The recognition of an enteropathy caused by olmesartan is recent. It was first described in 2012 by the Mayo Clinic, which presented 22 clinical cases. Olmesartan is a highly prescribed drug and the differential diagnosis of a sprue-like enteropathy is very wide, so it is important to be aware of this pathology.
We report a case of a 67-years-old man, with arterial hypertension under treatment with olmesartan, with a 4-months history of diarrhea and weight lost. He was admitted three times in our Department during this period of time. An initial diagnosis was made of lymphocytic colitis but he did not respond to treatment with corticosteroids. There was a high suspicion of celiac disease, so the patient started a gluten-free diet but still there were no symptomatic changes. The patient underwent several blood and imaging tests which were negative. Due to the suspicion of an enteropathy caused by drugs, olmesartan was stopped and the patient showed a significant improvement of his symptoms.
The exact pathophysiology of this entity remains to be elucidated. It may affect all gastrointestinal tract and mimic a refractory celiac disease as well as a lymphocytic colitis due to similar symptoms and histology. It is expected more cases like this in the future due to high use of olmesartan in current clinical practice.
So, it is important to all gastroenterologists to be aware of this pathology and take it into consideration when putting together a differential diagnosis.
A enteropatia por Olmesartan não estava reconhecida até ao ano de 2012 quando a “Mayo Clinic” apresentou 22 casos. Apesar de existirem poucos casos publicados, é importante familiarizar os clínicos com aspectos referentes a esta patologia, visto tratar-se de um fármaco muito prescrito e o diagnóstico diferencial da enteropatia “sprue-like” ser muito amplo.
Apresentamos o caso de um paciente em tratamento com Olmesartan por hipertensão arterial. É internado 3 vezes num prazo de 4 meses por quadro de diarreia e perda importante de peso. Inicialmente, é diagnosticado de colite linfocítica que não responde ao tratamento com corticóides e, posteriormente, de provável doença celíaca que não responde a dieta sem glúten. Realizam-se inúmeros exames complementários, analíticos e de imagem, sem concluir-se nenhum diagnóstico. Perante a suspeita de uma enteropatia por Olmesartan, retira-se o fármaco definitivamente e reinicia-se o glúten na dieta, confirmando-se uma melhoria espectacular do quadro clínico. Durante os internamentos não tomava Olmesartan, sendo o motivo da melhoria clínica durante os mesmos.
Os mecanismos associados a esta patologia são desconhecidos. Pode afectar todo o aparelho digestivo e mimetizar uma doença celíaca refractária e/ou colite linfocítica devido a semelhança dos sintomas e da anatomia patológica. Devido à elevada prescrição deste medicamento, é esperável que no futuro se diagnostiquem mais casos, motivo pelo qual, os clínicos, principalmente os gastroenterologistas, devem de considera-la no diagnóstico diferencial.
Sprue-like enteropathy is characterized by diarrhea, villous atrophy of the small bowel with lymphocyte infiltration, intestinal malabsorption, significant weight loss and negative celiac disease serology. This entity remains a challenge to all gastroenterologists due to a wide differential diagnosis, such as bacterial overgrowth, jejunitis, lymphoma, tropical sprue, protein-losing enteropathy or immunosuppressive drugs such as methotrexate, azathioprine and mycophenolate.1,2
Olmesartan is an angiotensin II receptor antagonist indicated for the treatment of hypertension since 2002. Olmesartan-associated enteropathy was first described in 2012 by Rubio-Tapia et al. of the Mayo Clinic when they reported 22 clinical cases.1
We describe a case of a sprue-like enteropathy due to olmesartan to draw attention to this disease, given the high frequency of use of this drug and the difficulty of diagnosis if the entity it is not known.
2Case reportWe report a case of a 67-years-old man, with hypertension under treatment with olmesartan 40mg/day. He presented with a 4-months history of diarrhea and significant weight loss of 22kg. He was hospitalised three times in our Gastroenterology Department during this period of time. At first, the patient described a 1-month history of diarrhea with a bowel frequency of 10 times/day, mostly by night, absence of blood or mucous in stools and a weight loss of 7kg. He had no abdominal pain, nausea, vomiting or fever. His blood tests were normal, with no changes in his complete blood cell count, coagulation, ionogram, hepatic or renal function. Stool culture and Clostridium difficile toxin were negative. Colonoscopy showed no macroscopic changes but the histological features of the biopsies showed intraepithelial lymphocytosis. The findings were suggestive of lymphocytic colitis. He started treatment with budesonide 9mg/day with good symptomatic improvement. He was then discharged and follow-up was made in clinic.
