We report a case of a 63-year-old-man presenting with chronic diarrhea and weight loss while on olmesartan treatment for hypertension. Investigation showed multiple nutritional deficiencies associated with diffuse intestinal villous atrophy. Serologies for celiac disease were negative and other causes of villous atrophy were excluded. Olmesartan as a precipitant agent was suspected and withdrawn. Clinical improvement occurred in days with no need for other therapeutic measures. Follow-up at three months showed clinical remission and almost complete recovery of intestinal atrophy.
Olmesartan is an angiotensin receptor blocker commonly prescribed for the management of hypertension. Spruelike enteropathy associated with this drug is a recently described entity with few cases reported. It presents with chronic diarrhea and intestinal villous atrophy and should be included in its differential diagnosis. This case intends to alert clinicians for the possibility of this event in a patient on treatment with this drug.
Apresentamos o caso de um homem de 63 anos com diarreia crónica e perda ponderal. Apresentava hipertensão arterial tratada com olmesartan. A investigação complementar mostrou múltiplos défices nutricionais associados a atrofia vilositária intestinal difusa. As serologias de doença celíaca foram negativas e outras causas de atrofia vilositária foram excluídas. Suspeitou-se do olmesartan como agente precipitante, sendo este suspenso. Observou-se melhoria clínica em dias, sem necessidade de outras medidas terapêuticas. No seguimento, aos 3 meses, constatou-se remissão clínica e recuperação quase completa da atrofia intestinal.
O olmesartan é um bloqueador dos recetores da angiotensina, geralmente prescrito no tratamento da hipertensão. A enteropatia “spruelike” associada a este fármaco é uma entidade recentemente descrita, com poucos casos reportados. Manifesta-se por diarreia crónica associada a atrofia vilositária intestinal, devendo ser incluída no seu diagnóstico diferencial. Com este caso pretende-se alertar os clínicos para a possibilidade deste evento em doentes sob tratamento com este fármaco.
The most common cause of villous atrophy is celiac disease.1,2 The villous atrophy results from injury to the small intestine and leads to loss of absorptive surface area, reduction of digestive enzymes, and consequential impaired absorption of micronutrients.3 Negative celiac serology or nonresponse to a gluten-free diet implies a broad and challenging differential diagnosis which includes Crohn's disease, enteric infections (e.g. Giardia lamblia), collagenous sprue, tropical sprue, common variable immunodeficiency, autoimmune enteropathy, hematological malignancies and medication-associated enteropathy.2 Regarding the latter, olmesartan medoxomil, an angiotensin receptor blocker for the management of hypertension, has been recently recognized as a cause of “sprue-like enteropathy”.4–6
We report a case of severe enteropathy associated with olmesartan use.
2Clinical caseA 63-year-old man was admitted to our department complaining of progressive diarrhea and significant weight loss (12kg) for one year. He reported between 5 and 7 daily episodes of bulky, watery and nonbloody diarrhea. Over the preceding two weeks, it was associated with severe fatigue, anorexia and vomiting. He denied abdominal pain, fever or other symptoms. There was no history of recent travels or sick contacts.
Apart from arterial hypertension, treated with olmesartan and hydrochlorothiazide (20/12.5mg) for two years, his past medical history was unremarkable.
The patient had already undergone total colonoscopy and abdominal computed tomography with no remarkable findings. A gluten and lactose-free diet were tried without improvement. He also failed initial conservative treatment with a trial of oral antibiotic for possible small bowel bacterial overgrowth.
On presentation at our department, his body mass index was 20kg/m2 (normal (N): 18.5–24.99kg/m2), close to the lower limit of normal. Muscle wasting was also seen without evident weakness. There was no peripheral edema or other relevant findings on physical examination.
