Despite their clinical utility and the importance that laboratory tests have in APS diagnosis, probably the most important drawback of such tests is the elevated intra- and inter-laboratory variation. The aim of the present work was to assess the multilaboratory performance of aCL and anti-β the multilaboratory performance of anti-cardiolipin (aCL) and antibeta 2 glycoprotein I antibodies (anti-β2GPI) assays and to assess the interlaboratory and inter-assay variability. Here, we report the most significant results from the Autoimmunity Workshop of the Spanish Society of Immunology (AWSEI). Seventeen sera from patients with antiphospholipid syndrome (APS) and/or probable APS were collected after written informed consent. Thirty-three laboratories participated and measured aCL and anti-anti-β2GPI. 61 and 49 results/serum for IgG/IgM aCL and anti- anti-β2GPI, respectively, were informed with 20 different assays. A high interlaboratory variation was found in quantitative results regardless the method used. Coefficient of variation ranged from 50% to 128% for aCL and from 9% to 200% for anti-anti-β2GPI. A limited consensus (defined as >90% agreement) was observed in semiquantitative results for IgG/IgM aCL and anti-β2GPI: 47%, 65%, 47% and 70%, respectively. In general, there was concordance between aCL and anti-β2GPI, yet 2 of the 17 sera were positive for anti-β2GPI only. In conclusion, interpretation of aCL and anti-β2GPI results from different laboratories may be done only in semiquantitative terms and its real value for clinical diagnosis of APS is still limited. Cut off values must be set in each laboratory.
A pesar de la indudable utilidad clínica y de la importancia de las pruebas de laboratorio en el diagnóstico del síndrome antifosfolípido (APS), probablemente el mayor defecto de dichas pruebas es su elevada variabilidad intra- e inter-laboratorio. El objetivo del presente trabajo fue evaluar el comportamiento de los ensayos para detección de anticuerpos anti-cardiolipina (aCL) y anti-beta 2 glicoproteína I (anti-β2GPI) entre laboratorios y determinar el grado de variabiidad inter-laboratorio e interensayo. En este trabajo se describen los resultados más significativos del Taller de Autoinmunidad de la SEI. 17 sueros obtenidos de pacientes con APS y/o probable APS se recogieron tras consentimiento informado. 33 laboratorios participaron y midieron los títulos de aCL y anti-β2GPI. 61 y 49 resultados/suero se informaron para aCL and anti-β2GPI (IgG/IgM), respectivamente, y medidos con 20 ensayos diferentes. Se encontró un coeficiente de variación (CV) elevado en los resultados cuantitativos, independientemente del método empleado. El CV fue del 50–128% para aCL y 9–200% para anti-β2GPI. Se obtuvo un consenso (definido como >90% de acuerdo) débil para los resultados semicuantitativos de IgG/IgM aCL y anti-β2GPI: 47%, 65%, 47% y 70%, respectivamente. En general, hubo una buena concordancia entre aCL y anti-β2GPI, aunque 2 de los 17 sueros fueron positivos para anti-β2GPI pero no para aCL. En resumen, la interpretación de los resultados de aCL y anti-β2GPI emitidos por distintos laboratorios puede hacerse solo en términos semicuantitativos y su valor real en el diagnóstico clínico del APS es aún limitada. Los puntos de corte para cada ensayo deben ser establecidos por el propio laboratorio.
The presence of anti-βhospholipid antibodies (APL) is mandatory in the diagnosis of antiphospholipid syndrome (APS)(1). The three clinically useful APL are lupus anticoagulant (LA), anti-cardiolipin antibodies (aCL) and anti-beta 2- glycoprotein I antibodies (anti-β2GPI)(2). Their utility is partially hampered by the deficient standardization of the assays to measure them(3). Whereas LA consists on several coagulation tests, aCL and anti-β2GPI are usually measured by ELISA that, in the beginning, used to be home-made. However, the importance of these tests, as judged by the number of orders, is exponentially increasing in the routine at the same time as APS suspicion from the clinicians is growing. This results in a high pressure on clinical laboratories, forcing them to use commercial kits instead of procedures developed in-house(4). These commercial assays are highly variable and with very different sensitivity/specificity. Altogether, the standardization of the results for aCL and anti-β2GPI antibodies remains a daily problem in the autoimmunity laboratories that perform these important assays for APS diagnosis.
