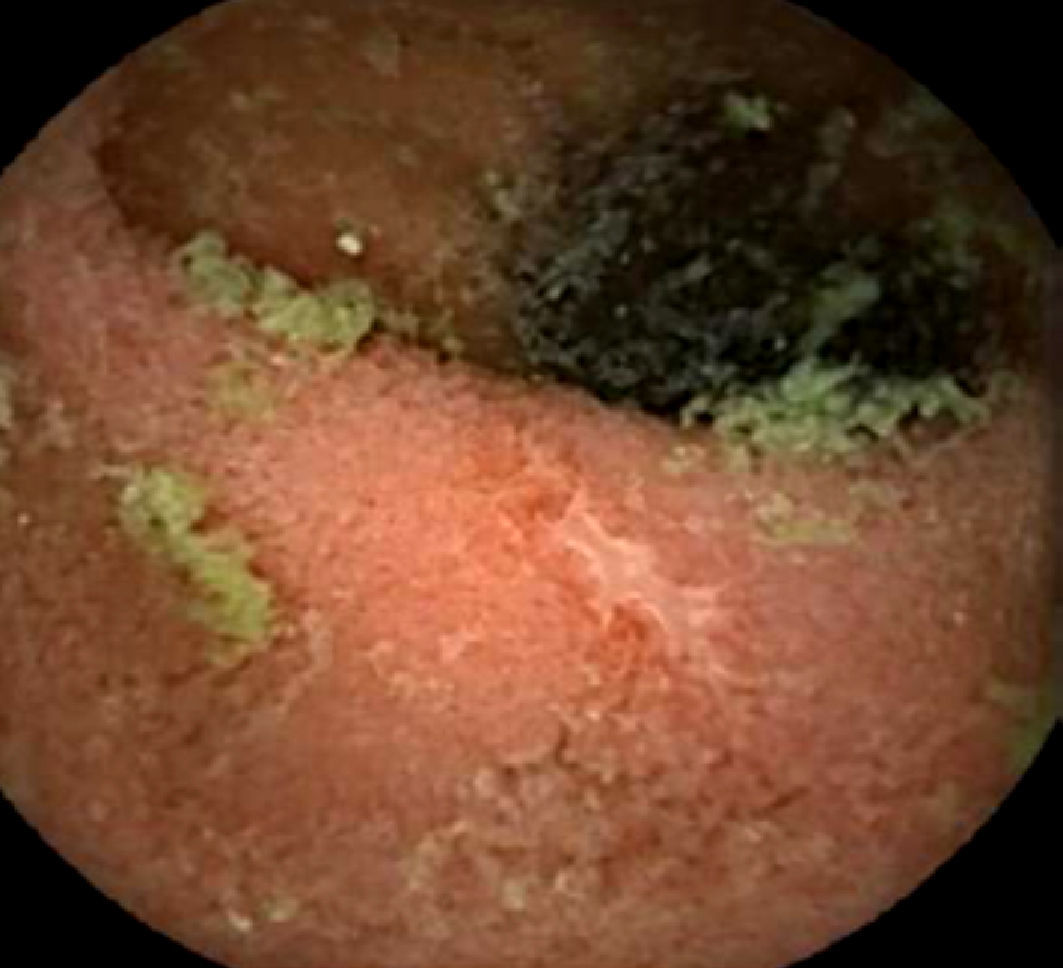
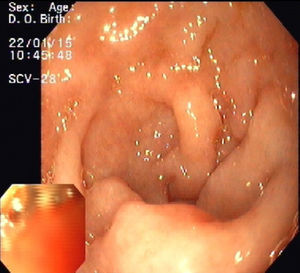
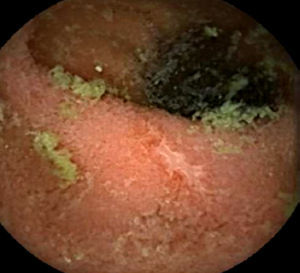
Upper endoscopy revealed patchy erythema of the antral mucosa and villous atrophy of the duodenal mucosa (Fig. 3). Biopsies showed attenuated villi with severe inflammatory aspects with no signs of granulomas, microorganisms, or malignancy. Capsule endoscopy showed jejunoileitis with mucosal ulceration (Fig. 4). A few days after endoscopy, the patient was admitted to our hospital because of the persistence of symptoms and development of acute renal failure. In this context, olmesartan was discontinued. Within 48h, a rapid improvement was observed with resolution of the diarrhoea. After discharge, the patient restarted olmesartan and immediate recurrence of diarrhoea was noted. Based on relapsing symptoms, simultaneous with long periods of drug treatment, olmesartan-induced diarrhoea was suspected and the drug was discontinued. A 9-month follow-up, he showed complete resolution of diarrhoea with no further relapses, despite consuming a gluten-containing diet.