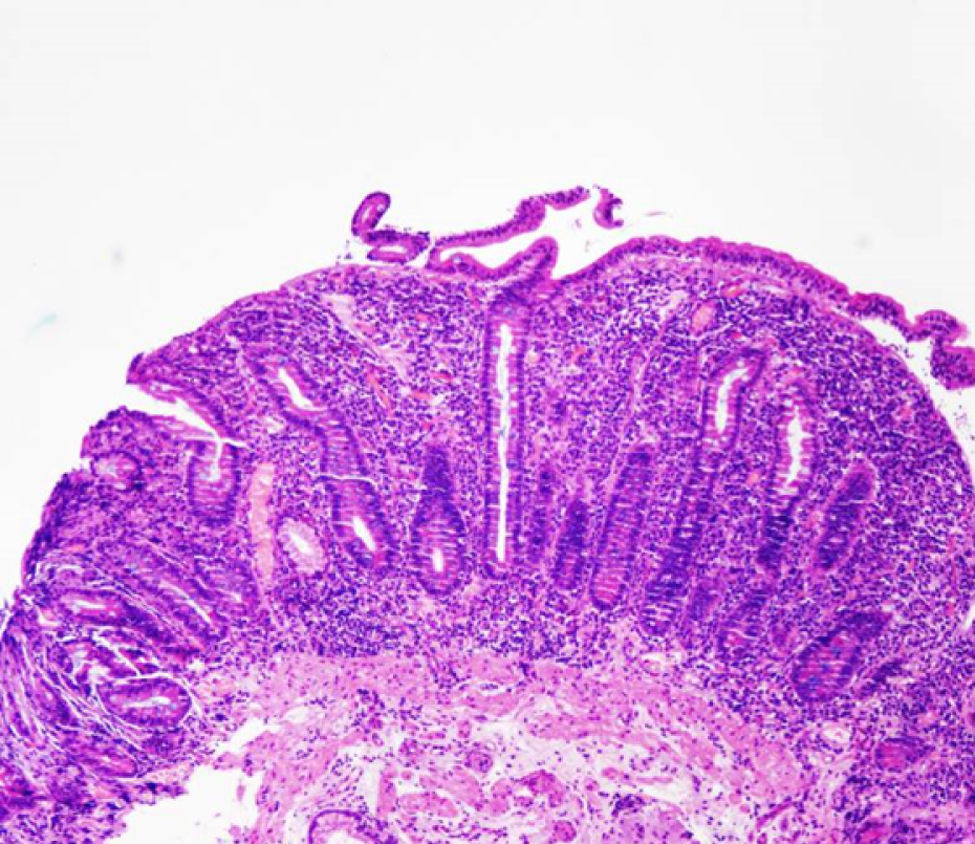
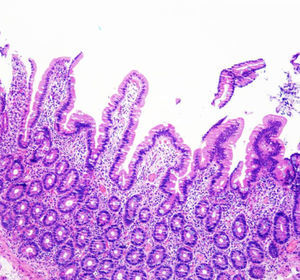
After one month, the patient was once again admitted in our Department due to symptomatic recurrence. He presented a total of 15kg weight loss. Blood tests showed hypokalemia of 2.9mmol/l, hypomagnesemia of 1.3mmol/l and hypoalbuminemia of 2.3g/dL. Complete blood cell count was normal. There were no changes in his D-Xylose absorption test as well as fecal pancreatic elastase and stool cultures. Abdominal CT and gastrointestinal transit showed significant dilatation of the small bowel but no stricture or stenosis were found. An endoscopic ultrasound showed an atrophic pancreas with a normal duct of Wirsung. Celiac disease serology (IgA anti-transglutaminase, anti-endomysial and anti-gliadin antibodies) was negative and the genetic test for HLA DQ2/DQ8 was positive. An endoscopy was also performed and duodenal biopsies showed increased intraepithelial lymphocytes, crypt hyperplasia and villous atrophy (grade IIIC according to the modified Marsh classification) (Fig. 1). Meanwhile, the patient showed clinical improvement and he was discharge with the diagnosis of celiac disease. His eating habits were changed during this admission and he started a gluten-free diet.
