Aging is accompanied by an impairment of the physiological systems, including the immune system. The age-related changes of immune functions, which are denominated immunosenescence, are the cause of the increased vulnerability to infections and inflammatory diseases of aged animals. Since the functional capacity of the immune cells has been proposed as a marker of health, our group, using mice with premature senescence, long-lived mice and human centenarians, has ascertained that several immune functions are good markers of biological age and predictors of longevity. Moreover, in agreement with the most widely accepted theory of aging, namely the oxidation theory, we have observed that upon aging the immune cells show increased oxidant and inflammatory compounds and decreased antioxidant and anti-inflammatory defenses. This chronic oxidative and inflammatory stress, has among its intracellular mechanisms the activation of the NF-κB in the immune cells. Based on these data, we have proposed the theory of oxidation-inflammation as the cause of aging. Accordingly, the chronic oxidative stress that appears with aging affects all cells and especially those of the regulatory systems, such as the nervous, endocrine and immune systems and the communication between them. This prevents an adequate homeostasis and, therefore, the preservation of health. Moreover, we have proposed a key involvement of the immune system in the aging process of the organism, specifically in the rate of aging. Since macrophages are cells of the innate immunity present in all animals, and they produce the highest levels of oxidant and pro-inflammatory compounds in aged individuals, the role of the immune system in aging could be of universal application. A confirmation of the role of the immune system in aging is the fact that several life style strategies, such as the administration of adequate amount of antioxidants improve the immune cell functions, decreasing their oxidative stress, and consequently increase the longevity of the subjects.
El envejecimiento supone un deterioro progresivo y generalizado de todos los sistemas funcionales, incluido el sistema inmunitario. Los cambios que con la edad experimenta el sistema inmunitario, lo que se denomina inmunosenescencia, son la causa de la mayor susceptibilidad a enfermedades infecciosas e inflamatorias que aparecen al envejecer. La capacidad funcional de las células inmunitarias ha sido propuesta como un excelente marcador de salud, y nosotros hemos comprobado, utilizando modelos de envejecimiento prematuro en ratones, así como ratones y humanos longevos, que varias funciones inmunitarias pueden ser indicadoras de la edad biológica del individuo y de su longevidad. También, en base a la teoría más aceptada del envejecimiento, la de la oxidación, hemos observado que, al envejecer, las células inmunitarias generan más compuestos oxidantes e inflamatorios y tienen menos defensas antioxidantes y antiinflamatorias. Este estrés oxidativo e inflamatorio crónico tiene entre sus mecanismos intracelulares una mayor activación del NF-κB de las células inmunitarias. Con base en lo indicado, hemos propuesto la teoría de la oxidación-inflamación del envejecimiento, según la cual el estrés oxidativo crónico que aparece al envejecer y que afecta a todas las células del organismo, lo hace especialmente en las de los sistemas reguladores como el nervioso, el endocrino y el inmunitario, a las que deteriora, así como a la comunicación que se establece entre esos sistemas. Ello impediría tener una adecuada homeostasis y consecuentemente mantener la salud. Además, hemos planteado que el sistema inmunitario, por su peculiar funcionamiento y por su alteración al envejecer, podría incidir en la velocidad de envejecimiento del organismo. Dado que son los macrófagos, células de la defensa innata presentes en todos los animales, los que al envejecer producen altos niveles de compuestos oxidantes e inflamatorios, esta implicación del sistema inmunitario en la tasa de envejecimiento puede tener carácter universal. Esto se ha ratificado en mamíferos al comprobar que diferentes estrategias de estilo de vida que regulan el estado redox y la funcionalidad inmunitaria al envejecer, como la ingestión de cantidades apropiadas de compuestos antioxidantes, entre otras, permiten una mayor longevidad.
To understand the role of the immune system (IS) in the aging process it is required, in the first place, to know several key concepts about this process. Aging may be defined as a progressive and general decrease of the organism functions that leads to a lower ability to adaptively react to changes and preserve homeostasis. This accumulation of adverse changes with age increases the risk of disease and eventually results in death. Thus, although aging should not be considered a disease, it strongly increases the chances of suffering many degenerative diseases. As indicated by Strehler(1), there are four rules defining aging: It is universal (practically all animal species including the metazoans showing sexual reproduction suffer aging), progressive (the rate of aging is similar at different ages after the adult state), intrinsic (since even if animals are exposed to optimal environmental conditions throughout life, they still experience the aging process at the rate characteristic for their species) and deleterious (aging is obviously detrimental to the individuals since it leads to their death). However, at the species level, the detrimental character of aging could be questioned, since it is counteracted by a continuous renewal of the population.
Currently, human aging is a problem in developed countries because the mean lifespan or mean longevity is very high. This mean longevity can be defined as the mean of the life span of the subjects of a group that have been born on the same date. Thus, with a mean life span of 75-83 years in these countries, and since we start the aging process at about 18 years of age, we spend most of the time of our life aging. The aging process is finished at the end of the maximum life span or maximum longevity (the maximum time that a subject belonging to a determined species can live), that for human beings is about 122 years, whereas in mouse and rat strains is only 3 and 4 years, respectively. We can increase the mean life span, with factors of style of life that allow to maintain good health and to approach the maximum life span in good conditions. However, presently it is impossible to increase the maximum longevity.
BIOLOGICAL AGEThe aging process is very heterogeneous. Thus, it is well known that the molecular and cellular disorganization and the decreased physiological performance associated with aging do not proceed at the same rate in all members of a population of the same chronological age. This justifies the introduction of the concept of "biological age", which has a better predictive value of longevity than the chronological age(2). To establish the "biological age", a number of biochemical, physiological and psychological parameters that change with age and that show the tendency to a premature death should be determined. The most complete investigation on biological age was performed by Borkan and Norris(2), on over one thousand men, in a longitudinal study on human aging of the Gerontological Center of Baltimore. The retrospective analysis of this study showed that the subjects presenting certain parameters "more aged" than those found in the majority of the subjects of the same chronological age had a shorter life expectancy. These biomarkers include those related to respiratory function, systolic arterial tension, and reaction times determined by psychometric tests. Most research on biological age such as "The Baltimore study" did not include immune parameters, which presently are considered essential and very representative of the "true" biological age of a subject. Thus, a positive relation between a good function of the T cells and the NK cells, as well as of phagocytic cells, with longevity has been shown(3-9).
HOW, WHERE AND WHY OF AGINGThe answers to the key questions in gerontology: how does aging happen?, where does aging start?, and why does aging occur?, have produced so many theories that justify the cynical comment that there are so many theories of aging as there are gerontologists. Indeed, more that 300 theories have been proposed to explain the process of aging(10). Most of those theories can be divided in two groups. The first group of theories, "the genetic program theories" propose that aging is the result of a purposeful program driven by genes. The second, the "epigenetic theories" suggest that aging is the result of events that are not guided by a program but are stochastic or random events, and it is not genetically programmed. At this moment, and despite the claim by many researchers to the contrary, there is no direct evidence that genes drive age changes. The aging process, which appears after reproductive maturation, is driven by random events not genetically programmed(11). Thus, theories like the mitotic limit of Hayflick or shortening of telomeres have to be seen as possible explanations of cell differentiation processes or replicative cellular senescence, but not as the base of organism aging. Most theories of aging indicate events that are consequences of the aging process but not its cause.