3DiscussionDrug-induced sprue-like enteropathy must be considered in the differential diagnosis of patients presenting diarrhoea, weight loss, and unexplained villous atrophy of the duodenal mucosa. In 2012, Rubio-Tapia et al.1 reported on 22 patients with severe olmesartan-induced sprue-like enteropathy. The most frequent symptoms are severe chronic diarrhoea with substantial weight loss (median, 18kg), but nausea, vomiting, abdominal pain, and fatigue can be present. Enteropathy can develop months to years after olmesartan initiation, although case reports of irbesartan and valsartan-associated enteropathy have also been described.2,6 In the majority of reported cases, patients were administered with 40mg/day olmesartan, but doses of 10mg/day might be sufficient to cause enteropathy.1 Laboratory evaluation usually shows evidence of malabsorption with normocytic normochromic anaemia, hypoalbuminaemia, and multiple electrolyte abnormalities. Dehydration and acute renal failure have been reported as the main causes of hospitalization,1–4 although a case of colonic perforation has also been documented.7
As in celiac disease, olmesartan-induced enteropathy is characterized histologically by intestinal villous atrophy with mucosal inflammation and lymphoid aggregation.1,3 However, sprue-like enteropathy can be differentiated from celiac disease by the absence of tissue transglutaminase and endomysial antibodies and the lack of response to a gluten-free diet. Rubio-Tapia et al.1 reported pathologic evidence of involvement of the stomach and colon, suggesting that this disorder may affect the entire gastrointestinal tract, as demonstrated here in the second case. Discontinuation of olmesartan led to clinical improvement and histological recovery in all patients who underwent follow-up biopsies approximately 18 months later.1
At present, the mechanisms responsible for the onset of enteritis after olmesartan use are unknown. The long delay between the onset of olmesartan therapy and the development of enteropathy suggests that the reaction is a localized, delayed hypersensitivity response that is cell-mediated and results in damage to the small intestinal brush border. In addition, angiotensin receptor blockers have been suggested to have inhibitory effects on transforming growth factor, which is responsible for gut immune homeostasis and maintaining a normal balance between proinflammatory and anti-inflammatory factors.1–5
Recent findings suggested that olmesartan-induced enteropathy had an immunological basis and affected predisposed individuals. Rubio-Tapia et al.1 found a prevalence of HLA-DQ2 in 68% of patients with olmesartan-associated enteropathy, significantly higher than the expected for the general population (25–30%). These findings were reinforced by Scialom et al.5 In their study, extraintestinal autoimmune diseases were found in three of seven patients who developed olmesartan-induced enteropathy, and immunosuppressive drugs induced remission in all patients before olmesartan discontinuation. Together these data support caution when using olmesartan in patients with an autoimmune background. In the first patient presented here, systemic sclerosis may have been a predisposing factor for the development of olmesartan-induced enteropathy.
Discontinuation of olmesartan should be considered in cases where another aetiology has not been identified. When symptoms disappear and sprue-like enteropathy is confirmed by biopsy, treatment with olmesartan should not be restarted because of the life-threatening nature of the syndrome.
4ConclusionThese cases highlight the importance for clinicians to maintain a high index of suspicion for olmesartan as a cause of spruelike enteropathy, since this entity may not be so rare. The early recognition of such relationship is important due to widespread use of this drug, to avoid delay in establishing a correct diagnosis and to avoid frustrating, unnecessary and often expensive investigation.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data.
Right to privacy and informed consentThe authors declare that no patient data appear in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.