Existen diversas poblaciones de linfocitos T con funciones inmunoreguladoras: linfocitos naturales CD4+CD25+Foxp3+, y linfocitos T reguladores adquiridos, como linfocitos reguladores de tipo 1 (Tr1) o Th3. Entre otras características, los linfocitos Tr1 se diferencian en las citocinas que inducen su desarrollo y proliferación. Las células Tr1 son generadas gracias a la presencia de citocinas antiinflamatorias, sobretodo IL-10. Estas células inmunomoduladoras ejercen su función, de manera primordial, liberando citocinas tales como IL-10 o TGF-®. En concreto, las células Tr1 se caracterizan por secretar grandes cantidades de IL-10 al medio. Se han encontrado alteraciones, ya sea en número o en función, de esta población linfocitaria en desórdenes inmunológicos tales como enfermedades autoinmunes (esclerosis múltiple, artritis reumatoide, diabetes), enfermedades alérgicas o rechazo de trasplantes. En dichas enfermedades se han observado alteraciones en la liberación de IL-10 por las células Tr1. En esta revisión nos vamos a centrar en alteraciones de las células Tr1 en diferentes enfermedades, en el potencial de este grupo celular para el desarrollo de inmunoterapia específica y las dificultades encontradas para utilizar linfocitos Tr1 en la atención de pacientes individuales.
Many cell subsets with regulatory function have been described, including natural CD4+CD25+Foxp3+ T cells and acquired regulatory T cells such as regulatory type I cells (Tr1) or Th3 cells. They are generated from different precursors and have different mechanisms of action. Tr1 cells are induced in the presence of high levels of IL-10 and they exert their immunomodulatory activity through the production of large amounts of IL-10 and TGF-β. Tr1 cell function is impaired in several immunological diseases such as autoimmune diseases (multiple sclerosis, rheumatic disease, psoriasis, or type I diabetes), allergic diseases, or graft rejection. The impairment of Tr1 function can be related to the decrease of IL-10 production by Tr1 cells or by an alteration in the IL-10 signalling pathway. In this work, the characteristics of Tr1 cell are reviewed, highlighting the potential of these regulatory T cells for immunotherapy.
Many cell subsets with regulatory function have been described, including natural CD4+CD25+Foxp3+ T cells(1–3) and acquired regulatory T cells such as regulatory type I (Tr1) cells(4,5), or Th3 cells. These regulatory T cell subsets are different in various biological aspects(6). Regulatory T cells control immune responses and prevent the appearance of immune pathology due to an excessive immune response, and play crucial roles in the induction of peripheral tolerance to self and foreign antigens(7–9). These cells are involved in the immunological dysregulation underlying autoimmune disease, chronic inflammatory diseases, allergic disease, as well as cancer and transplant rejection. Tr1 cells are a promising cellular therapy although there are some unsolved questions. Even if their mechanisms of action are rather known (immunosuppression through IL-10), there are other aspects not fully understood. More studies are needed in order to make them an ideal candidate for immunotherapy.
IMMUNOLOGICAL PROPERTIES OF Tr1 CELLSTr1 cells are defined by their ability to produce high levels of IL-10 and transforming growth factor-β (TGF-β)(10). IL-10 was first described as a Th2 cytokine but nowadays it is considered as a cytokine that plays a central role in suppressing T cell responses. IL-10 controls the inflammation mechanisms and it is implicated in maintaining immunological tolerance(11). IL-10 indirectly suppresses cytokine production and proliferation of antigen-specific CD4+ T effector cells, and modulates the stimulatory capacity of dendritic cells and other antigen presenting cells (APC). IL-10 downregulates the expression of MHC-class II, co-stimulatory and adhesion molecules in effector T cells. Also, IL-10 induces long-lasting antigen-specific anergy in both CD4+ and CD8+ T cells. Antigen-specific T cell suppression by IL-10 is essential in peripheral tolerance to autoantigens, allergens, tumour antigens and transplantation antigens(12). On the basis of these characteristics, immunomodulation through IL-10-producing cells seems an excellent candidate for cell therapy in immunological diseases(13,14).