Laboratory evaluation revealed: hemoglobin 11.6g/dL (normal: 13–17) with normal mean corpuscular volume and mean corpuscular hemoglobin; serum potassium 1.8mmol/L (N: 3.6–5.1), phosphorus 1.7mg/dL (N: 2.3–4.7), magnesium 1.3mg/dL (N: 3.6–5.1), corrected calcium 8.6mg/dL (N: 8.8–10); albumin 2.9g/dL (N: 3.4–4.8), total protein 4.9g/dL (N: 6.2–8.5), aspartate aminotransferase 205UI/L (N: 5–34), alanine aminotransferase 106 UI/L (N<55) and protein C-reaction 25mg/L (N: <5mg/L). The prothrombine time (PT) was increased (26.6s; N: 9.4–13) as well as activated partial thromboplastin time (aPTT) (50s; N: 20–40).
Other laboratory work-up was unremarkable including leucogram, serum glucose, B12 vitamin, folic acid, transglutaminase antibodies, serum immunoglobulins, thyroid stimulating hormone and serology for human immunodeficiency virus. Platelets, bleeding time and fibrinogen were also normal.
Other causes for hypertransaminasemia were additionally excluded (no alcohol consumption; bilirubin, alkaline phosphatase, gamma-glutamyl transpeptidase, serum iron, ferritin, transferrin saturation, cholesterol and triglycerides were normal; hepatitis B and C serologies were negative; antinuclear antibodies and smooth muscle antibodies were negative and abdominal ultrasound excluded liver or biliary abnormalities).