Among all the aging theories, the free-radical concept proposed by Harman(12) has attracted a great deal of attention and probably is now the most widely accepted to explain how the aging process occurs. This epigenetic theory, that was further developed by Harman(13), Miquel et al.(14-16), and others(17,18) proposes that aging is the consequence of accumulation of damage (by deleterious oxidation) in biomolecules caused by the high reactivity of the free radicals and reactive oxygen species (ROS) produced in our cells as a result of the necessary use of oxygen. Since oxygen is mainly used in respiration to support the life-maintaining metabolic processes, the mitochondria, and specifically their DNA (mtDNA), are probably the first target of oxidation. As first pointed out by Miquel et al.(14-16), it is in the fixed post-mitotic cells, which cannot fully regenerate these organelles where the aging process starts. Moreover, the rate of mitochondrial oxygen radical generation, as well as the degree of membrane fatty acid unsaturation, and the oxidative damage to mtDNA are lower in long-lived than in short-lived species(19). Thus, the mitochondrial damage caused by free radicals results in a loss of bioenergetic competence that leads to aging and death of cells and therefore of the organism(14-16,20).
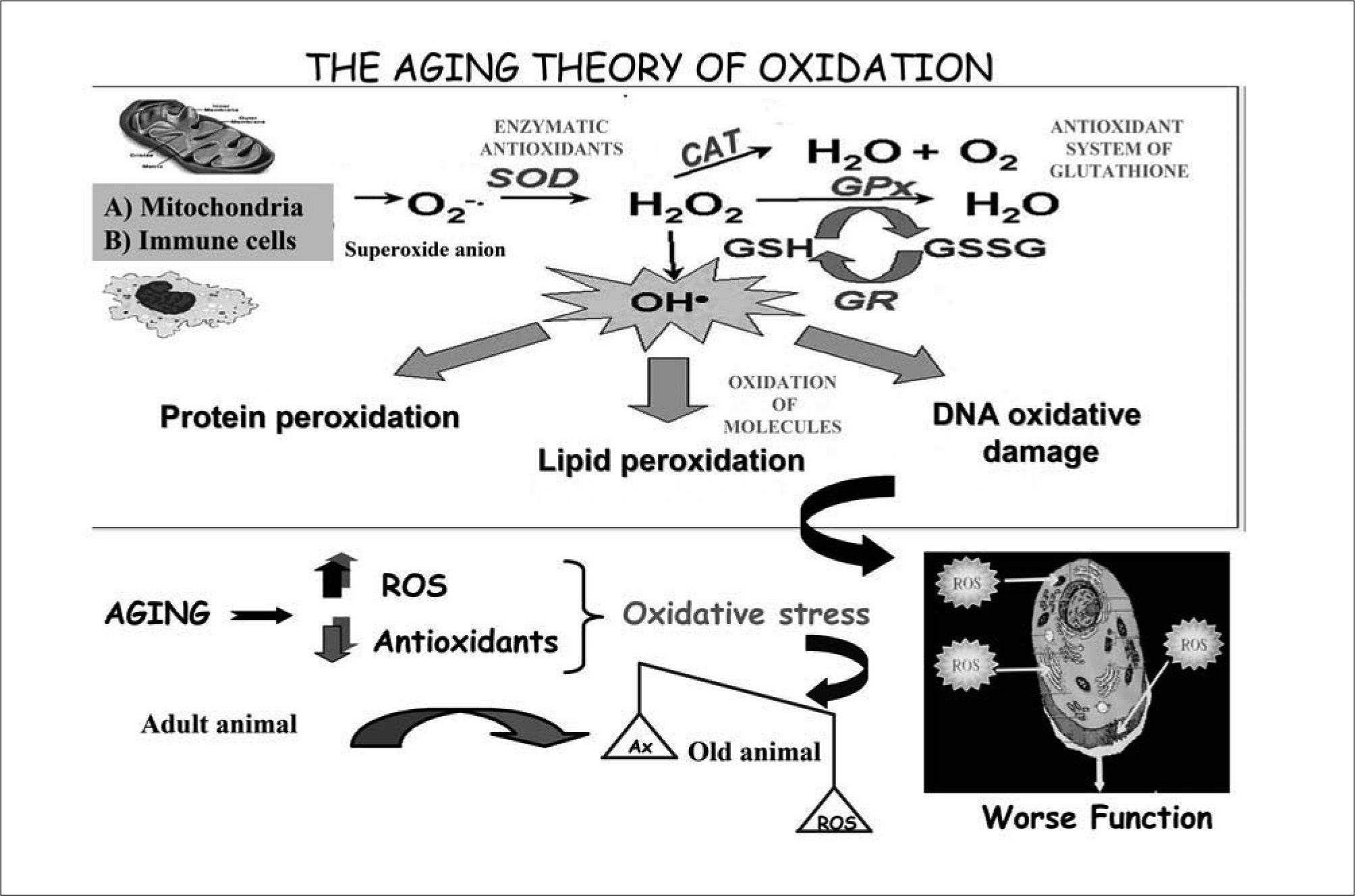
To protect themselves against oxygen toxicity, cells have developed a variety of antioxidant mechanisms that prevent the formation of ROS or neutralize them after their generation. However, these defensive systems are not perfect, so when the amount of ROS exceeds the antioxidant protection, an oxidative stress situation appears resulting in cell injury(21). Despite this, we should consider that oxygen is essential for life and that ROS, in certain amounts, are needed for many physiological processes which are essential for our survival(22,23). Therefore, the functions of our organism are based on a perfect balance between the levels of ROS and those of antioxidants. It is the loss of this balance, because of an excess in the production of ROS or an insufficient availability of antioxidants, that leads to the oxidative stress than underlies ROS-related diseases and aging(18) (Figure 1).
The aging theory of oxidation. Aging is the consequence of accumulation of oxidative damage in biomolecules caused by the high reactivity of the free radicals and reactive oxygen species (ROS) produced in our cells, specially in mitochondria, as a result of the necessary use of oxygen. The immune cells also produce important levels of ROS. The first oxygen free radical appearing in cells is the superoxide anion (O2–•), which produces hydrogen peroxide (H2O2) and hydroxil radical (OH•), the most reactive free radical, which carries out the oxidation of biomolecules such as proteins, lipids and DNA. The cells, in order to protect themselves against oxygen toxicity, have developed a variety of antioxidant mechanisms that prevent the formation of ROS or neutralize them after they are produced. Thus, superoxide dismutase (SOD) catalyzes the inactivation of superoxide anion and catalase (CAT) inactivates hydrogen peroxide. The reduced glutathione (GSH) is the most important antioxidant in the organism and neutralizes peroxides using the glutathione peroxidase (GPx) and in this action it is transformed to oxidized glutathione (GSSG). The antioxidant enzyme glutathione reductase (GR) is used to catalyze the reduction of glutathione. In healthy adult animals there is a balance between the amount of ROS produced and antioxidant defence levels. We should considerer that oxygen is essential for life and that ROS, in certain amounts, are needed for many physiological processes that are essential for our survival. Therefore, the functions of our organism are based on a perfect balance between the levels of ROS and those of antioxidants. However, with aging a loss of the balance appears, with an excess in the production of ROS or an insufficient availability of antioxidants, which leads to the oxidative stress. This situation of oxidative stress results in oxidative cell injury and therefore in a worse function of cells.
