Schwannoma is a benign tumor arising from Schwann cells that form the neural sheath. Primary schwannoma of the colon is rare and a few cases have been reported. We report a case of schwannoma of the colon and present the differential diagnosis that must be considered in the evaluation of colonic subepithelial lesions.
O schwannoma é um tumor benigno com origem nas células de Schwann que formam as bainhas nervosas. O schwannoma primário do cólon é uma lesão rara e poucos casos foram descritos. Apresenta-se o caso de um schwannoma do cólon e faz-se referência aos diagnósticos diferenciais que devem ser considerados na avaliação das lesões subepiteliais do cólon.
Subepithelial lesions represent abnormal growing tissue underneath the normal mucosa of the gastrointestinal (GI) tract. The term ‘submucosal lesion’ is often recognized and used as synonymous. However, it is inappropriate and should be avoided, because many of these lesions do not arise from the submucosa.1 Subepithelial lesions may arise from any layer of the GI tract wall (intramural growth) and should be distinguished from subepithelial-like lesions that have origin in neighboring tissue.2,3 They are more frequent in upper GI tract and are usually asymptomatic.1,4 The diagnosis is mainly incidental during endoscopic or radiologic examinations.4
Schwannomas of GI tract are subepithelial lesions arising from GI autonomic nerves that are uncommonly found and are very rarely seen in the colon.2,6 Despite the unspecific appearance at endoscopic and radiologic exams, it is important to differentiate these from another subepithelial and subepithelial-like lesions.3,7
We report a case of schwannoma of the colon that was confirmed by pathology after surgery.
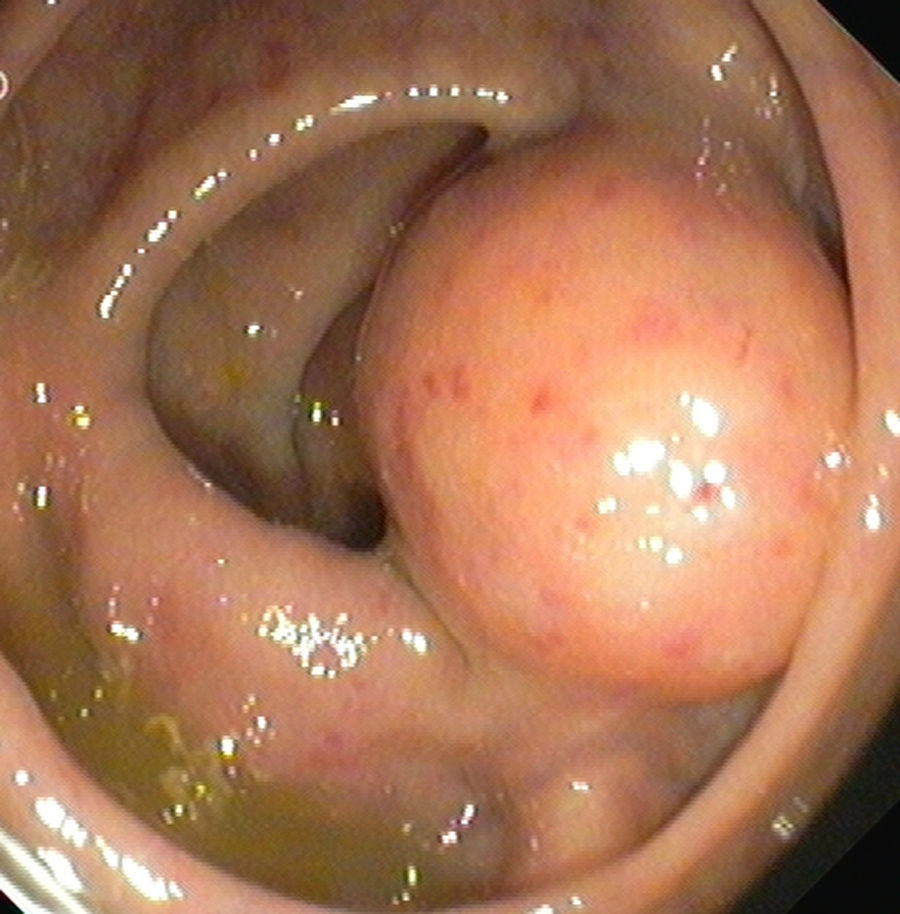
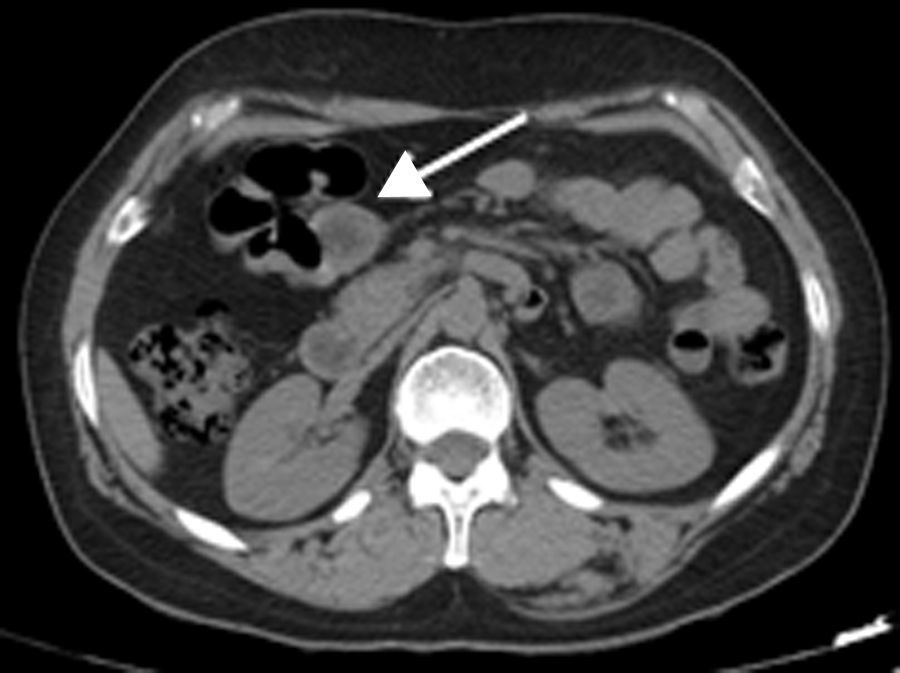
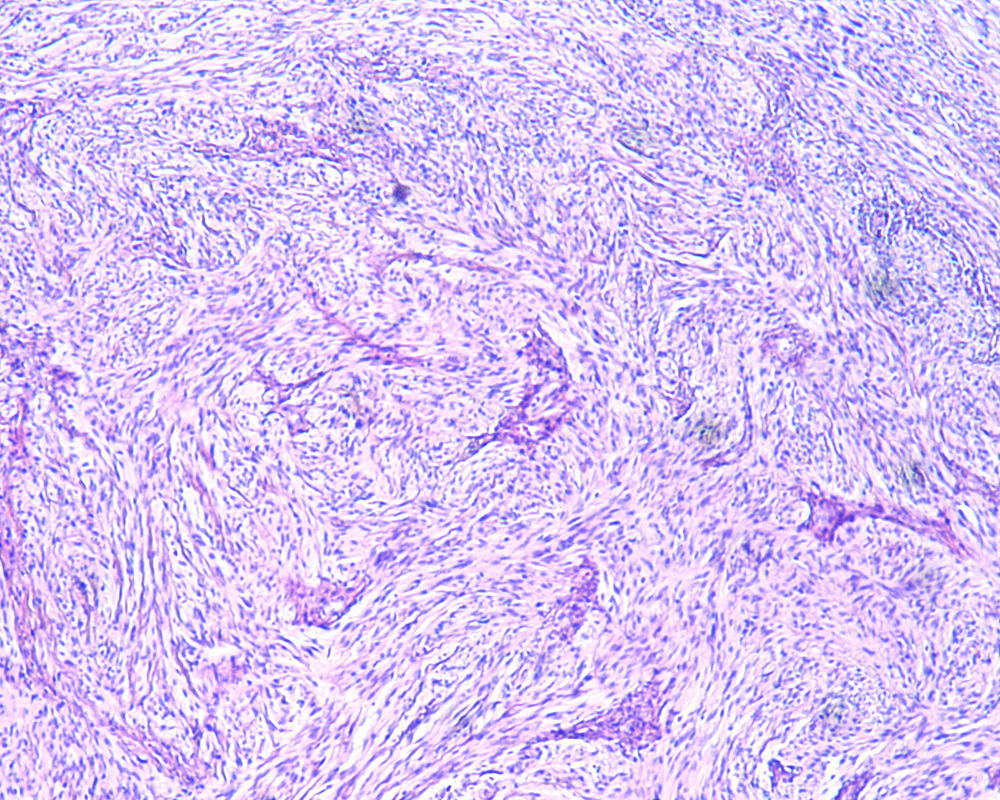
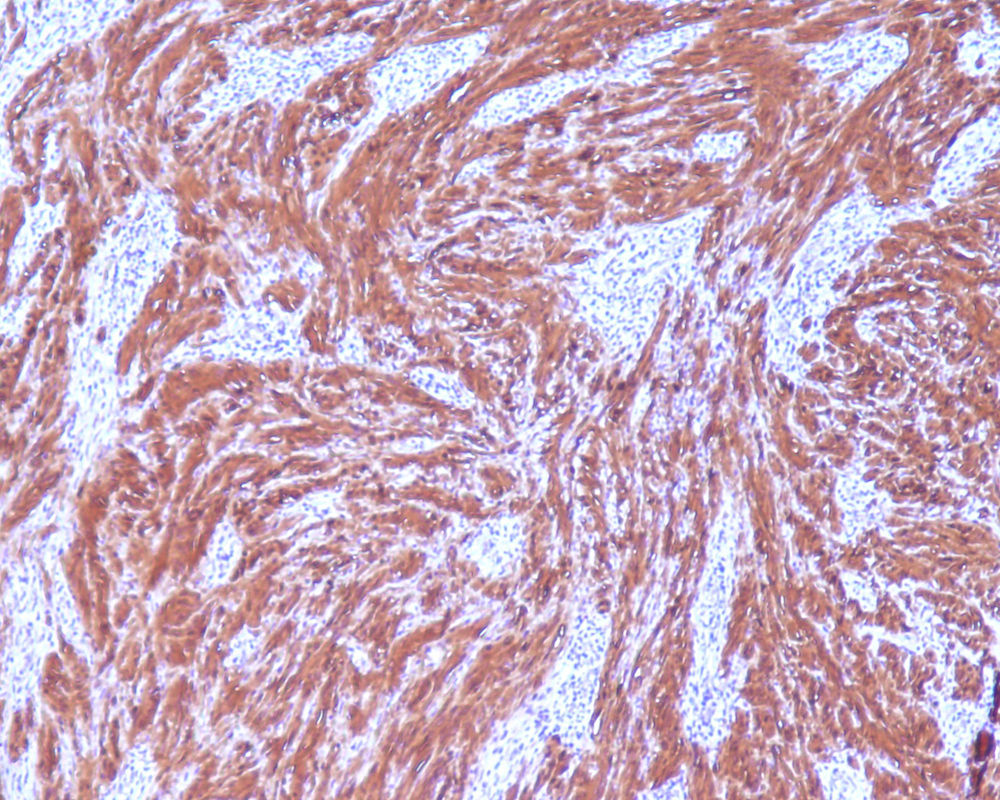
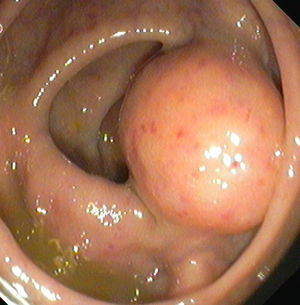
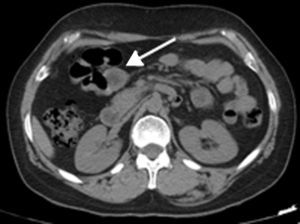
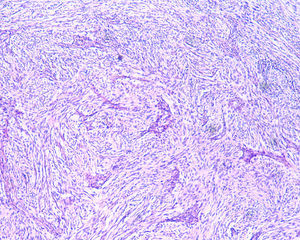
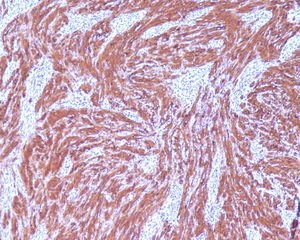
2Clinical caseA 49-year-old female was admitted to the Gastrenterology department for an elective colonoscopy. The patient had left-sided ulcerative colitis in clinic remission (S0, Montréal classification of disease activity). There was no family history of colonic tumors or neurofibromatosis. On colonoscopy, a subepithelial mass (3.0cm-sized) with normal overlying mucosa in the ascending colon was found (Fig. 1); the remaining colon was normal (ulcerative colitis in endoscopic remission). There was a report of a previous normal colonoscopy (three years ago). Abdominal examination revealed no abnormality. Contrast-enhanced abdominopelvic computed tomography showed a 2.9cm-sized well-circumscribed mass contiguous with intestinal wall that protruded intraluminally and exhibiting homogenous enhancement (Fig. 2). As the lesion was deeply located in intestinal wall and due to uncertainty of a definitive diagnosis, a right hemicolectomy and lymph node resection was performed. Histopathological evaluation was compatible with schwannoma. The mass was composed of benign spindle cells arranged in bundles, with dense distribution of nucleus forming palisades in dense fibrillar stroma and mitotic count was low (Fig. 3). Immunostaining revealed strong positivity for S100; muscle markers, vimentin, CD34 and KIT (CD117) were negative (Fig. 4). The lymph nodes were normal. The patient's postoperative evolution was uneventful.