The aim of the Autoimmunity Workshop of the Spanish Society of Immunology (AWSEI) was to evaluate the multilaboratory performance of aCL and anti- β2GPI assays and to assess inter-laboratory and inter-assay variability. Here, we report the most significant results from the workshop.
MATERIAL AND METHODSParticipantsThe report includes the test data results submitted by 33 laboratories from the 43 centers all over Spain that participated in the AWSEI.
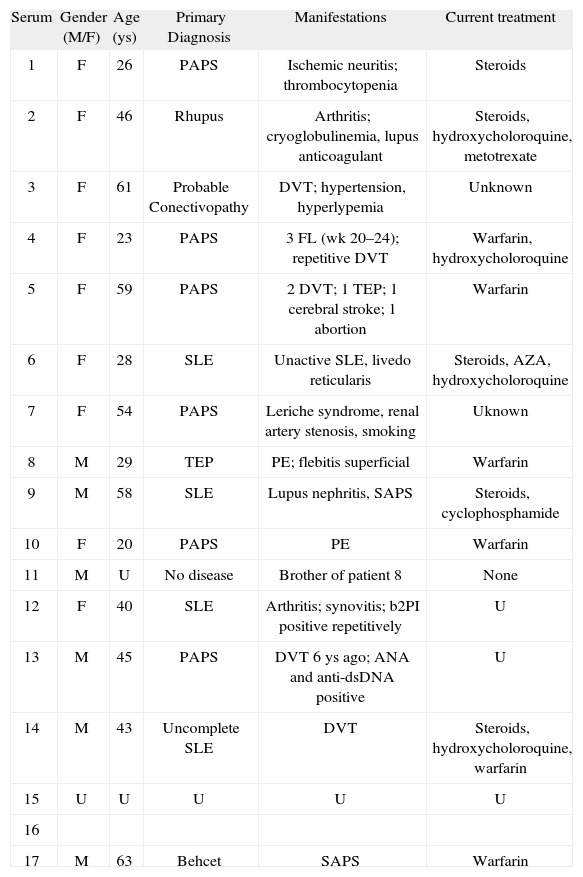
Serum samples and patientsSeventeen sera from patients with APS and/or probable APS (Table I) were collected after written informed consent, sent to the coordinator laboratory, aliquoted, and frozen at −80°C until distribution. Frozen aliquots were distributed among participating laboratories in only one package. Aliquots contained serum volume (200–500 μl) enough to perform multiple assays (and not only those routinely done in each laboratory).
Clinical and demographic characteristics of patients whose sera were studied for APL in the workshop
| Serum | Gender (M/F) | Age (ys) | Primary Diagnosis | Manifestations | Current treatment |
| 1 | F | 26 | PAPS | Ischemic neuritis; thrombocytopenia | Steroids |
| 2 | F | 46 | Rhupus | Arthritis; cryoglobulinemia, lupus anticoagulant | Steroids, hydroxycholoroquine, metotrexate |
| 3 | F | 61 | Probable Conectivopathy | DVT; hypertension, hyperlypemia | Unknown |
| 4 | F | 23 | PAPS | 3 FL (wk 20–24); repetitive DVT | Warfarin, hydroxycholoroquine |
| 5 | F | 59 | PAPS | 2 DVT; 1 TEP; 1 cerebral stroke; 1 abortion | Warfarin |
| 6 | F | 28 | SLE | Unactive SLE, livedo reticularis | Steroids, AZA, hydroxycholoroquine |
| 7 | F | 54 | PAPS | Leriche syndrome, renal artery stenosis, smoking | Uknown |
| 8 | M | 29 | TEP | PE; flebitis superficial | Warfarin |
| 9 | M | 58 | SLE | Lupus nephritis, SAPS | Steroids, cyclophosphamide |
| 10 | F | 20 | PAPS | PE | Warfarin |
| 11 | M | U | No disease | Brother of patient 8 | None |
| 12 | F | 40 | SLE | Arthritis; synovitis; b2PI positive repetitively | U |
| 13 | M | 45 | PAPS | DVT 6 ys ago; ANA and anti-dsDNA positive | U |
| 14 | M | 43 | Uncomplete SLE | DVT | Steroids, hydroxycholoroquine, warfarin |
| 15 | U | U | U | U | U |
| 16 | |||||
| 17 | M | 63 | Behcet | SAPS | Warfarin |
AZA: Azathyoprine; DVT: Deep venous thrombosis; PAPS: Primary antiphospholipid syndrome; PE: Pulmonary embolism; SAPS: Secondary antiphospholipid syndrome; U: Unknown.