Taking this into account, we could know how the aging process happens: the oxidation produced by the oxidative stress is in the base of the multitude of age-related changes affecting many aspects including morphology, physiology and behaviour at all levels of organization, i.e. molecular, cellular, tissular, organic and that of the whole individual. Thus, we have a reasonable answer to the question of where aging starts: in the mitochondria from fixed postmitotc cells. But, why does aging happen?. The answer seems to be found in several evolutionary theories and concepts published a long time ago. Thus, we agree with the concept of Williams(24) that aging is the consequence of characters selected by evolution as an advantage for the young subjects of the species, allowing them to reach the reproductive age in the best condition and thus maintain those species. These characters are a disadvantage for old people. Thus, the selection acts before the adult age and the maintenance of the species is biologically more relevant than the longevity of the individual. The resulting loss of bioenergetic competence and physiological performance is involved in the senescence and death of the members of metazoan species, whose genes, housed in a series of "disposable somas"(25), have an unlimited survival in their normal habitat thanks to sexual reproduction.
INTEGRATIVE THEORY OF AGINGSince the process of aging is very complex, a theory based on just one mechanism cannot give a satisfactory explanation of all its aspects. This justifies the proposal of a theory integrating early concepts that offered partial explanations of the mechanism of aging with more recent ones like those shown in Table I(26-28), which allow us to state that aging results from the differentiation process shown by somatic cells, especially those unable to divide such as the neurons, in contrast with germ cells. This process is linked to the appearance of mitochondria with very high levels of oxygen consumption and resulting oxidative damage to their molecules, particularly the mtDNA. It is evident that what improves functions in the reproductive age, namely oxygen utilization for cell energy production, is also the main cause of functional impairment afterwards. Thus, aging would not be a programmed effect of the high levels of oxidative stress in the differentiated cells, since it is irrelevant from the view point of species survival through sexual reproduction.
"Classic" and modern theories that attempt to explain the aging process at several levels of biological organization and are the base of the integrative theory of senescence
| Author(Year of publication) | Key concept/proposed cause of aging |
| Weissman (1891) | Division of work in metazoans between germ cells, with great regenerative and mitotic potential, and somatic differentiated cells that perform specialized work |
| Minot (1907) | "Prize paid for cell differentiation" |
| Pearl (1928) | Side-effect of metabolism |
| Willians (1957) | Result of genes favourable to obtain the maximal performance at the age of reproduction and are noxious thereafter |
| Harman (1956) | Damaging effects of oxygen free radicals |
| Miquel (1980, 1991) | Vulnerability of the mitochondrial genome to oxidative injury in differentiated postmitotic cells |
Recently, we have proposed a new theory of aging that provides more precision to the unifying concepts mentioned above. This new theory proposes that the immune system could play a key role not as the basic cause of aging, but as a mechanism that modifies the rate of senescence. The concepts that have led us to this new theory will be discussed below.
IMMUNOSENESCENCEAs mentioned above, aging is accompanied by a decline of the physiological systems, including the immune functions. In fact, it is well known that with the passage of time there is a decrease in the resistance to infections and an increasedincidence of autoimmune processes and cancer, indicating a less competent immune system. In fact, the increased death rate found in aged populations is due in great proportion to infectious processes(29). Thus, the immune system is impaired with age, and this exerts a great influence on the increasing morbidity and mortality observed in aging human subjects(30). However, although there are conflicting observations on this subject, it is presently accepted that almost every component of the immune system undergoes striking age-associated restructuring, leading to changes that may include enhanced as well as diminished functions, involving the components of the immune system as well as their interactions(9,31-36). This fact is denominated immunosenescence. As it has been recently indicated, understanding the specific mechanisms and targeting interventions depend on research to elucidate the relationship between frailty-associated impaired immunity and immunosenescence in developing an impaired immunity(37).
Immunosenescence results in a pronounced decrease in T-cell functions, particularly T-helper cells, which affects humoral immunity and causes an impaired B-cell function(33,34,38). However, not all immune cell types, or all functions of an immune cell show a significant decrease. In fact, several cell types and functions within a cell are more activated with age, whereas other cell types and functions do not show significant age-related changes. Thus, a cell type that has been relatively neglected in studies of age and immunity is the Treg subset. With age, these cells maintain their function capacity and increase their number, and this could explain the greater suppressive activity in the elderly(39,40). The age-related alterations in cells from innate immunity have been less studied than those of lymphocytes. Although the evidence accumulated over the last decade supports the profound impact of aging on this immunity, the results obtained on the age-related changes of the functions of cells from innate immunity are often contradictory(31,32,41,35,42-45). In general, NK cells, one of the cellular mediators of innate defence more extensively studied in the elderly, show a decreased cytotoxicity and cytokine production(41,44). NKT cells also change in number and function with age(45). The phagocytic cells such as neutrophils and macrophages show a significant decrease in several of their functions(7-9,35,41-45). Thus, phagocytes, whose age-related changes were studied by us a long time ago(46), were thought to play a less critical role in the immune dysfunction that occurs throughout aging. However, recent studies point out that the general decline in the functional activities of these cells is one major reason for the susceptibility and vulnerability to bacterial and viral infections among aged subjects. This stands out as the most common causes of illness and death in aging(35,41-45). Moreover, since the innate and adaptive immune systems co-operate to ensure an optimal immune response, we have to consider that any decline in innate immunity will have an impact on the function of the adaptive immune system, and vice-versa(35,41,45,47). Thus, at the level of a key component of T cell immunity as is antigen presentation, the age-related changes in antigen presenting cells like macrophages and dendritic cells could play a relevant role in the altered initiation and outcome of T cell immune responses(41). Furthermore, dendritic cells have been implicated in the age-related change to a predominant Th2 response instead of the predominant Th1 of the adult(48). In fact, this change from predominant Th1-type to predominant Th2-type antigen responses, with an accompanying shift in cytokine profiles, has been proposed as a mechanism for age-related immune dysfunction(38).
Although several of the age-related changes observed in the immune response have been attributed to the modifications of immune cell subpopulations with aging(38), the age-related quantitative variations in a given type of immune cell is not necessarily related to its functions. For example, the number of NK cells increase with age, but have decreased tumoral cytotoxic capacity(38,41). However, the presence of a higher ratio of memory T lymphocytes with respect to virgin T cells could explain the decrease of several immune functions(38,48,49). Despite the rapid accumulation of new data on immunosenescence(31-49), the puzzle of all the changes in the different aspects of the immune function with age has not yet been solved, and the specific role played by the immune system in aging is not wholly understood.
THE PSYCHONEUROENDOCRINE-IMMUNE NETWORK AND THE LOSS OF HOMEOSTASIS IN AGINGWe know that there is a "neuroendocrine-immune" system that allows the preservation of homeostasis and therefore of health(50,51). With age all regulatory systems involved in homeostasis, i.e., the nervous, the endocrine and the immune systems, as well as the communication between them, are impaired(35,52,53). This important observation justified the proposal of another theory of aging, according to which, the changes in this communication between the immune system and the nervous system (and concomitant loss of homeostasis and resistance to stress) is the probable cause of physiological senescence(54). Recent studies of our group support this hypothesis, as well as the idea that the impairment of the immune system with aging could affect the functions of the other regulatory systems through an increased oxidative and inflammatory stress, resulting in the age-related alteration of homeostasis and increased morbidity and mortality(9,35).