Schwannomas or neurilemmomas are uncommon GI autonomic nerve tumors arising from Schwann cells of the neural sheath and may occur anywhere in the body.6 The GI tract involvement is rare (0.1% of benign tumors), and solitary schwannomas of the colon are even rarer.8 In the GI wall, they may present as subepithelial lesions with origin in the Auerbach's or Meissner's nerve plexus, in the muscularis propria (4th layer) and in submucosal (3rd layer), respectively.1,8 Differential diagnosis that must be considered in the evaluation of colonic subepithelial lesions is shown in Table 1.3,7,9
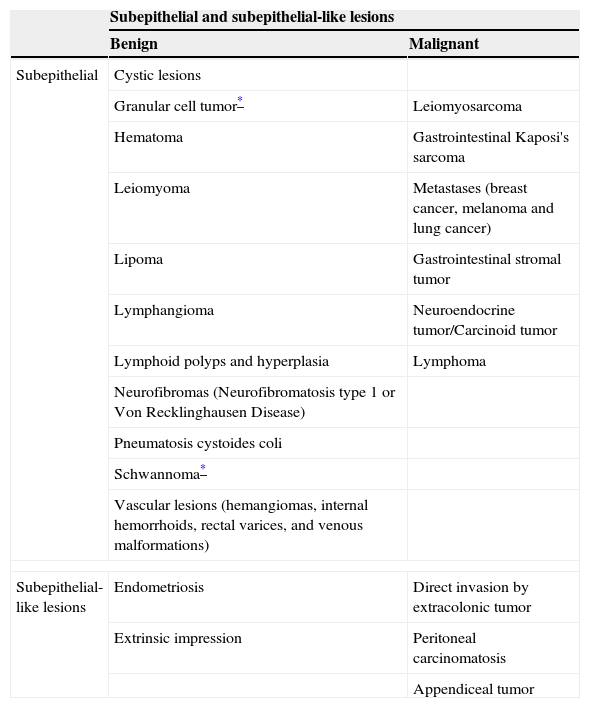
Subepithelial and subepithelial-like lesions of the colon and rectum.1,3,5,7,9
| Subepithelial and subepithelial-like lesions | ||
|---|---|---|
| Benign | Malignant | |
| Subepithelial | Cystic lesions | |
| Granular cell tumor* | Leiomyosarcoma | |
| Hematoma | Gastrointestinal Kaposi's sarcoma | |
| Leiomyoma | Metastases (breast cancer, melanoma and lung cancer) | |
| Lipoma | Gastrointestinal stromal tumor | |
| Lymphangioma | Neuroendocrine tumor/Carcinoid tumor | |
| Lymphoid polyps and hyperplasia | Lymphoma | |
| Neurofibromas (Neurofibromatosis type 1 or Von Recklinghausen Disease) | ||
| Pneumatosis cystoides coli | ||
| Schwannoma* | ||
| Vascular lesions (hemangiomas, internal hemorrhoids, rectal varices, and venous malformations) | ||
| Subepithelial-like lesions | Endometriosis | Direct invasion by extracolonic tumor |
| Extrinsic impression | Peritoneal carcinomatosis | |
| Appendiceal tumor | ||
Schwannomas are mesenchymal tumors, which can be classified in three types: gastrointestinal stromal tumors (GISTs), leiomyoma, and schwannoma. GISTs are the most common GI mesenchymal tumors.1 The GI schwannoma to GISTs ratio is approximately 1:50–100.10
Schwannomas are usually asymptomatic and diagnosed as incidental findings during diagnostic procedures, surgery or autopsy. Symptoms, when present, are nonspecific and similar to those caused by other intestinal tumors: abdominal pain, altered bowel habits, obstruction, hemorrhage, intussusception or systemic symptoms.6,8 There is a rare association of Schwannomas with neurofibromatosis and adrenal ganglioneuroma.8