Stool examination namely cultures, Clostridium difficile toxin assay, ova and parasites was unrevealing.
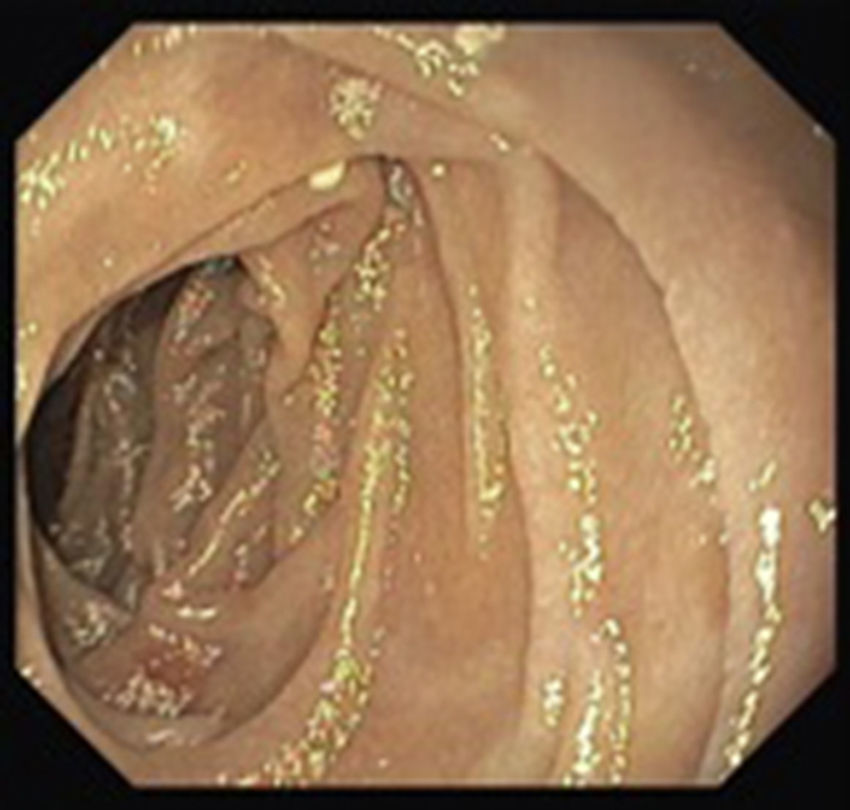
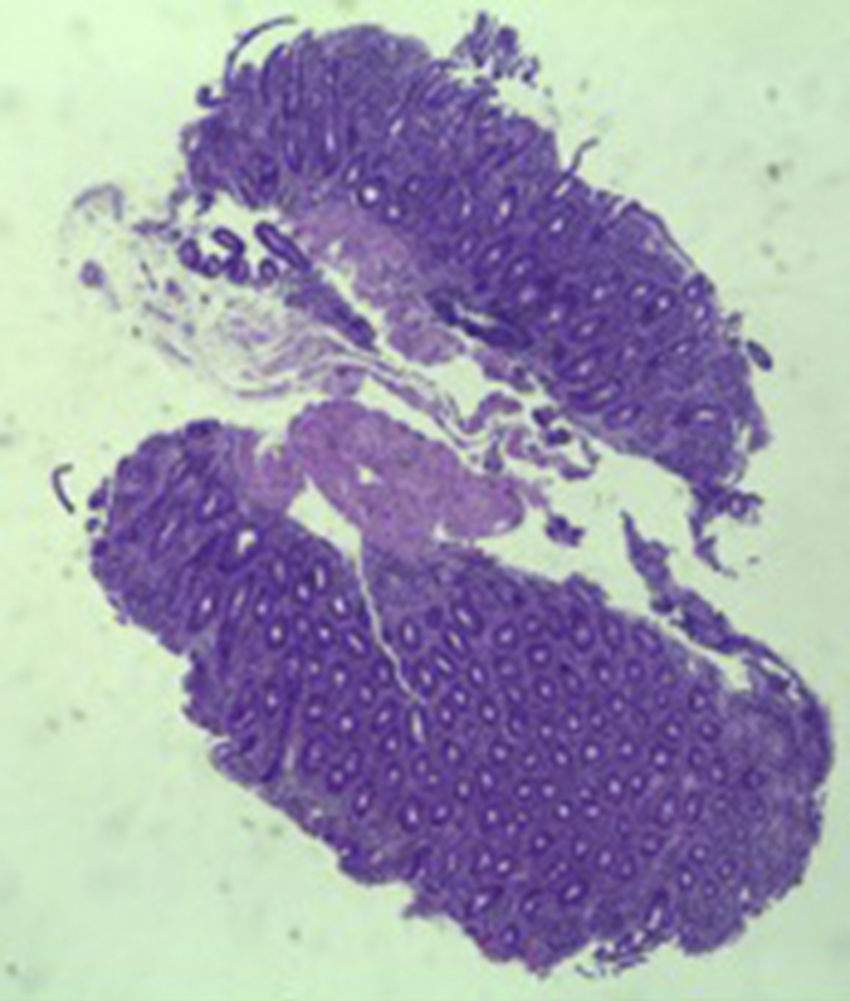
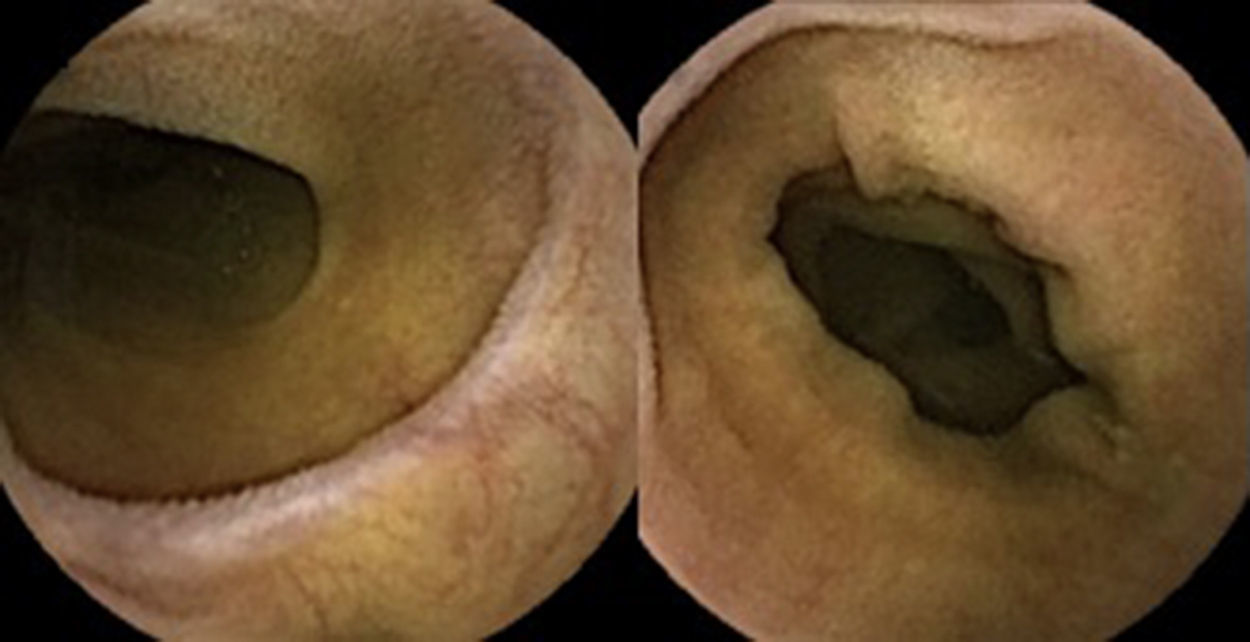
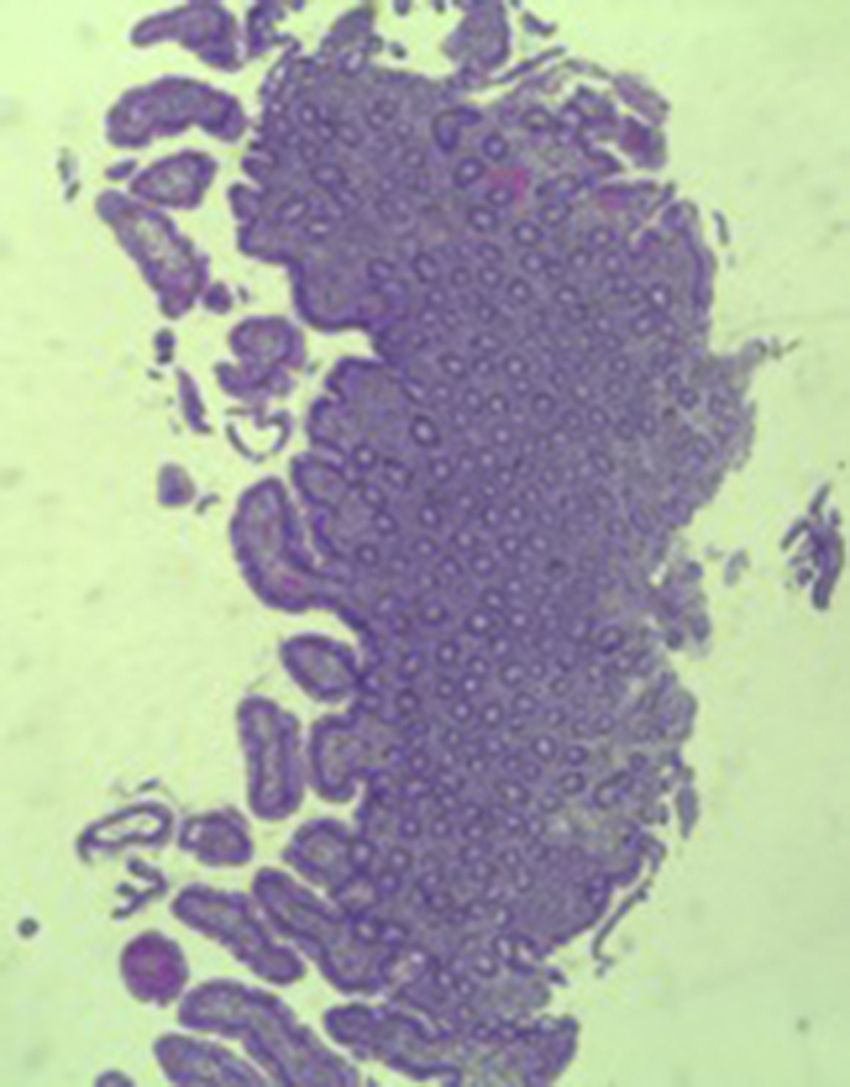
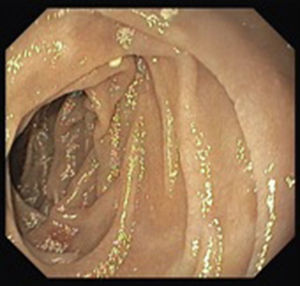
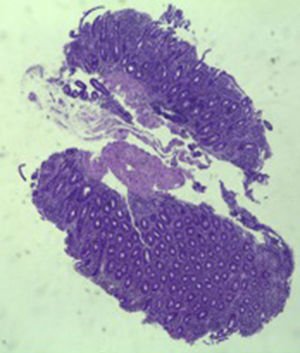
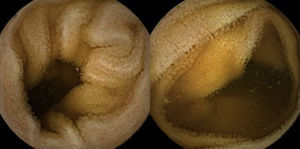
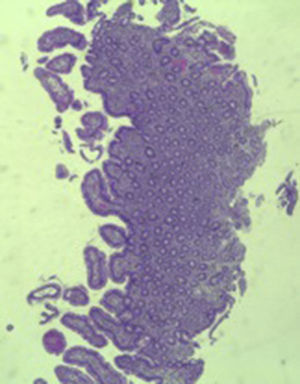
A colonoscopy was repeated and, despite all efforts, the terminal ileum could not be intubated. Colonic random biopsies excluded microscopic colitis or other abnormalities. Upper endoscopy evidenced a discrete attenuation of duodenal villous pattern without other findings (Fig. 1). Histopathological examination confirmed a partial villous atrophy and chronic lymphocytic infiltration of the lamina propria (Fig. 2). Capsule endoscopy was performed and demonstrated a diffuse flattening of the small bowel villi (Fig. 3).
We suspected of olmesartan-associated sprue-like enteropathy. This drug was therefore withdrawn along with replacement of electrolytes and vitamin K administration. Prompt improvement was achieved within a few days. One week after hospital admission, the patient was discharged without diarrhea or need for nutritional/electrolyte support and began to gain weight. Olmesartan was switched to amlodipine.