In relation to the above, an inadequate response to stress is one of the factors leading to an acceleration of aging accompanied by poor condition of the immune and other physiological systems(55-57). Thus, our group has shown that mice with chronic hyperreactivity to stress and anxiety show a premature immunosenescence and they age prematurely(58). We have also recently observed that mice exposed to the stressful condition of isolation have behavioural responses that reveal an impairment of cognition, certain degree of depression and a more evident inmunosenescence than control animals of the same age housed in groups (Arranz L, De la Fuente et al., submitted). Likewise, human subjects suffering chronic anxiety(59) or depression show a significant premature immunosenescence.
It is difficult to determine whether, upon aging, neural changes induce immunological changes or whether an altered immune system induces nervous changes, or whether both processes occur simultaneously, which is the most likely mechanism according to some authors(53). In addition, we have observed that the in vitro response of immune cells to a wide range of neurotransmitter concentrations changes with the age of the subject(60,61). This demonstrates that although the levels of neurotransmitters that come into with the immune cells are maintained with age, the response of the immune system cells to them could be different.
THE IMMUNE SYSTEM AS A MARKER OF BIOLOGICAL AGE AND PREDICTOR OF LONGEVITY: RESEARCH ON A MODEL OF PREMATURE AGING IN MICE, IN VERY LONG-LIVING MICE AND IN HUMAN CENTENARIANSIt has been demonstrated that the competence of the immune system is an excellent marker of health(30) and several age-changes in immune functions have been linked to longevity(3-8,35). Thus, the level of immunological parameters such as an excess of CD8+CD27-CD28-T cells, low T cell proliferative responses in vitro and low IL-2 secretion predicted mortality, and together with increased IL-6 levels and a CD4:CD8 ratio <1, defined an "Immune Risk Profile" in humans(62). Based on this, we decided to find out if some immune functions could be useful as markers of biological age or "biomarkers" and therefore as predictors of longevity. We felt that this project was worthwhile since biological age is a more adequate parameter than chronological age to measure the rate of aging of a subject, although the proposed batteries of biomarkers have seldom included immune functions.
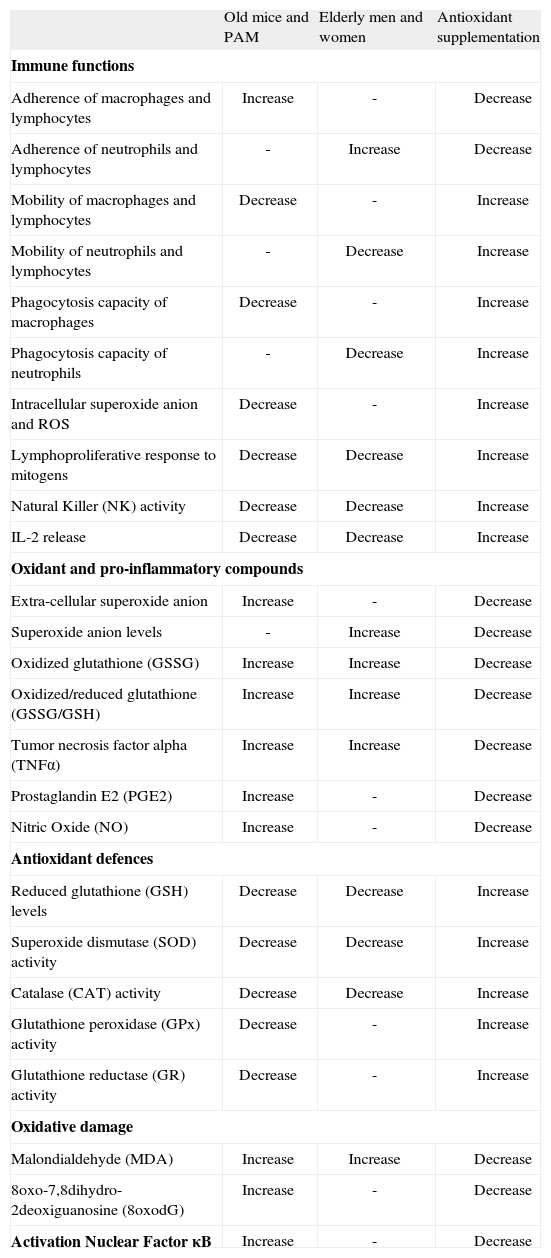
Among all functions of immune cells, we have focused on those listed in Table II. Thus, in lymphocytes these include their ability to adhere to the vascular endothelia, migrate towards the site of antigen recognition (chemotaxis), proliferate in response to mitogens and release cytokines like IL-2. In phagocytes they include the process of adherence to tissues, chemotaxis, ingestion or phagocytosis of foreign particles and destruction of pathogens by means of the intracellular production of free radicals such as the superoxide anion and other ROS located in the phagosome of these cells. Further, in the NK cells we have analyzed their capacity to destroy tumoral cells of the same animal species investigated. The same parameters have been determined along various decades in human subjects from the adult age of twenty to eighty, in leukocytes of peripheral blood, and throughout the life of mice in their peritoneal leukocytes. This type of longitudinal studies, which despite their high cost can be carried out in mice (that have a mean life span of two years), are almost impossible to perform in humans. Surprisingly, our results show that in both species similar age-related changes of the above mentioned immune parameters occur. Thus, with aging there are decreased lymphoproliferative responses and NK activity that protect us against tumoral cells. There is also a decline of IL-2, as well as chemotaxis, phagocytosis and adequate levels of ROS in the phagosomes. In addition, there is an increase of the functions that could become noxious in excess, such as those promoting adherence of immune cells to tissue that may prevent their arrival to the site where they have to perform their organism-protecting task. Also, there is an increase with age in other potentially harmful immune functions that will be dealt with below, such as the extracellular release of superoxide anion and proinflammatory cytokines such as TNFα(8,9,35) (Table II).