INDUCTION AND PROLIFERATION OF Tr1 CELLSAntigen-specific Tr1 cells arise in vivo through the differentiation from naive CD4+ T cells after activation in an specific environment. Stimulating naive CD4+ T cells in the presence of high levels of IL-10 results in the generation of Tr1-cell clones, which produce large amounts of IL-10, TGF-β, and IL-5, but low amounts of IFN-γ and no IL-4(15,16). It has been shown that co-engagement of CD3 and the complement regulator CD46 in the presence of IL-2 also induces a Tr1 phenotype in human CD4+ T cells(17). Also, murine T cells stimulated with anti-CD3 plus anti CD28 mAbs in the presence of vitamin D3, dexamethasone, anti-IFN-γ, -IL-4, and -IL-12 mAbs differentiate into Tr1 cells, which express granzyme B(17). More recently, IL-27 has been identified as a potent inductor of IL-10 production by effector T cells(18). Although IL-10 is considered the main force for Tr1 generation, it seems that IL-10 is necessary but it may be not sufficient for their differentiation. Tr1 cells are inducible, antigen-specific and need to be activated via their TCR in order to exert their suppressive functions. Immature dendritic cells (DCs) are able to induce human Tr1 cells in vitro(10). In addition to immature DCs, specific tolerogenic DCs can prime Tr1 cells(10). Once they are activated they can suppress in an antigen-non-specific manner. Probably this bystander suppression is due to the immunosuppressive cytokines released such as IL-10 and TGF-β(5).
Tr1 cells are able to suppress both naive and memory T-cell responses in vitro and in vivo. Tr1 cells can inhibit naive and memory Th1 and Th2 responses through the production of IL-10. Also, Tr1 cells participate in the maintenance of peripheral tolerance by halting the activation of autoreactive T cells. In vitro studies indicate that Tr1 cells are anergic and do not strongly proliferate upon antigenspecific stimulation. The inhibitory effect of IL-10 may contribute to this anergic profile(19). Although Tr1 cells have a low proliferative capacity, they can be expanded in the presence of IL-2 and IL-15 or IL-27, even in the absence of TCR activation. However, it is still unkown whether they can proliferate in vivo.
Currently, there are not specific markers expressed by Tr1 cells useful to discriminate them from naturally occurring CD4+CD25+Foxp3+ Treg cells or even for confirming that all Tr1 cells obtained with different protocols are the same cell subtype. In contrast with natural Treg cells, adaptive Treg cells exert suppression mostly through soluble factors (IL-10 and/or TGF-β), and their suppressive function is not strictly associated to high-level Foxp3 expression. Indeed, the transcription factor Foxp3 is not constitutively expressed by Tr1 cells, but it can be up-regulated upon activation to levels similar to those of activated CD4+ CD25-T cells(20).
Tr1 CELLS AND IMMUNOTHERAPYBecause T cells can be considered as minifactories that might release the right combinations of cytokines in the right place and time, and they are able to sense the tissue environment, they seem to be an ideal candidate for immunotherapy. In this respect, Tr1 cells might be good candidates for cell immunotherapy since they can be obtained in humans by stimulating naive CD4+ cells with different protocols. Moreover, their mechanism of action (mainly based on IL-10 secretion) is well defined in order to monitor the response to therapy. Another advantage of using these cells is that the target antigen does not need to be known because of the immunosuppressive bystander effect they exert through IL-10, as is indeed the case in most immunological diseases. The need for sustained high-dose IL-10 administration at sites of potential inflammation indicates that IL-10 primarily acts by blocking the entry and/or activity of pathogenic T cells in the target tissue(21), an effect that might be accomplished using Tr1 cells.
One major concern when clinically manipulating the levels of IL-10 is the critical role of this cytokine in immune homeostasis. Long-term application of IL-10 could cause immunodeficiency, whereas continuous use of anti-IL10 may lead to hyperimmune reactions(22). Because Tr1 cells do not induce general immunosupression, they are less prone to result in opportunistic infections. However, recent studies have shown impairment of Tr1 cell function in diverse immune diseases, such as autoimmunity(23), graftversus-host disease, tumour cell growth, parasite survival, chronic infections(24), and the development of acquired immune deficiency syndrome(22).
