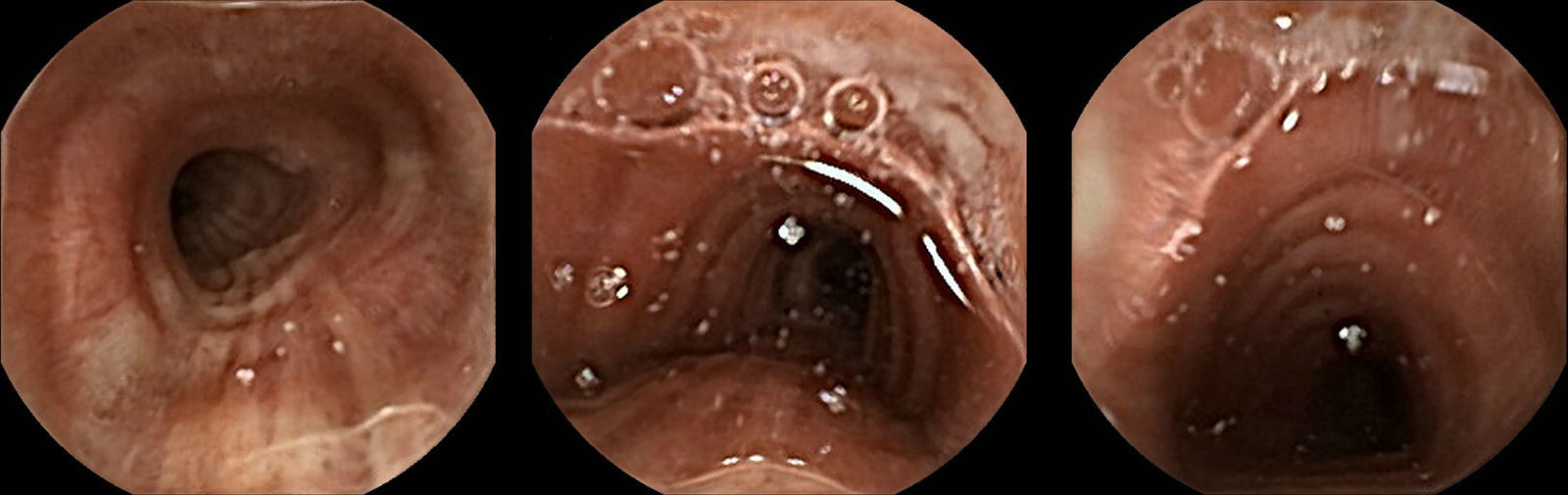
A 92-year-old man was referred to our endoscopy department for overt obscure gastrointestinal bleeding (OGIB) investigation, thus a capsule endoscopy (CE) – PillCam@ SB2 (Given Imaging Ltd., Yoqneam, Israel) was performed. As co-morbidities, he had Parkinson disease and diabetes mellitus. The patient had no prior history of dysphagia or cerebrovascular events and the device was ingested under the surveillance of an experienced nurse. No complaints were mentioned at the time CE was ingested. Four hours later, the patient felt a sudden dyspnea compelling him to a vigorous cough. After some minutes the CE device was expelled intact and the patient, asymptomatic, returned the device to our department. The recorded video photograms showed that the device was persistently in the mouth and pharynx of the patient for almost four hours and then entered the trachea (Fig. 1). The typical features of this structure are easily recognizable (the incomplete cartilaginous rings anteriorly and laterally, and a straight membranous wall posteriorly).
CE is considered a safe and effective method for studying OGIB.1 Although, as with any other medical diagnostic device, it is not devoid of potential complications. Our case presents a very rare but potentially fatal complication. After the first reported case in 2003, only 25 cases are described in the literature2 and most of them had a spontaneous resolution, after vigorous cough. Older patients and those with neurological diseases are prone to such complication and, as recommended by some authors, very old patients and those with known dysphagia or neurological diseases should have the device introduced via a delivery system (simple net or the AdvanCE delivery device).3 Recently, the Real-Time Viewer4 was introduced with the aim of inform, in real-time, the clinician where is the device and if a delivery system or a prokinetic should be employed to aid the passage to the small-bowel. Physicians and their teams should identify these patients at higher risk of CE aspiration in order to prevent a potentially fatal outcome.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the relevant clinical research ethics committee and with those of the Code of Ethics of the World Medical Association (Declaration of Helsinki).
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflicts of interestThe authors have no conflicts of interest to declare.
Authors’ contributionPedro Magalhães-Costa is the author guarantor. The manuscript is approved by all Authors. All authors have contributed to and agreed on the content of this manuscript. Pedro Magalhães-Costa wrote the manuscript, Pedro Magalhães-Costa and Miguel Bispo diagnosed, treated and followed the patient, Cristina Chagas critically revised the manuscript.