After approximately 45 days, the patient was admitted due to severe recurrence of symptoms. He had a total weight loss of 22kg then. The blood tests showed a prothrombin time of 50%, total cholesterol of 85mg/dL, triglycerides of 73mg/dL, hypoalbuminemia of 1.9g/dL and transferrin of 184mg/dL. Stools cultures were re-done and once again negative. All food allergies tests turned out negative. He started a hyperproteic parenteral nutrition and a gluten-free diet.
Due to a sprue-like enteropathy with negative serology for celiac disease, we decided to search in the literature for similar cases. We found some studies describing cases of olmesartan-associated enteropathy. Following this, olmesartan was discontinued. The patient had an impressive outcome and all clinical signs ceased one week later.
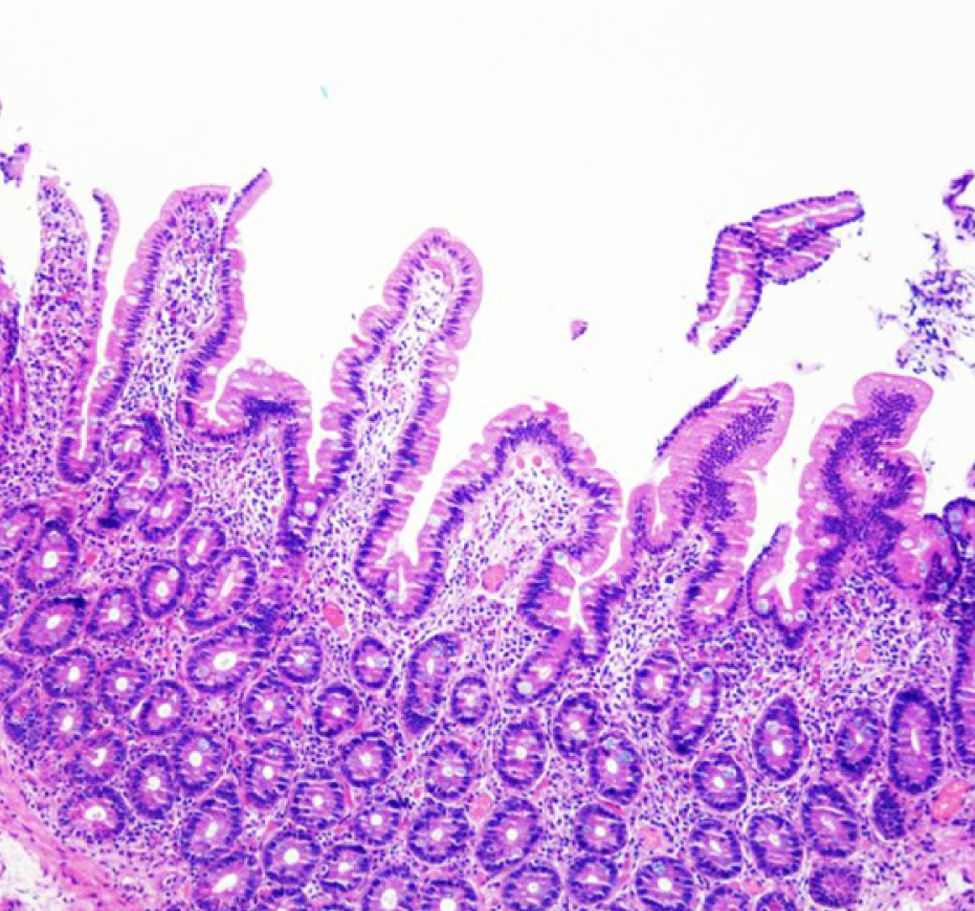
A close follow-up was made in our clinic during the following two months. The patient presented normal bowel habits and had gain weight, although he claimed to have reintroduced gluten to his diet during this time. Subsequently, we choose to let the patient have a no restriction diet. No changes were noted in his clinical status during the following consults. Four months later, the patient showed a weight gain of 20kg and normal bowel habits. A histopathological review was made and duodenal biopsies showed a significant improvement of the villous architecture (Fig. 2).