Manufacturers were kindly invited to provide their ELISA kits to the participant laboratories. Table II shows the listing of the manufacturers that contributed with their kits to the AWSEI.
Participating laboratories were asked to give the results as quantitative and semiquantitative (negative, low-, mediumor high-positive titer). When quantitative results were reported as greater than a given number, the numerical value was taken as the next highest whole number. Variation in numerical results between laboratories was determined using the calculated coefficient of variation (%CV), by dividing the standard deviation obtained for the pooled numerical results of the sample by the mean numerical value for that serum. Variations in both quantitative and semiquantitative results were calculated from all reported results and separately for each manufacturer when the number of results reported was ≥5.
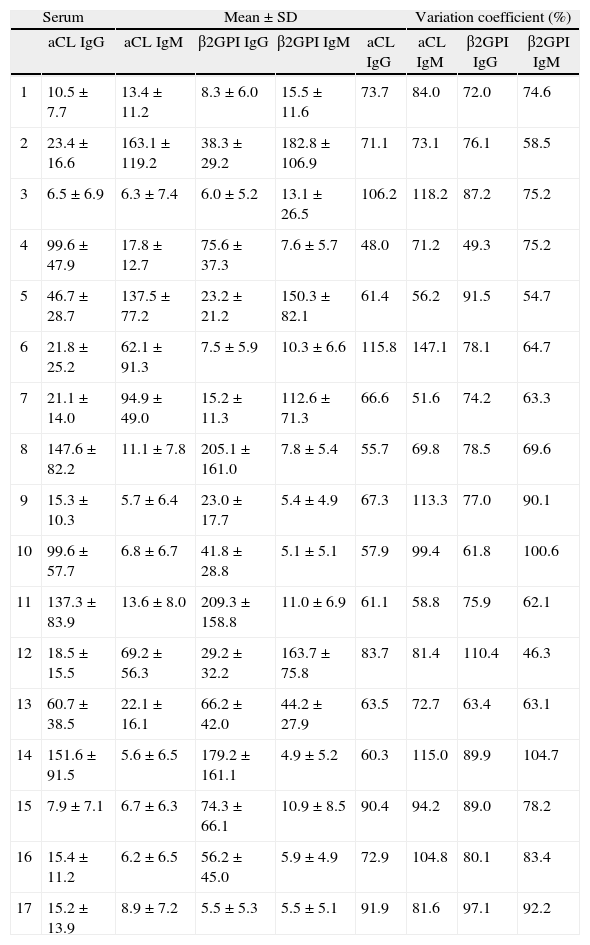
RESULTSThe quantitative results show a wide interlaboratory variabilityThe quantitative results reported from the participant laboratories for aCL and anti-β2GPI were pooled to determine the inter-laboratory variability regardless of the method used (Table III). Globally, the results showed a very poor agreement between laboratories when using quantitative data to report APL results. The range of % CV was 48.02-115.00, 51–147.1, 49.34-110.35, 46.29-100.54 for aCL IgG, aCL IgM, anti-β2GPI IgG, and anti-β2GPI IgM, respectively. Table III shows that the higher % CV were obtained when antibody titers were low. The interlaboratory variability remained high even when results were grouped by manufacturer (not shown).