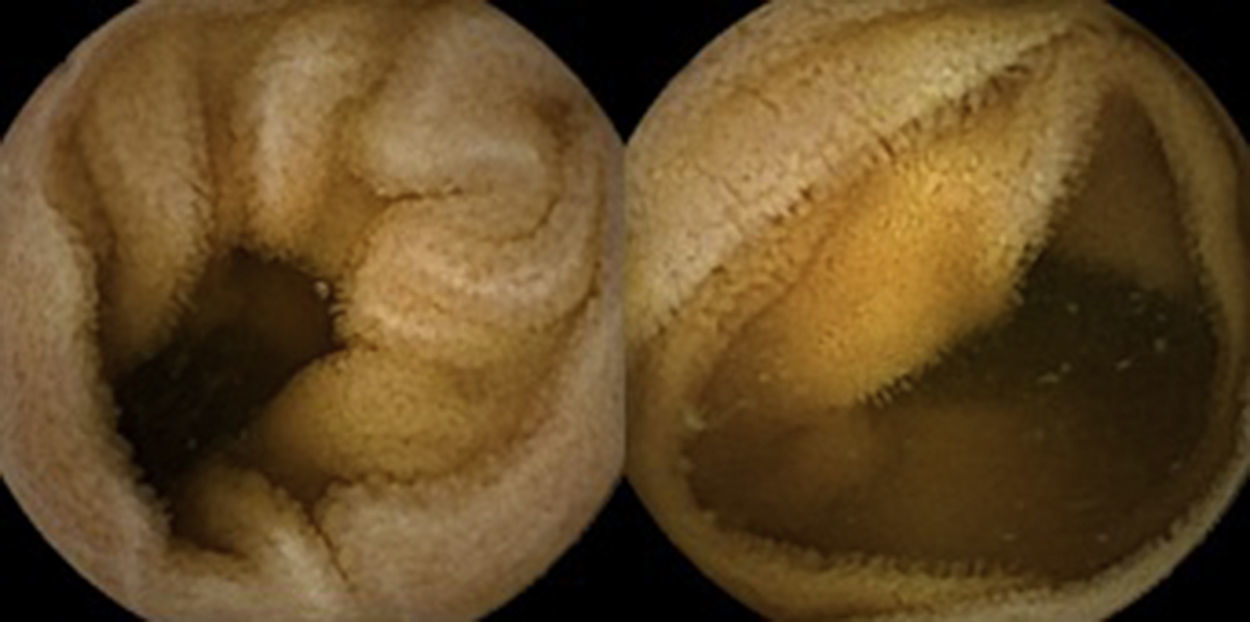
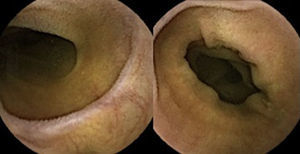
Three months later, a complete recovery of weight (12.5kg) was seen along with full normalization of laboratory tests (hemoglobin, electrolytes, albumin, TP, aPTT, protein-C reaction and aminotransferases). Upper endoscopy and capsule endoscopy (Fig. 4) were, again, performed and showed normal small bowel appearance. Histopathological analysis of duodenal biopsies confirmed an almost complete recovery of duodenal villi and no lymphocyte infiltration (Fig. 5). At sixth month follow-up, the patient remained asymptomatic with no laboratory abnormalities.
3DiscussionWe described a case of a patient presenting with chronic diarrhea and malabsorption as evidenced by multiple nutritional deficits including electrolyte imbalance and reduced serum albumin. Prolonged PT and aPTT in a patient not taking vitamin K antagonists, with no evidence of liver disease, biliary obstruction or disseminated intravascular coagulation suggested, in this clinical setting, vitamin K malabsorption. In addition, villous atrophy was present throughout the entire small bowel as demonstrated by capsule endoscopy, which explains the malabsoption.
