Editado por: Óscar F. Gonçalves
Más datosThe present study aimed to establish and develop an online de novo conditioning paradigm for the measurement of conditioned disgust responses. We further explored the effects of explicit instructions about the CS-UCS contingency on extinction learning and retrieval of conditioned disgust responses.
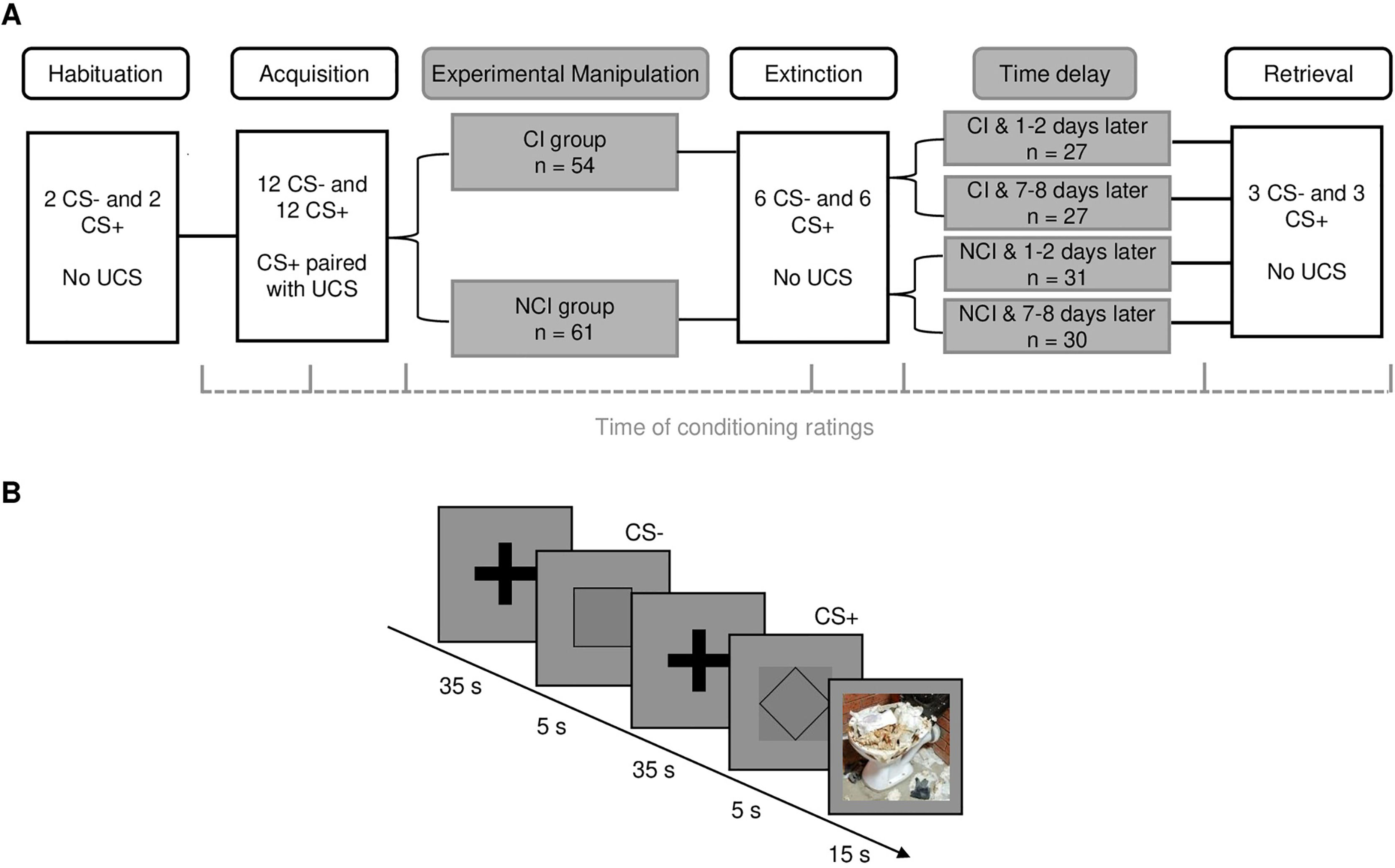
MethodThe study included a sample of 115 healthy participants. Geometric figures served as conditioned stimuli (CS) and disgust-evoking pictures as unconditioned stimuli (UCS). During disgust conditioning, the CS+ was paired with the UCS (66% reinforcement) and the CS- remained unpaired; during extinction and retrieval, no UCS was presented. Half of the participants (n = 54) received instructions prior to the disgust extinction stating that the UCS will not be presented anymore. 1-2 days or 7-8 days later participants performed a retrieval test. CS-UCS contingency, disgust and valence ratings were used as dependent measures.