Changes in immune functions and oxidative stress parameters in peritoneal leukocytes from old mice versus adults, and PAM versus NPAM, as well as in peripheral blood leukocytes from elderly versus adult men and women. Effects of a diet supplemented with antioxidants in old mice, in PAM and in elderly men and women
| Old mice and PAM | Elderly men and women | Antioxidant supplementation | |
| Immune functions | |||
| Adherence of macrophages and lymphocytes | Increase | - | Decrease |
| Adherence of neutrophils and lymphocytes | - | Increase | Decrease |
| Mobility of macrophages and lymphocytes | Decrease | - | Increase |
| Mobility of neutrophils and lymphocytes | - | Decrease | Increase |
| Phagocytosis capacity of macrophages | Decrease | - | Increase |
| Phagocytosis capacity of neutrophils | - | Decrease | Increase |
| Intracellular superoxide anion and ROS | Decrease | - | Increase |
| Lymphoproliferative response to mitogens | Decrease | Decrease | Increase |
| Natural Killer (NK) activity | Decrease | Decrease | Increase |
| IL-2 release | Decrease | Decrease | Increase |
| Oxidant and pro-inflammatory compounds | |||
| Extra-cellular superoxide anion | Increase | - | Decrease |
| Superoxide anion levels | - | Increase | Decrease |
| Oxidized glutathione (GSSG) | Increase | Increase | Decrease |
| Oxidized/reduced glutathione (GSSG/GSH) | Increase | Increase | Decrease |
| Tumor necrosis factor alpha (TNFα) | Increase | Increase | Decrease |
| Prostaglandin E2 (PGE2) | Increase | - | Decrease |
| Nitric Oxide (NO) | Increase | - | Decrease |
| Antioxidant defences | |||
| Reduced glutathione (GSH) levels | Decrease | Decrease | Increase |
| Superoxide dismutase (SOD) activity | Decrease | Decrease | Increase |
| Catalase (CAT) activity | Decrease | Decrease | Increase |
| Glutathione peroxidase (GPx) activity | Decrease | - | Increase |
| Glutathione reductase (GR) activity | Decrease | - | Increase |
| Oxidative damage | |||
| Malondialdehyde (MDA) | Increase | Increase | Decrease |
| 8oxo-7,8dihydro-2deoxiguanosine (8oxodG) | Increase | - | Decrease |
| Activation Nuclear Factor κB | Increase | - | Decrease |
In order to identify the above parameters as markers of biological age and predictors of longevity we need to demonstrate that the levels that they show in a particular subject reveal his real health and senescent conditions. This has been achieved in two ways:
- A)
Ascertaining that the individuals with those parameters showing levels older than those of most subjects of the same population, sex and chronological age die before their counterparts. The confirmation that a premature immunosenescence in those parameters may predict a premature death can be tested only in experimental animals. This was performed using a model of premature senescence in mice proposed by our group. The animals that we have denominated PAM (prematurely aging mice), in contrast to NPAM (nonprematurely aging mice) of the same population, sex and chronological age, are identified by their poor response in a simple T-maze exploration test. This provides strong support for the concept that the nervous and the immune systems are closely linked. In mice showing premature aging we have observed that the above mentioned immune functions performed at the levels characteristic of older mice. In addition to a more significant inmunosenescence, the PAM showed high levels of anxiety and a brain neurochemistry similar to older animals. Nevertheless, the most convincing evidence that the above mentioned parameters are useful markers of biological age is that the PAM showed a shorter life span than their counterpart NPAM of the same, sex and chronological age(6,7,35,58) (Table II).
- B)
An additional way to confirm the key role of the immune system in health and longevity is the finding that the subjects reaching a very advanced age preserve the immune functions at similar levels to those of the adults. This has been shown in both humans and experimental animals such as mice. In human subjects our group has shown that in healthy centenarians the above mentioned immune functions perform as well as in young adults (30-years old) and much better than in 70-years old human subjects(63). A similar finding of a "youthful" condition has been obtained in peritoneal immune cells of very long-living mice(61).
All these results confirm that the immune system is a good marker of biological age and a predictor of longevity. Moreover, since the evolution of the parameters shown in Table II is similar in mice and human subjects, we can assume that men and women showing the above immune parameters at the levels of older subjects have a higher biological age and a shorter longevity.
WHY DOES IMMUNOSENESCENCE OCCUR? THE ROLE OF OXIDATIVE STRESSIf, as it is generally accepted, the mechanisms underlying aging must be of general application, it follows that the cause of inmunosenescence is the same as that responsible for the senescence of the other cells in the organism, namely the oxidative disorganization linked to the unavoidable use of oxygen to support cellular functions. Moreover, we should remember that the immune cells need to produce free radicals and other oxidant and inflammatory compounds in order to perform their defensive functions(9,22,23,35). This, together with the membrane characteristics of the immune cells, make them very vulnerable to oxidative damage(9,35). Therefore, if any cell needs to maintain a balance between the production of oxidants and the antioxidant defences to prevent an excess of the first and the resulting oxidative stress, this balance is even more essential to preserve the functional capacity of imzmune cells and, thus, the health of the organism. In addition, there is a close link between oxidative stress and inflammation, since excess or uncontrolled free radical production can induce an inflammatory response, and free radicals are inflammation effectors(64). In fact, some agerelated pathologies, such as hypertension and endothelial disfunction(9,65), atherosclerosis(66), and neurodegenerative diseases(67) are now considered to include in their pathogenesis not only an oxidative process, but also an inflammatory component. In fact, the levels of pro-inflammatory enzymes and molecules, such as cyclooxigenase 2, several cytokines and prostaglandins, seem to be increased with age(68). Further more, the increase of inflammatory compounds can explain several aspects of immunosenescence(69). Thus, a balance of pro-inflammatory compounds, needed to cope with damaging agents and crucial for survival, and anti-inflammatory markers, is also essential for an appropriate immune function, health condition and successful aging(70,71).
Based on the above, our group decided to investigate the age-related changes in the redox and the inflammatory state of immune cells. Thus we have analyzed in these cells different oxidant and inflammatory compounds (extracellular superoxide anion, oxidized glutathione GSSG, TNF-α, PGE2), and anti-inflammatory and antioxidant protectors, namely IL-10, reduced glutathione (GSH), glutathione peroxidase (GPx), glutathione reductase (GR), superoxide dismutase (SOD) and catalase (CAT), as well as oxidative damage to biomolecules such as lipids and DNA (Table II). Our results indicate that aging leukocytes suffer oxidative and inflammatory stress, resulting in higher levels of the parameters of oxidation and inflammation, decreased antioxidant defences, and increased oxidative damage to lipids and DNA(9,35) (Table II). Moreover, an increased oxidative and inflammatory stress has been also found in the immune cells of PAM with respect to those of NPAM, and in the leukocytes of male mice with respect to those of female mice(58) (Table II).
In addition, very long-living mice and human centenarians show a redox condition in their immune cells similar to that of healthy adult subjects. In fact, recent studies have pointed to a lower expression of genes resulting in inflammation and oxidation in human centenarian, who show preserved immune functions(63,70,71). Thus, centenarians seem to have a peculiar compromise between pro-and anti-inflammatory compounds and they are remarkably free of most age-related diseases with an inflammatory component(70,71). Although these conditions have not been adequately studied in exceptionally long-living experimental animals, we have observed that peritoneal immune cells from very old mice not only preserve in general their function in response to stimuli(61), but show controlled oxidative-inflammatory stress (Arranz L, de la Fuente et al., submitted).
THE ROLE OF NF-κB IN IMMUNOSENESCENCEAge-associated increase of oxidants and inflammatory compounds could be related to an up-regulation of the nuclear factor-κB (NFκB) activation, which in turn has been related to many chronic inflammatory disease states(69,72-74). Moreover, NF-κB is down-regulated by glutathione precursors such as N-acetylcysteine, which thereby prevent excessive oxidation and inflammation in the above situations(73,74). However, the activation of that factor in leukocytes with aging has been scarcely studied(68,69,75). Therefore, we have analyzed NF-κB activation, in resting conditions, in peritoneal immune cells through aging. Our results show that the immune cells from exceptionally long-living mice have levels of activation of NF-κB similar to those of younger animals. Besides, in old mice, only animals with controlled basal NF-κB activation in leukocytes achieved longevity, and the adult animals with a very high activation of NF-κB in their peritoneal leukocytes died early. Thus, the level of activation of that factor in leukocytes is significantly related to the life expectancy of the subjects from which the cells were obtained (Arranz L, de la Fuente et al., submitted).