In our case, celiac disease, the most common cause of villous atrophy,1,2 was excluded by serology methods and the lack of clinical response to a gluten-free diet. After excluding other causes of villous atrophy, we considered an olmesartan-associated enteropathy.
Olmesartan medoxomil is an angiotensin II receptor blocker approved for the treatment of hypertension since 2002.7 A sprue-like enteropathy associated with olmesartan was first reported by Rubio-Tapia et al.4 and since then, similar cases have been described, although mainly as case reports or small case series.6,8–15 As a result, United States Food and Drug Administration reported this olmesartan associated adverse event via a MedWatch alert in July 2013.
Clinical presentation of this entity include chronic diarrhea, vomiting, abdominal pain, bloating, weight loss and fatigue.4,6,11 More severe cases with dehydration,4,9,13 acute renal failure9 and a case of colonic perforation11 have been reported. According to previous descriptions, the duration of exposure to olmesartan before the onset of diarrhea has varied between several months and years.4,6 In our case, it took one year to present symptoms, which is in accordance with the timing reported.
Laboratory investigation may show normocytic, normochromic anemia, hypoalbuminemia and one or multiple electrolyte abnormalities,4 as evidenced in our case. Human leukocyte antigen (HLA) assessment, when performed, may reveal a higher prevalence of DQ2 or DQ8 haplotypes than expected for the general population, which suggests a possible role for genetics in this enteropathy.4,6
Upper endoscopy may be normal, show a nodular appearance of the duodenal mucosa or flattening of villi.6 In our case, only a discrete attenuation of duodenal villous pattern was observed. Capsule endoscopy, however, highlighted a diffuse and obvious flattening of the small bowel villi.
The most common histopathological finding is intestinal villous atrophy (either total or partial), which may be associated with variable degrees of mucosal inflammation. In contrast to celiac disease, flattening of villi is not always associated with increased intra-epithelial lymphocytes and inflammation.6 In addition, involvement of the stomach and colon with lymphocytic aggregation was also reported by some authors,4,8,15 suggesting that this disorder may affect the entire gastrointestinal tract.