At endoscopy, schwannomas appear as a wide-based sessile lesion with a gradually sloping contour and normal overlying mucosa. Nevertheless, it can be pedunculated and ulcerated.6,11 Computed tomography and magnetic resonance imaging do not provide additional information for the distinction from other mesenchymal tumors of the GI tract and lesions smaller than 10mm may not be detected.9
Endoscopic ultrasonography (EUS) is considered to be the best imaging procedure to characterize subepithelial GI lesions.5 EUS has high sensitivity but low specificity in identifying the location (intramural or extramural) of subepithelial lesions. It can also provide detailed information about intestinal wall structure or adjacent organs, samples for cytologic or histologic analysis and discrimination between benign and malignant lesions.1,5
The colonic EUS is usually performed with electronic radial echoendoscopes or ultrasonic miniprobes under colonoscopy.4 Only the electronic linear echoendoscopes enables ultrasound-guided fine-needle aspiration. The progression with electronic radial or linear echoendoscopes is limited, but may be possible with guide wire and overtube.4,12 Despite ultrasonic miniprobe under colonoscopy being useful in the characterization of any lesion in the colon (mainly the proximal ones), it does not allow ultrasound-guided fine-needle aspiration.4,13 In the rectum, radial or linear inflexible echoendoscopes may be used.4 In EUS, Schwannomas usually arise from 4th (or 3rd layer), leiomyomas from the 4th (or 2nd) and GISTs from the 4th (or 2nd, 3rd, 5th). Ultrassonographic characteristics are similar: hypoechoic, round or oval, well demarcated lesions.1,5 Differentiation between leiomyomas, schwannomas and GISTs is extremely difficult by imaging modalities, even EUS.1 Recent study shows difference in echogenicities of mesenchymal tumors: GISTs and schwannomas may have a marginal hypoechoic halo which is not found in leiomyomas.14 EUS imaging alone is insufficient to accurately diagnose 3rd and 4th layer hypoechoic masses and still will not differentiate a schwannoma from other mesenchymal tumors of the GI tract.6,15
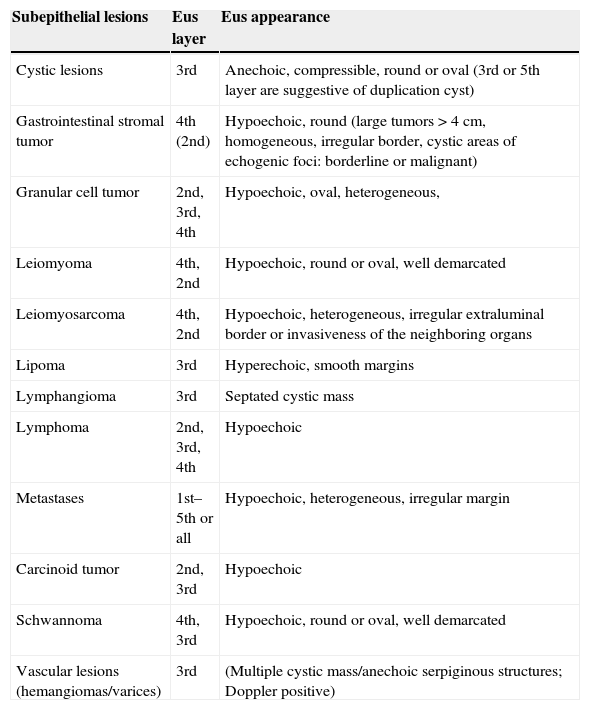
EUS can recognize characteristics suggestive of malignancy, like tumor size of more than 4cm, irregular borders, echogenic foci, cystic spaces, ulcerated mucosae, lymph nodes and exophytic growt.4 Characteristics of lower GI subepithelial lesions at EUS are shown in Table 2.