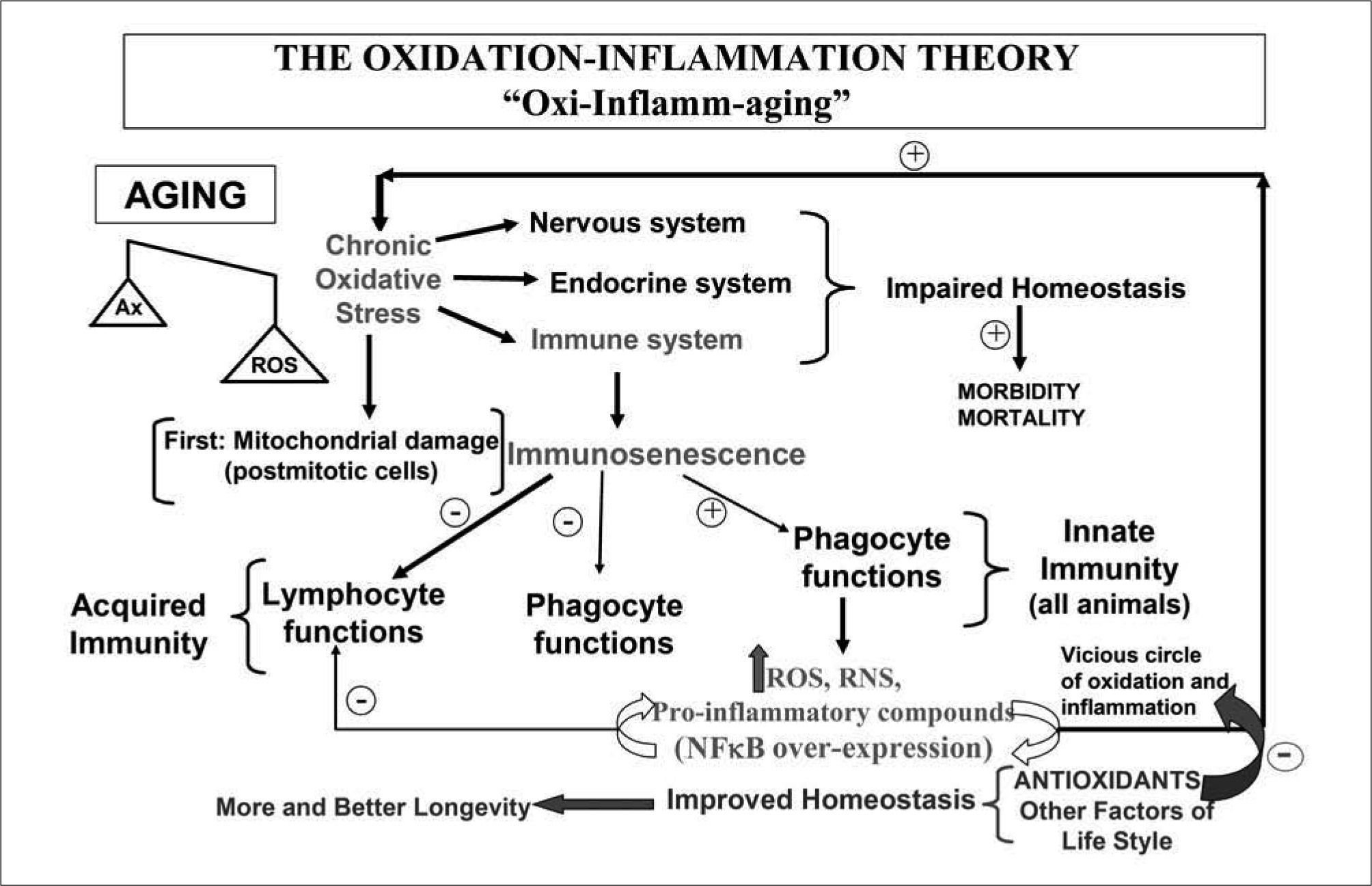
THE OXIDATION-INFLAMMATION THEORY OF AGING. ROLE OF THE IMMUNE SYSTEMWith all the above results obtained by us and others in the immune system, and in agreement with other published data supporting the idea of an inflammation and oxidation condition in aging(68,76), we have proposed the oxidation-inflammation theory of aging(9,35). We suggest that aging is linked to a chronic oxidative stress, which affects all cells in the organism, but particularly those of the regulatory systems, and thus the nervous, endocrine and immune systems would show the greatest oxidative damage. consequently, they would be unable to preserve their redox balance, and suffer functional losses incompatible with an adequate preservation of homeostasis, with a resulting increase in morbidity and mortality such as that found in old age. In addition, our immune system, because of its need to continuously generate oxidative and inflammatory compounds could activate, if it is not well regulated, factors such as the transcription NF-κB, which after reaching a certain level of activation, stimulates the expression of genes programming the production of higher amounts of those compounds. Thus, it is likely that if the production of oxidative and inflammatory compounds is not well regulated the organism may enter a "vicious circle" where the greater amounts of oxidant and inflammatory compounds produced by the immune system would further activate the production of the same noxious compounds through factors such as the above mentioned NF-κB. If this harmful circle is not well controlled, the noxious compounds from the immune system would in time disorganize not only the immune cells, but also all other cells of the organism, thus contributing to maintenance of its chronic oxidative stress. In view of this, it seems evident that the immune system can play a role in the uncontrolled oxidation and inflammation process linked to aging and thus affect the rate of aging (Figure 2).
A more developed scheme of the oxidation-inflammation theory than that previously published (9). Aging is a chronic oxidative stress condition (more oxidant than antioxidant compounds are present) affecting all cells, specially those of the regulatory systems, i.e, the nervous, endocrine and immune systems, and therefore the communication among them. This explains the impaired homeostasis and the increase of morbidity and mortality found in old age. According to the oxidative mitochondrial theory of aging, the differentiated postmitotic cells are the first to suffer damage with age. In animals with a complex immune system are the T lymphocytes, and specially the memory T cells (which are the most abundant T cells in aged subjects and are also the most postmitotic cells of the immune system), those that suffer the effects of the oxidative stress of aging. In the age-associated re-structuring of the immune system or immunosenescence, there is a decrease of several lymphocyte and phagocyte functions (more related to the acquired immunity), but an increase in other functions, specially those carried out by phagocytic cells generating continuously oxidative and inflammatory compounds (cells and functions responsible for the innate immunity and present in all animals). These compounds produced in order to eliminate foreign agents, could activate the factor of transcription NF-κB, which after reaching certain level of activation stimulates the expression of genes programming the production of higher amounts of those compounds. If this fact is not well regulated a vicious circle of oxidation-inflammation could be established, increasing the oxidative stress and consequently accelerating aging. Thus we could conclude that in all animal species the immune cells modulate the rate of aging of each subject. In agreement with this concept, the administration of antioxidants has been shown to cut the above mentioned vicious circle, improving both the nervous and immune functions, decreasing their oxidative stress, and consequently improving homeostasis and increasing longevity. Moreover, other factors of life style such as the practice of mental and physical exercise and overcoming emotional stress can also help to improve homeostasis and thus maintain health and increase mean longevity.
We can say that when an animal shows a great oxidative stress in its immune cells, they have a worsened function and that particular animal shows a decreased longevity. On the contrary, if the immune cells of a subject maintain their redox state better, their function will also be better and this subject will live longer. Thus, there is a relation between the redox state of the immune cells, their functional capacity and the life span of the subject (Figure 3).