Regarding treatment, previous reports have demonstrated clinical remission in all patients after discontinuation of olmesartan.4,6,8–13,15 Moreover, almost all patients have shown histological recovery of the duodenum after drug withdrawal (although follow-up intestinal biopsies have not been done systematically in all patients).4,6,10,12 In contrast to other enteropathies, such as celiac disease that may take years to achieve histological recovery, despite adequate treatment,16,17 olmesartan-induced enteropathy is associated with a quick mucosal recovery (median eight months from the suspension of the drug to follow-up biopsies).4
In our case, the complete resolution of symptoms and malabsorption, the intestinal villous atrophy improvement without any other therapeutic/dietary measures besides discontinuation of the drug and the absence of other causes of enteropathy support this diagnosis. Deliberate rechallenge test with olmesartan was not performed because of the life-threatening nature of the disease and was not clinically pertinent.
One additional interesting finding, in our case, was the hypertransaminasemia that normalized after the discontinuation of the drug. After excluding other causes for this finding, we believe that this could be linked to the enteropathy, similar to that described for celiac disease. Celiac patients with hypertransaminasemia have an important increase in intestinal permeability compared with those whose liver enzymes are normal.18 One proposed explanation for hepatic involvement in these patients is that the increased intestinal permeability may ease the entry of toxins, antigens, and inflammatory substances (cytokines and/or autoantibodies) to the portal circulation that, subsequently, play a role in liver injury.19 In our case of severe olmesartan-induced enteropathy we speculate that similar mechanisms could be on the basis of hypertransaminasemia. The report of more cases of this drug-induced enteropathy is crucial to clarify these findings.
The pathogenesis of this entity is still unclear. The long interval between the beginning of olmesartan therapy and the onset of diarrhea suggests a cell-mediated immune mechanism.4,11 Some reports have indicated a potential inhibitory effect of angiotensin receptor blockers on transforming growth factor, which is responsible for gut immune homeostasis.20,21 Another proposed theory is related to a pro-apoptotic effect of angiotensin (AT) II on intestinal epithelial cells. Angiotensin II binds to two receptor forms, AT1 and AT2, with different properties and different gastrointestinal distribution. AT1 receptor is expressed throughout the whole alimentary tract, while AT2 receptor is expressed particularly in the duodenum and jejunum.22 Sun et al. suggested that angiotensin II through binding to AT2 receptor up-regulates pro-apoptotic proteins, such as Bax and GATA-6, in association with a down-regulation of Bcl-2, an anti-apoptotic protein.23 Olmesartan shows high affinity for AT1 receptors. In case of saturation of this receptors, circulating angiotensin II could bind to AT2 receptor resulting in a pro-apoptotic effect and, consequently, to villous atrophy.6 Further investigations are needed to elucidate the physiopathological mechanisms underlying this entity.
Another issue to clarify is if other angiotensin II receptor blockers can have a similar adverse reaction. Recently, valsartan was implicated in a case of “sprue-like” enteropathy.24 Nevertheless, cases of enteropathy related to other angiotensin II receptor inhibitors seem to be much less frequent than olmesartan-induced enteropathy.5 The selective role of olmesartan might be explained by its conversion into the active form in the intestine, its longer half-life and higher efficacy compared to other sartans.25
In conclusion, this report intends to alert the clinical community for this probably underreported problem,11 since this condition is serious and olmesartan is commonly prescribed for the treatment of hypertension. Negative serology for celiac disease and absence of response to a gluten-free diet on patients taking olmesartan should aware clinicians for the possibility of this condition. We show iconography namely capsule endoscopic images, that document the endoscopic findings associated with this entity and can add information on the hallmarks of this entity. Increasing reports of this drug-induced enteropathy may lead to the identification of more cases, a better characterization of this entity and an earlier diagnosis, with obvious benefits to patients.