3DiscussionWe describe a case of severe sprue-like enteropathy due to olmesartan.
The exact pathophysiology underlying remains to be elucidated. The current data sustain the hypothesis of a cell-mediated immune response3 based on the presence of antinuclear antibodies and circulating activated T cells, and to the fact that this entity presents a good response to corticosteroid and/or immunosuppressive treatment.4 The pathway behind the inhibition of the transforming growth factor beta has also been considered.5 Nevertheless, this is a question that requires further investigation.
Sprue like enteropathy can be severe in up to 50% of all cases and sometimes can lead to perforation.3
Olmesartan-associated enteropathy may mimic celiac disease. However, these patients do not respond to a gluten-free diet and celiac disease serology is negative. In our study HLA DQ2/DQ8 testing was positive. Overall, and according to Rubio-Tapia et al., DQ2/DQ8 is present in 68% of patients with olmesartan-associated enteropathy, prevalence higher than the 25–30% expected for the general population, suggesting that the presence of HLA DQ2/DQ8 may increase the risk of immune-mediated damage in these patients.1,6 Despite its association with DQ2/DQ8 genes, olmesartan does not trigger celiac disease, unlike other drugs such as interferon or ipilimumab.4
A microscopic colitis could also be a potential differential diagnosis of this entity due to similar histopathological findings. Colonic biopsies showed a dense lymphocyte infiltration and a thick collagenous subepithelial layer. However, the patient did not respond to treatment with corticosteroids.1,5,8
In 2012, Pallav et al. published a study in which 33% of patients presented a non-specific enteropathy with negative serology.7 In 2013, in a report by DeGaetani et al., 14% of patients presented with the same pathology and they showed that this number could have been increased up to 30% if an olmesartan-associated enteropathy had not been described in the literature.8
Marisa DeGaetani et al. reported a study with 72 patients with villous atrophy and negative serology for celiac disease. She realized that 26% of her cases were drug-associated, being olmesartan responsible for 22.2%. If we only take into consideration the data regarding the drug-associated enteropathy cases, we can conclude that 84% of all cases were related to olmesartan followed by mycophenolate mofetil (11%) and methotrexate (5%). This study shows how frequent this entity is and enhances its importance when putting together a differential diagnosis.8
In this clinical case we did not reintroduce olmesartan to confirm the diagnosis because of the patient's severe clinical condition. However, whenever the patient was admitted, olmesartan was discontinued due to normal arterial pressure. Everytime our patient was discharged olmesartan was reintroduced. This event probably justifies his clinical remission and relapses. This theory is confirmed by Marthey et al. In this specific study she described 36 patients with olmesartan-associated enteropathy. She reported 10 cases of olmesartan interruptions which were followed by reintroductions before steroids or immunosuppressant drugs. Interruptions were followed by remissions (9/10), and reintroductions were followed by relapses (9/9). Here clinical symptoms occurred 28 months after the first exposition to olmesartan.4 In this case report, our patient was exposed to olmesartan for 36 months before presenting any symptoms. This suggests the need for a prolonged exposure time, maybe months or even years, until the onset of symptoms.
Overall, cases of enteropathy related to other angiotensin II receptor antagonists seem to be less frequent and further investigations about its mechanism of action is still required. Until now, only one case of irbesartan-associated enteropathy has been described.4 This case was very similar to olmesartan-associated enteropathy in clinical presentation as well as its response to treatment and drug suspension. Subsequently, nowadays it is not recommended the use of angiotensin II receptor antagonists in patients presenting olmesartan-associated enteropathy.