Relation of the oxidative state of leukocytes, their function and individual longevity. High levels of oxidative stress in the immune system results in an impaired function of its cells and a lower longevity of the subjects having these cells, whereas the contrary situation, namely low oxidative stress and better function of immune cells, is accompanied by high longevity. In agreement with the above, the models of premature and long-living mice and human subjects indicated in this figure are linked to low and high oxidative stress, respectively, in their immune cells.
As examples supporting that idea and, consequently, the role of the immune system in aging, we can mention again what happens in our mouse model of premature aging. The PAM, with a shorter life span than the NPAM, show a greater oxidative stress not only in their immune system but also in the brain, liver, heart and kidneys(58). Moreover, mammalian females, that usually have better immune functions than males(77), also have a longer mean life span owing to the effects of the estrogens that allow them to live in a less oxidized condition(78). This is reflected in the better redox state of their leukocytes (Baeza and De la Fuente et al., manuscript in preparation). In addition, human centenarians(63), and laboratory mice with a high mean-life expectancy (Arranz and De la Fuente et al., submitted) are those that better maintain the redox state of their immune cells and therefore their immune functions (Figure 3).
CAN THE ROLE OF THE IMMUNE SYSTEM IN AGING HAVE AN UNIVERSAL APPLICATION?It is well known that from the time we are born our immune system has to face a great variety of foreign agents and, in order to protect the organism against them, it needs to release toxic oxidant and inflammatory compounds. An adequate production of these noxious products is needed to increase the probability that a subject will survive to reach the age of reproduction, which is essential for the survival of the species. Obviously, the immune system has not been well prepared to preserve its defensive functions for a long time, especially after the reproductive period. In the case of the members of the human species living in developed countries that show considerable increases in their mean life span, they usually suffer the consequences of a very activated immune system during a long period of time(35,70,71,79). Thus, the participation of the immune system in oxi-inflammaging seems evident. As happens with oxygen use, wich allows a very active life style but has as its "side effect" the production of noxious ROS, a very active immune system is designed to provide protection against the risk of contracting infections and tumours to which we are chronically exposed, but it has a "price", i.e., that if the oxygen-inflammationsupported functions and resulting stress are not well controlled or the aging organism lacks a satisfactory individual adaptation to this stress, the senescent process accelerates(35).
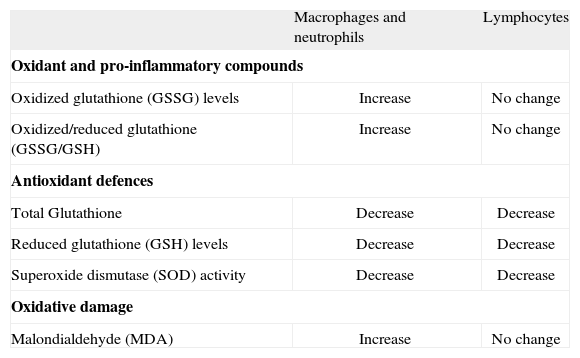
This evolution-related view of inmunosenescence could explain why the acquired immunocompetence, which is more specialized and obtained more recently in evolution, is the most impaired with age whereas the innate immune response, older and less specific, is better preserved and may be even stimulated in excess in aging organisms(80) (Figure 2). Thus, "the immune theory of aging" as originally proposed(81), can not be accepted since this theory proposed as the cause of organism senescence the impairment of the immune system, and this concept do not follow the principle of universality of Strehler. We should keep in mind that not all animal species have immune systems as complex as those of mammals. Nevertheless, it seems evident, based on all the above information, that leukocytes can play a fundamental role in aging. Confirmation of this concept could be found in the fact that the immune cells producing oxidant and inflammatory compounds in the highest amounts, and specially with aging, are those responsible for the innate immunity, especially the phagocytic cells that are found, with different denominations, in all animals, including invertebrates. These animals produce inflammation-like responses and cytotoxic free radicals during infection and other defensive actions, through a cellular and humoral immune system similar to the innate immune system of vertebrates(82-85). In invertebrates, whose immune system has been scarcely studied, there is no equivalent of a lymphoid linage and thus, as it has been shown in insects, they lack an acquired immunity based on the presence and function of lymphocytes(84). In agreement with the above, we have observed that in the peritoneal immune cell populations of mice, and in blood immune cells of human subjects, macrophages and neutrophils respectively generate higher levels of oxidant compounds than lymphocytes, and these levels significantly increase with age in these phagocytic cells(35) (Table III). Thus, it is likely that macrophages, which as already pointed out, are found in all animals, are implicated in the chronic oxidative stress of senescence due to their high age-related increase in the production of ROS and inflammatory cytokines(9,32,43). Hence, in all animal species the immune cells could modulate the rate of aging of each subject.
Comparative age-related changes in the levels of oxidants, pro-inflammatory compounds, antioxidant defences and oxidative damage to lipid in peritoneal macrophages and lymphocytes from mice and in neutrophils and lymphocytes from human peripheral blood.
| Macrophages and neutrophils | Lymphocytes | |
| Oxidant and pro-inflammatory compounds | ||
| Oxidized glutathione (GSSG) levels | Increase | No change |
| Oxidized/reduced glutathione (GSSG/GSH) | Increase | No change |
| Antioxidant defences | ||
| Total Glutathione | Decrease | Decrease |
| Reduced glutathione (GSH) levels | Decrease | Decrease |
| Superoxide dismutase (SOD) activity | Decrease | Decrease |
| Oxidative damage | ||
| Malondialdehyde (MDA) | Increase | No change |
This theory can be supported by research showing that a regulation of the immune system by strategies of life style allowing to cut the "vicious circle" of oxidation-inflammation injury results in an increased longevity.
The ingestion of a diet enriched in antioxidants seems adequate to maintain an optimum redox balance and therefore to protect the aging organism against oxygen stress. So, the administration of compounds such as vitamins C and E, polyphenols and thiolic antioxidants (taurine, thioproline and N-acetylcisteine, among others), which are precursors of reduced glutathione (GSH), alone or in nutritional formulations containing several compounds, may be recommended, because of their antioxidant and antiinflammmatory action, for use in both laboratory animals and human subjects. In fact, these antioxidants show important favorable effects on health, acting on the immune system(8,9,35,44,74,86-88). In addition, in the performance of their function the leukocytes may exhaust their reserves of antioxidants(89). This might help to explain why in both laboratory animals and human subjects, the homeostasislinked functional competence of the immune system in the adult age improves after diet supplementation with appropriate amounts of antioxidants like vitamin C, vitamin E, thiol antioxidants and polyphenols(90,91). Moreover our research has shown that, in elderly men and women, in control mice chronologically-old, and in PAM, the above antioxidant compounds exert a favorable effect improving the functional capacity of the immune system as well as neutralizing the redox state, leaving them at levels similar to those of human adults and non prematurely aging healthy adult mice(8,9,35,44,58,86,88,92) (Table II). In fact, our group has observed that the lymphocytes from old mice, after ingestion of a diet supplemented with thiol antioxidants, did not show the lipofuscin pigments present in control animals(35). These pigments are the end products of the oxidative disorganization and autodigestion of mitochondria, and they appear in cells of aging animals(93).