Quantitative results reported for each of the 17 sera studied showing the interlaboratory variation and considering all the methods as a whole
| Serum | Mean±SD | Variation coefficient (%) | ||||||
| aCL IgG | aCL IgM | β2GPI IgG | β2GPI IgM | aCL IgG | aCL IgM | β2GPI IgG | β2GPI IgM | |
| 1 | 10.5±7.7 | 13.4 ±11.2 | 8.3 ±6.0 | 15.5 ±11.6 | 73.7 | 84.0 | 72.0 | 74.6 |
| 2 | 23.4±16.6 | 163.1 ±119.2 | 38.3 ±29.2 | 182.8±106.9 | 71.1 | 73.1 | 76.1 | 58.5 |
| 3 | 6.5±6.9 | 6.3 ±7.4 | 6.0 ±5.2 | 13.1 ±26.5 | 106.2 | 118.2 | 87.2 | 75.2 |
| 4 | 99.6±47.9 | 17.8±12.7 | 75.6 ±37.3 | 7.6±5.7 | 48.0 | 71.2 | 49.3 | 75.2 |
| 5 | 46.7 ±28.7 | 137.5 ±77.2 | 23.2 ±21.2 | 150.3±82.1 | 61.4 | 56.2 | 91.5 | 54.7 |
| 6 | 21.8 ±25.2 | 62.1 ±91.3 | 7.5 ±5.9 | 10.3±6.6 | 115.8 | 147.1 | 78.1 | 64.7 |
| 7 | 21.1±14.0 | 94.9 ±49.0 | 15.2 ±11.3 | 112.6 ±71.3 | 66.6 | 51.6 | 74.2 | 63.3 |
| 8 | 147.6±82.2 | 11.1 ±7.8 | 205.1 ±161.0 | 7.8±5.4 | 55.7 | 69.8 | 78.5 | 69.6 |
| 9 | 15.3±10.3 | 5.7 ±6.4 | 23.0±17.7 | 5.4 ±4.9 | 67.3 | 113.3 | 77.0 | 90.1 |
| 10 | 99.6±57.7 | 6.8 ±6.7 | 41.8 ±28.8 | 5.1±5.1 | 57.9 | 99.4 | 61.8 | 100.6 |
| 11 | 137.3±83.9 | 13.6 ±8.0 | 209.3±158.8 | 11.0 ±6.9 | 61.1 | 58.8 | 75.9 | 62.1 |
| 12 | 18.5±15.5 | 69.2 ±56.3 | 29.2 ±32.2 | 163.7±75.8 | 83.7 | 81.4 | 110.4 | 46.3 |
| 13 | 60.7 ±38.5 | 22.1 ±16.1 | 66.2 ±42.0 | 44.2 ±27.9 | 63.5 | 72.7 | 63.4 | 63.1 |
| 14 | 151.6 ±91.5 | 5.6 ±6.5 | 179.2 ±161.1 | 4.9±5.2 | 60.3 | 115.0 | 89.9 | 104.7 |
| 15 | 7.9±7.1 | 6.7 ±6.3 | 74.3 ±66.1 | 10.9 ±8.5 | 90.4 | 94.2 | 89.0 | 78.2 |
| 16 | 15.4±11.2 | 6.2 ±6.5 | 56.2 ±45.0 | 5.9 ±4.9 | 72.9 | 104.8 | 80.1 | 83.4 |
| 17 | 15.2±13.9 | 8.9 ±7.2 | 5.5 ±5.3 | 5.5 ±5.1 | 91.9 | 81.6 | 97.1 | 92.2 |
Figure 1 shows the results reported for each of the 17 sera as semiquantitative results (i.e., negative, low-, mediumor high-positive titres). To determine the consensus between laboratories in reporting the results, a >90% agreement was established for each antibody and each serum. Thus, negative and low-positive results were considered as a negative result whereas medium- and high-positive results were considered as positive results, as recommended by international consensus(1). A limited consensus (defined as ≥90% agreement) for semiquantitative results was observed: aCL IgG (47%), aCL IgM (65%), anti-β2GPI IgG (47%), anti-β2GPI IgM (70%).