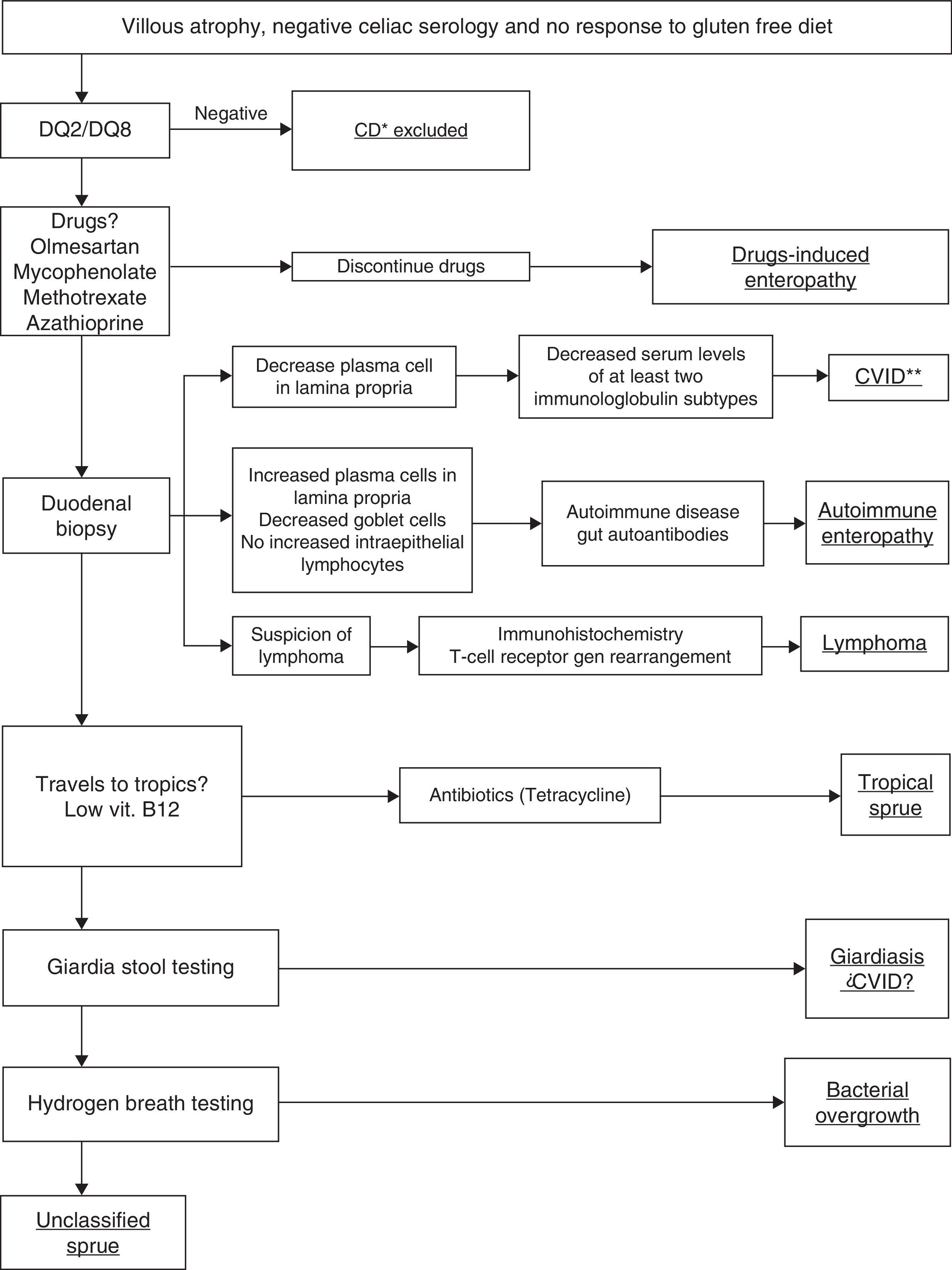
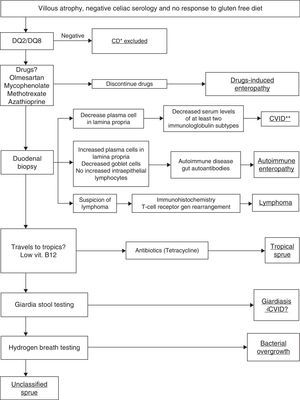
We suggest an algorithm to help making a diagnosis in patients who present villous atrophy, negative serology for celiac disease and no response to gluten free diet (Fig. 3).
In conclusion, it is expected more cases like this in the future due to high use of olmesartan in current clinical practice for treatment of hypertension. Olmesartan-associated enteropathy may be considered as a clinical entity, and should be included in the differential diagnosis of a sprue-like enteropathy, particularly when serology for celiac disease is negative.
Ethical disclosuresRight to privacy and informed consentThe authors declare that no patient data appear in this article.
Confidentiality of dataThe authors declare that no patient data appear in this article.
Protection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Conflicts of interestThe authors have no conflicts of interest to declare.