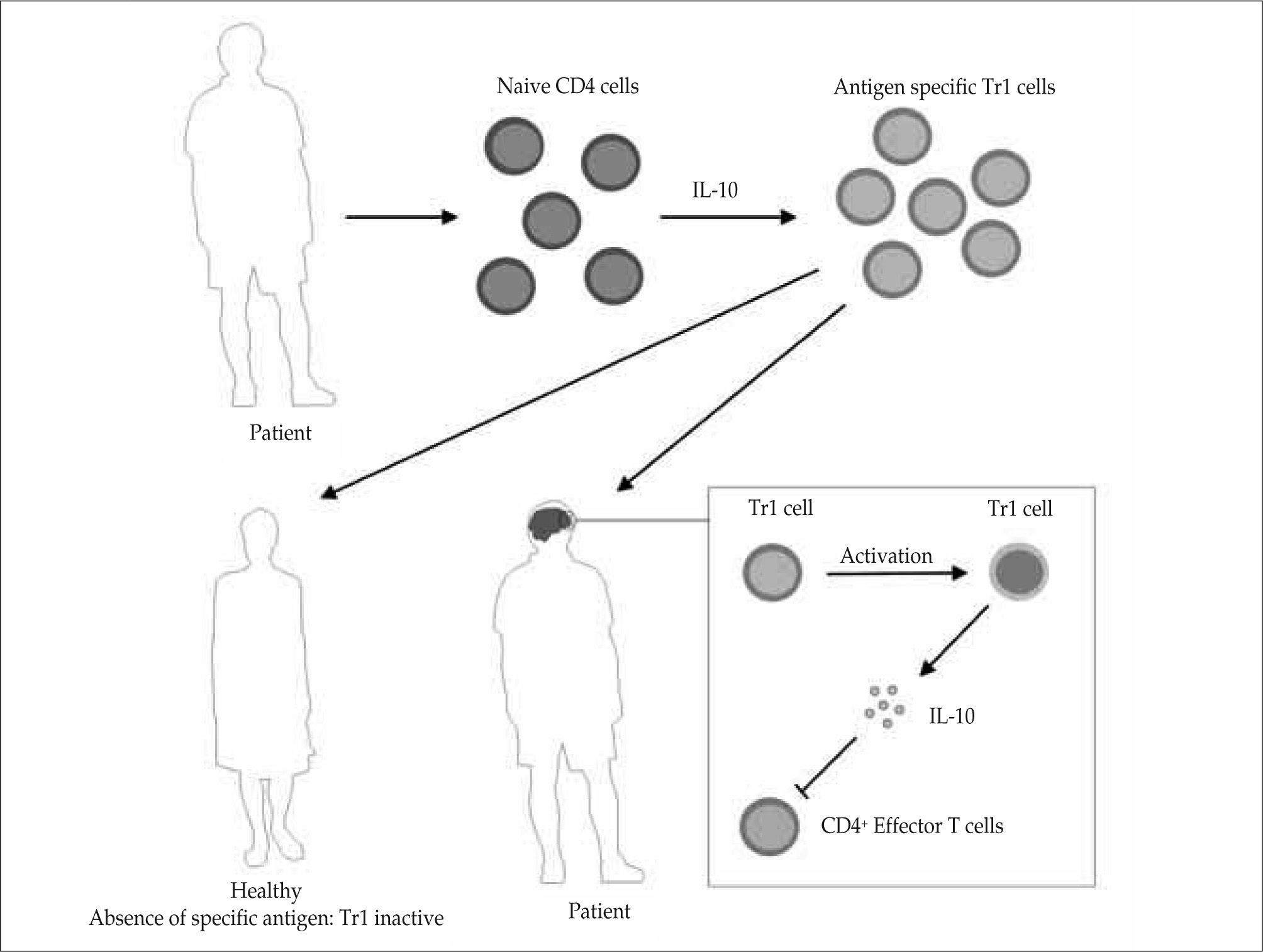
AutoimmunityIn several autoimmune diseases, including rheumatoid arthritis, type I diabetes, psoriasis, myasthenia gravis, sarcoidosis, and multiple sclerosis (MS), the number of Tr1 cells is decreased and its function is impaired(21). Recently, we reported that Tr1 cells isolated from patients with MS produced less amounts of IL-10 than healthy controls(25). We also observed that CD4+ cells from MS patients were less prone to differentiate to functional Tr1 cells after induction with anti-CD46 monoclonal antibodies. In the same way, Tr1 cell function was defective in MS patients as we evaluated IL-10 regulatory activity with suppression assays, quantitative PCR, flow cytometry and western-blot. IL-10 obtained from Tr1 cell culture supernatants was unable to suppress CD4+ cell proliferation in MS patients. We then analyzed IL-10 signaling pathway, finding that Stat-3 phosphorylation dynamics is altered in MS patients. This down-regulation of Stat-3 phosphorylation could be dependent on the overexpression of Socs-3, an inhibitor of cytokine signaling. In summary, our results and previous studies indicate that the function and IL-10 signaling pathway of Tr1 cells is altered in MS patients. We speculate that in order to restore Tr1 activity in MS, it is necessary to use IL-10 signaling pathway modulators capable of changing the altered dynamic observed in MS patients (Fig. 1). Indeed, our results help to explain why the clinical trial with recombinant IL-10 failed in MS patients, due to the impaired IL-10 signaling pathway in these patients(25).