Pie graphs showing the semiquantitative results reported by all the participant laboratories. Four results for each serum are depicted: aCL IgG (upper left pie), aCL IgM (upper right pie), anti-β2GPIIgG (lower left pie) and anti-β2GPIIgM (lower right pie). The number in the middle indicates the serum identification. Results were reported as negative, low-, mediumor- high-positive as indicated in the lower right corner of the figure. To evaluate the congruence of reports as semiquantitative results, negative and low-positive sera were considered as negative whereas medium- and high-positive sera were considered as positive.
When looking at the overall semiquantitative results depicted in Figure 1, several findings are observed. First, most sera were both positive for aCL and anti-β2GPI of the same isotype, but for sera 5 and 6. Serum 5 was aCL and anti-β2GPI IgM positive in several laboratories but only aCL IgG positive in more than 50% of the reported results. Serum 6 was aCL IgM positive but not anti-β2GPI IgM.
Thus, these two sera could be considered as aPL positive cofactor-independent. However, sera 15 and 16 were mostly reported positive only for anti-β2GPI IgG but not aCL IgG. On the other hand, APL IgG (sera 4, 8, 11, 13, and 14) were more represented than APL IgM (sera 2, 5, and 7). Finally, serum 17 from a patient diagnosed with Behcet and APS was reported as negative by almost all the participants.
DISCUSSIONMore than 25 years after aCL testing in the diagnosis of APS has led us to a number of changes in laboratory assays as well as in APS management. As a consequence APS diagnosis has become more effective, although there is still an important field for improvement in both clinical management and laboratory tests(5,6). Laboratory testing is based in aCL, LA and, more recently, anti-β2GPI. The present scenario indicates that aCL are the most sensitive autoantibodies for the diagnosis of APS but have a very poor specificity. On the other hand, LA and anti-β2GPI seem to be more specific but not as sensitive as aCL.
Despite their clinical utility and the importance that laboratory tests have in APS diagnosis, probably the most important drawback of such tests is the elevated intra- and inter-laboratory variation(3),(4). As a result, a patient classified as APS in a hospital may not be considered as APS in another centre where the APL testing may be very different. Interlaboratory results are not interchangeable even if measured with the same commercial kit because there is still a high inter-laboratory coefficient of variation (data not shown but obtained in the AWSEI), particularly for quantitative results. Such variability in quantitative results has been assumed for anti-β2GPI results since there is no international calibrator to give results in the same units as occurs for aCL (i.e., GPL or MPL). Nonetheless, the high inter-laboratory variation was observed for both aCL and anti-β2GPI within the same commercial kit in the AWSEI, which does not make sense for the previous explanation. When semiquantitative results were reported (Figure 1), variability remained elevated. A possible explanation for this limited reproducibility might reside in the use of different values to define low-, mediumor high-positive titres among laboratories. International consensus establishes that medium/high titres of APL are levels above 40 GLP or MPL units or higher than the 99th percentile of normal subjects(2). The use of different values among laboratories, without a uniform criterion, may be one of the causes explaining the limited inter-laboratory consensus when reporting the results as semiquantitative. The establishment of cut-off values is still a matter of debate since there could be different cut-off levels depending on the clinical subtype of APS patient considered.
An additional message to draw from the present workshop is the variable spectrum of APL observed. As expected, most sera were positive for aCL and anti-β2GPI at the same time due to the cofactor dependence of aCL. On the other hand, there were two sera (15 and 16 in Figure 1) negative for aCL but positive for anti-β2GPI. In the last years, these autoantibodies are gaining importance as prognostic markers of the disease, particularly high avidity anti-β2GPI antibodies(7). Finally, one serum (from patient 6 with SLE) was aCL IgG and IgM positive but anti-β2GPI IgG and IgM negative, and serum from patient 10, diagnosed with primary APS, was more frequently reported as medium-high positive for aCL IgG than for β2GPI IgG. These data bring the message of the importance of reporting the four parameters in the revised criteria established in Sidney for the diagnosis of APS.
In conclusion, despite the necessary use of the APL test for APS diagnosis, there is still a long way to walk to perform these laboratory assays in a more precise and reproducible manner. It is probable that such a desired reproducibility would come with the description of the true epitope/ antigen involved in the APS.
CONFLICT OF INTERESTThe authors declare no financial conflict of interest.