Characteristics of lower gastrointestinal subepithelial lesions at EUS.1,5
| Subepithelial lesions | Eus layer | Eus appearance |
|---|---|---|
| Cystic lesions | 3rd | Anechoic, compressible, round or oval (3rd or 5th layer are suggestive of duplication cyst) |
| Gastrointestinal stromal tumor | 4th (2nd) | Hypoechoic, round (large tumors>4cm, homogeneous, irregular border, cystic areas of echogenic foci: borderline or malignant) |
| Granular cell tumor | 2nd, 3rd, 4th | Hypoechoic, oval, heterogeneous, |
| Leiomyoma | 4th, 2nd | Hypoechoic, round or oval, well demarcated |
| Leiomyosarcoma | 4th, 2nd | Hypoechoic, heterogeneous, irregular extraluminal border or invasiveness of the neighboring organs |
| Lipoma | 3rd | Hyperechoic, smooth margins |
| Lymphangioma | 3rd | Septated cystic mass |
| Lymphoma | 2nd, 3rd, 4th | Hypoechoic |
| Metastases | 1st–5th or all | Hypoechoic, heterogeneous, irregular margin |
| Carcinoid tumor | 2nd, 3rd | Hypoechoic |
| Schwannoma | 4th, 3rd | Hypoechoic, round or oval, well demarcated |
| Vascular lesions (hemangiomas/varices) | 3rd | (Multiple cystic mass/anechoic serpiginous structures; Doppler positive) |
Although EUS can suggest the diagnosis, it cannot replace histopathologic classification.15 Limitations of conventional imaging and lack of sufficient biopsy material in endoscopy usually make an accurate diagnosis prior to surgical intervention difficult.1,11 EUS can provide adequate tissue samples for diagnostic purposes using EUS-guided fine needle aspiration technique and EUS-guided trucut biopsy.15
Histopathological examination and immunohistochemical studies allow definitive diagnosis. Schwannoma tumors show spindle cells arranged in bundles, with dense distribution of nucleus forming palisades in dense fibrillar stroma. Cells have positive immunoreactivity to the S-100 protein and were negative for CD34, KIT, desmin, cytokeratins (AE1/AE3), and chromogranin.1,2 GISTs tumors are KIT positive and leiomyoma demonstrate α-smooth muscle actin, desmin protein but not KIT expression.1
EUS is recommended for subepithelial lesions more than 1cm in diameter. For hypoechoic lesions less than 3cm in diameter, histologic evaluation is recommended. However, when GISTs are suspected (hypoechoic, 3rd and 4th layer masses) and the lesions are small (less than 3cm), periodic endoscopic, EUS follow-up or surgical resection are recommended. Surgery is recommended for subepithelial lesions more than 3cm.1
Recent techniques for endoscopic resection have been proven useful in subepithelial lesions treatment (especially those arising from mucosal and submucosal layers), including endoscopic mucosal resection, endoscopic band ligation, endoscopic submucosal dissection, endoscopic submucosal enucleation, endoscopic full-thickness resection and endoscopic submucosal tunneling dissection. These techniques have also been proven feasible and useful in selected cases, but not without increasing risk for hemorrhage and perforation.4 It usually requires highly skillful manipulation by experienced specialists and relatively longer procedure times.4
Although schwannomas were considered benign, they may recur locally if excision is incomplete. Malignant transformation has been occasionally reported.6 Tumor size greater than 5cm and mitotic activity rate of more than 5 mitoses per 50 high-power field tend to be associated with more aggressive behavior.2 Standard treatment for schwannomas is complete surgical resection.6,11 Lymph node resection is not recommended, because the risk of malignant change is low.6
The surgical option adopted in the present case is controversial and not the consensual strategy. Decision toward surgery was made because of the possibility of a malignant lesion (near 3cm) and EUS was not available.
Conflicts of interestThe authors have no conflicts of interest to declare.
FundingNone.