Since the favorable action of the antioxidants on the immune system is expressed as an increase of the functions that are depressed and a decrease of those that are excessively active, the antioxidants cannot be considered general immunostimulants. In fact, they may bring each immune function and redox state to its optimum level, thus acting as immunomodulators(44,88,94). This modulating ability appears to be focused at the level of the ubiquitous intracellular factors implicated in oxidation and inflammation, such as NF-κB(74). The regulatory role of the antioxidants would be performed not only in the immune system, but also in the other regulatory systems, including the nervous system, where oxidative stress also underlies its senescent impairment(95). Thus, in the PAM the ingestion of thiolic antioxidants not only improves the immune function, but also the behavioral response(58,96). Moreover, interestingly, this immune "rejuvenation" as well as the improvement of the nervous system are apparent in the laboratory mice showing the greatest longevity(9). A possible explanation is that since all cell functions are highly dependent on the redox reactions of the thiol compounds, the preservation of adequate levels of GSH or of other thiolic compounds during aging is essential for an adequate activity of cells in general and, especially, for those of the nervous and immune systems, and therefore for health of the aging subjects. In fact, it has been shown that the organelles and cells of aged animals contain less GSH than those of young ones, and this decrease becomes more striking at the age when mortality shows a marked increase. Thus, we have shown an age-related decrease of GSH levels in leukocytes from mice, which is more striking in those from PAM(35,58), and that the administration of GSH precursors, such as tioproline and N-acetylcysteine in both mice and humans, increases those levels as well as improves the immune cell functions and its redox state(35,58,88), being able to increase life expectancy in normal laboratory animals(97) and in PAM(35,58).
Recently, we have shown that the administration of N-acetylcysteine to postmenopausal women improves their immune cell functions and the redox state of these cells, bringing the values closer to those found in adult young women, which reveals a decrease of the biological age(88). This suggests that the oxidative and inflammatory stress that appears to play a fundamental role in the aging of both the immune system and the nervous system, can be counteracted to certain degree by administration of antioxidants, and that antioxidant diet supplementation may be useful to neutralize or retard age-related homeostatic impairment (Figure 2). In view of all the above, it seems reasonable to propose that the administration of adequate amounts of antioxidant compounds may be effective to neutralize or slow down the loss of homeostasis that occurs with age. It is convenient to realize that the effectiveness of the antioxidants depends on the administered amount of these compounds, and that there is an age-related change in the dose appropriate to improve the immune response(90).
Since physical activity is deemed to have a beneficial effect on health, this is another of the lifestyle factors that can improve health and quality of life in old subjects(98). There is a wealth of information on the effects of physical exercise on the immune function of adult experimental animals and humans. Although conflicting results have been obtained, depending on the type of exercise, immune function studied or state of the subject, it is generally accepted that acute or very strong training induces an inflammatory response with selective activation or depression of immune cell functions, whereas moderate training exercise leads to adaptations of the immune cells with improvement of their functions(99,100). Thus, physical activity does not have to be vigorous to benefit health and specifically the immune system. In fact, it is accepted that strenuous physical exercise may be a significant oxidative stress because it increases consumption of molecular oxygen for respiration and may generate higher amounts of ROS. According to several reviews of the broader field of exercise and oxidative damage(101), vigorous exercise is accompanied by the involvement of immune cells, specially phagocytic cells, in the generation of oxidants through the activation of factors such as NF-κB(102). Thus, an over-stimulation of the innate immune response could be harmful to those individuals with a high inflammatory state. However, a well controlled and regulated stimulation of the innate immune mechanisms during moderate exercise can help to prevent infections, and as reviewed by Johnson(103), a decrease in oxidative stress and a resistance to oxidative damage appears in response to repetitive or graded exercise training. This fact may be due to exercise induced changes in antioxidant enzymes not only in the skeletal muscle, which is recognized as a major source of free radical generation, but also in other tissues and cells. Although the results show discrepancies, depending on the species, tissue, type and subtype of cell, age of the animal and training regimen, in general an up-regulation of antioxidant defences appears with moderate exercise training(103). As regards to the immune cells, the downregulation of ROS release and the adaptation of antioxidant mechanisms to regular exercise has been observed in phagocytic cells by us and other authors(102,104). Moreover, we have recently reviewed exercise-induced neuroimmunomodulation and the role of catecholamines as "stress mediators" and/or "danger signals" for the innate immune response during physical exercise(105). Although in old animals or elderly humans the effects of physical exercise on the immune functions have been scarcely studied, available data show that the practice of moderate exercise is an important candidate for improving the immune function in the elderly. In fact, several authors and our own group have shown that, in old animals, regular exercise training improves immune functions(99,106). In addition, we have observed that the favorable effects of exercise are higher in old than in young mice(106), and similar results have been obtained by us in humans(9). We have observed that elderly men and women who performed a moderate physical exercise for 6 months (3 sessions of 45 minutes per week), improved significantly their immune system regarding neutrophil, lymphocyte and NK functions shown in Table II, bringing the values close to those in adults(9). It is posible that this effect of physical exercise improving the immune cell functions is carried out through a direct effect on these cells of the factors released in response to exercise and through an increase of their above mentioned antioxidant defences(9,103). In fact, we found that ascorbate content in macrophages increases after exercise in both young and old mice(104), and in humans, after a moderate physical exercise it not only improves several functions of peripheral blood neutrophils and lymphocytes but it also increases their antioxidant defences(9). Moreover, the production of pro-inflammatory and anti-inflammatory cytokines decreases and increases, respectively, after a moderate physical exercise(107), and the inflammatory markers are lower in older adults performing exercise than in those without physical activity(108). Thus, moderate physical exercise could decrease the rate of aging through recovering of the balance oxidant/antioxidant of the immune cells(9) and decrease of the inflamma-aging (Figure 2).
Other intervention systems aimed at decreasing the rate of aging that we are studying are the mental activity as well as overcoming of emotional stress. Our preliminary results have shown that mice with environmental enrichment show an improvement of the functions and redox state of their immune cells, and that they increase their longevity(109) (Figure 2).
CONCLUSIONS AND RECOMMENDATIONSThe immune system seems to have a very important role in the rate of aging, and the functional situation of the immune cells, which depends on their redox state, is a good marker of biological age and mean longevity. Therefore, maintaining an immune function in a condition similar to that of adults can assure a better health and a longer life span. Thus, strategies such as a good nutrition, with the appropriate amount of antioxidants, the practice of mental and physical exercise, and overcoming emotional stress, the effectiveness of which can be measured through their effects in improving the immune function, can help to maintain our health and increase our mean longevity.
DISCLOSUREThe author declares no financial conflict of interest.
The author thanks Dr. J. Miquel for his expert comments on some aspects of this review. The author would also like to express her gratitude to Dr. Medina, Dr. Victor, Dr. Vallejo, Dr. Guayerbas, Dr. Puerto, Dr. Alvarado, Dr. Alvarez, Dr. Alonso, Ms. Arranz and Ms. Baeza for their invaluable help in performing the experiments that have allowed me to arrive at the ideas expressed in this article. This work was supported by grants of the MEC (BFU 2005-06777), MCINN (BFU2008-04336), UCM Research Group (910379ENEROINN) and RETICEF (RD06/0013/0003) (ISCIII).