Tr1 based immunotherapy for autoimmune diseases. CD4+ naive T cells are isolated from patients. Tr1 cells are generated and expanded in vitro and then re-injected into the patient, so that they migrate to the inflamed tissue. In the absence of the specific antigen they will not be activated, remaining inactive. The encounter of the antigen at the site of inflammation (for example the brain in patients with MS) will induce the specific activation of regulatory T cells and the local secretion of anti-inflammatory cytotínes, such as IL-10, that will restore the immune system balance.
Tr1 cells have an anti-inflammatory capacity and infíltrate injured tissues to control inflammation (through the release of IL-10 and TGF-β) and tissue destruction (through the release of TGF-β) locally. Tr1 cells could be used as a promising target for the development of new therapeutic agents, as well as in cellular therapy for peripheral tolerance modulation in allergy and autoimmunity. It seems reasonable to isolate Tr1 antigen-specific cells from an affected patient, expand them in vitro and then re-inject the Tr1 cells to the patient. Regulatory T cells should migrate to the inflamed tissue. Once Tr1 cells discover the antigen at the site of inflammation, they will get spedfically activated and induce anti-inflammatory cytokine secretion. However, if they do not encounter the specific antigen they will stay inactive(26). For example, Prakken and colleagues showed that feeding rheumatoid arthritis patients with a peptide that induces inflammatory responses induced deviation to a regulatory phenotype, with large amounts of IL-10 production(27–29). Other studies have argued that patients with rheumatic disease exhibit defective IL-10 responses as compared with healthy individuals, suggesting the inability to generate IL-10. Thus, it is logical to assume that IL-10 deficit might contribute to disease pathogenesis if defects in function and/or number of Tr1 cells were responsible for autoimmunity(30,31).
Transplantation and other diseasesAfter allogenic hematopoietic stem cells transplantation, patients without acute graft versus host disease (GVHD) produce large amounts of IL-10 in response to recipient alloantigens. In contrast, low IL-10 levels were found in those patients with severe GVHD. This suggests an association between the concentration of IL-10 and the suppression of CD4+ effector responses against the graft. There is a clinical trial currently under way in which donor cells anergized ex vivo in the presence of IL-10 are used as post-transplant cellular therapy in hematological cancer patients undergoing HLA-haploidentical haematopoietic stem cells transplantation(32,33).
In vivo induction of antigen-specific Tr1 cells is useful to restore and maintain immune tolerance. Tr1 cells are an alternative to cure a variety of immune-mediated diseases(45,46). Roncarolo et al. have shown that Tr1 cells specific for alloantigens can be induced in vivo in murine models of pancreatic islet transplantation. The administration of rapamycin and IL-10 leads to the induction of alloantigenspecific Tr1 cells capable of blocking acute allograft rejection. In this setting, Tr1 cells also contribute to long-term tolerance(47). However, generation of antigen-specific Tr1 cells is not sufficient and down-modulation of effector T cells is also necessary(37).
Allergic diseases, such asthma, rhinitis, and atopic dermatitis are characterized by chronic inflammatory disorders caused by Th2-type immune responses against common environmental antigens(38). It is now clear that during the early course of allergen specific immunotherapy, IL-10 and/or TGF-β Tr1 cells are induced by high and increasing doses of allergens. Allergen specific Th1 and Th2 responses are down-regulated by these Tr1 cells. A recent study showed that in healthy individuals, Tr1 cells represent the dominant subset for common environmental allergens, whereas a high frequency of allergen-specific IL-4-secreting T cells (Th2-like-cells) is found in allergic individuals(39). The frequency of memory effector T cells or regulatory T cells is crucial in the development of allergy or a healthy immune response. Thus, induction of antigen-specific Tr1 cells can inhibit the development of allergic Th2 cell responses and play an important role in allergen-specific immunotherapy(40).
Besides the crucial role of Tr1 cells in autoimmunity, allergic diseases and transplantation(32,33), it has been postulated that in atherosclerosis there is an imbalance between regulatory and pathogenic immunity, and that such a disequilibrium promotes both plaque development and plaque inflammation. The European Vascular Genomics Network is working to develop and validate protocols of vaccination against atherosclerosis in mice, based on the stimulation of plaque antigen-specific regulatory T cells (CD4+CD25+ and Tr1 cells) and mediators like IL-10, which inhibit the formation of atheroma plaques.
Tr1 CELL ANTIGEN-SPECIFIC IMMUNOTHERAPY: TRADE-OFFSAlthough Tr1 therapy would be beneficial in some circumstances such as autoimmunity or hypersensitivity reactions, it may be also detrimental in other cases such as cancer, chronic infection and tissue remodelling. Recent evidence has demonstrated that regulatory-T-cell mediated immunosuppression is one crucial tumour immune-evasion mechanism and the main obstacle for successful immunotherapy and cancer vaccination. Functional regulatory T cells are found in the tumour microenvironment(50); these include thymus-derived "natural" Treg cells and locally induced Tr1 cells(51). IL-10 is also found in the tumour microenvironment, where it induces tumour-specific IL-10-expressing Tr1 cells and it is implicated in their locally differentiation and expansion. Tr1 cells and IL-10 may be responsible, in part, for the immune response failure against the tumor(52,53). In other words, tumour microenvironment promotes the generation of Tr1 cells, which may play a critical role in cancer progression and may mediate systemic immunosuppression. A better understanding of these mechanisms is essential for a future control of Tr1 cell expansion and their contributions to tumour escape(54).
Tr1 cells suppress immune responses and might result in the manifestation of a systemic or infectious disease. So, the best approach would be one in which the main antigens responsible for the pathogenesis are chosen. Tr1 cells would therefore be activated at the appropriate site to mediate local bystander suppression. In this way, the effects of Tr1 cell therapy would remain local and specific. From a technical point of view, there is a considerable challenge to generate sufficient quantities of cells to treat ongoing immune responses in humans. The small number of Tr1 cells in the circulation and their low grade of proliferation limit the use of Tr1 cells in clinical settings. Moreover, because this subset is defective and/or deficient in many autoimmune diseases, an in vitro antigen-specific proliferation rescue protocol would be necessary. Open questions regarding clinical usage of Tr1 remain unsolved. For instance, their life-span, their migratory ability in vivo, their toxicity, or their chance to promote tumour development are unknown.
CONCLUSIONSTr1 cell-based immunotherapy in humans is a promising strategy for treating immunological diseases(46,47). Furthermore Tr1 cells have the advantage of being inducible and expanded ex vivo. However, they are hard to purify because they lack specific surface markers. In addition, once Tr1 cells are generated, it is difficult to avoid contamination with nonregulatory T cells, and there is not a feasible method of selection. Another unresolved question is whether the phenotype of Tr1 cells generated in vitro is stable once they are transferred in vivo to patients. As explained above, Tr1 cells produce large amounts of the immunosuppressive cytokine IL-10 after encounter with their specific antigen, but once activated they can suppress in a non-antigen-specific manner and they can mediate some level of bystander suppression limited to the site where Tr1 cells are localized.
Although Tr1 cells do not induce generalized immunosuppression, it is reasonable to generate Tr1 cells specific for other antigens that are not the responsible of one given disease. On the basis of this assumption, once these Tr1 cells are transferred to patients, they will be activated and suppress not only antigen-specific T effector cells but also pathogenic T cells with different antigen-specificity. Since in many diseases the antigen causing the pathogenesis is unknown (for instance, in autoimmune diseases such as MS), this approach represents an interesting strategy(48).
Finally, it is tempting to speculate about the physiological role of Tr1 cells. One possibility is that they represent a way for modulating the immune system and preventing immunopathology. After immune system activation, including the counter-activation of professional Treg, Tr1 cells would be implicated in an antigen-specific delayed response. As Treg cells release IL-10 in the microenvironment, it is reasonable to think that Tr1 cells could be the result of an adequate regulatory cytokine milieu established by other T cell subtypes. Then, Tr1 cells would facilitate the control of immune responses, avoiding inflammation and tissue damage. Turning to cell immunotherapy, this translates into a two stages treatment. Mimicking its physiological function, at first it would be necessary to induce a professional Treg response (induction phase) continuing with the Tr1 phase of treatment (maintenance). Clinical data will be necessary to clarify the role and possibilities of Tr1 cells modulating and controlling the immune system balance.
DISCLOSURESThe authors declare no financial conflicts of interest.