ResultsSuccessful acquisition of conditioned disgust response was observed on the level of CS-UCS contingency, disgust and valence ratings. While some decline in valence and disgust ratings during the extinction stage was observed, contingency instructions did not significantly affect extinction performance. Retrieval one week later revealed that contingency instructions increased the discrimination of the CSs.
ConclusionsExtinction of conditioned disgust responses is not affected by explicit knowledge of the CS-UCS contingencies. However, contingency instructions prior to extinction seem to have a detrimental effect on long-term extinction retrieval.
Conditioning processes contribute to the development and maintenance of anxiety disorders. De novo fear conditioning paradigms allow the assessment of differences in acquisition and extinction of conditioned fear in patients with anxiety disorders and high-risk individuals (Blechert et al., 2007; Norrholm et al., 2011; Orr et al., 2000). Exposure therapy, which is the first-choice treatment for anxiety disorders, is presumably based on mechanisms related to (fear) extinction learning (Graham & Milad, 2011). Although exposure is certainly effective (Hofmann & Smits, 2008), a subgroup of patients does not respond to this form of intervention or show a relapse of symptoms over time (Craske & Mystkowski, 2006). Thus, there is a need of improving the effects of exposure.
Patients with anxiety disorders (Lissek et al., 2005) and high trait-anxious subjects (Caulfield et al., 2013; Mosig et al., 2014) show enhanced conditionability, either evidenced by an enhanced acquisition and/or delayed extinction of conditioned fear responses (Duits et al., 2015). Studies on fear conditioning indicate that explicit knowledge about the CS-UCS association affects the course and magnitude of fear learning (Mertens et al., 2021). Explicit instructions given to participants to increase conscious information about the CS-UCS occurrence have been shown to affect fear acquisition and extinction (reviewed in Luck & Lipp, 2016; Mertens et al., 2018). Providing participants with contingency instructions prior to fear acquisition supports the discrimination between CS+ and CS- and leads to more pronounced fear responding to the CS+ relative to the CS- (Duits et al., 2017; Javanbakht et al., 2017; Mertens et al., 2021). Explicit instructions about the CS-UCS contingency given prior to extinction learning accelerate fear extinction (Wendt et al., 2020). Furthermore, explicit instructions also reduce the strength of the conditioned response to the CS+, which leads to a smaller difference between the responses to the CS+ as compared to the CS- (Hugdahl & Öhman, 1977; Javanbakht et al., 2021; Rowles et al., 2012; Sevenster et al., 2012). The effects on extinction retrieval, however, are generally inconclusive (Duits et al., 2017; Javanbakht et al., 2017; Sevenster et al., 2012; Wendt et al., 2020), and evidence for unconscious fear conditioning without explicit knowledge about the CS-UCS association is rather weak (Mertens & Engelhard, 2020). Nevertheless, altogether, these findings suggest that top down (conscious) processing affects the acquisition and extinction of fear responses (Hofmann, 2008).
In addition to fear, disgust has been proposed to play an equally important role in the psychopathology of different anxiety disorders, in particular, specific phobia (Knowles et al., 2018) and obsessive-compulsive disorder (OCD; see a review by Cisler et al., 2009). Patients with anxiety disorders exhibit enhanced disgust sensitivity (Berle, & Phillips, 2006; de Jong et al., 2002; Schienle et al., 2003) which has been linked to an increased conditionability and poorer extinction of (conditioned) disgust responses (Mason & Richardson, 2010; Olatunji & Tomarken, 2022). Likewise, high contamination concern has been related to higher disgust responses towards the reinforced conditioned stimulus during extinction (Armstrong & Olatunji, 2017). Similar to fear responses, conditioned disgust responses are equally difficult to extinguish (Mason & Richardson, 2010; but see Engelhard et al., and Olatunji (2014); Olatunji et al., and Cherian (2007) and Klucken et al., and Stark (2013) for a detailed picture of results). Counterconditioning has been shown to reduce disgust responses towards the CS+ (Engelhard et al., 2014), whereas other attempts to stimulate disgust extinction by direct exposure to the CS+ (Bosman et al., 2016), by UCS memory devaluation (Mertens et al., 2021) or by cognitive reappraisal of the UCS (Olatunji et al., 2017) were not successful. In contrast to fear conditioning, the neurobiological substrates and cognitive processes mediating disgust conditioning are less well explored (Klucken et al., 2012). For instance, the extent to which contingency awareness, i.e. the ability to consciously recognize the pairing between conditioned stimulus (CS) and unconditioned stimulus (UCS), relates to disgust conditioning remains elusive. The effects of contingency instructions on CS-UCS occurrence during disgust conditioning have not been investigated yet.
Studying the malleability of extinction learning via explicit instructions is of high clinical significance. While previous studies were exclusively conducted under laboratory conditions we asked whether such processes can also be assessed remotely. In the present study, we have investigated the effects of contingency instructions in an online de novo conditioning paradigm for the measurement of conditioned disgust responses. The online version of this task allows remote assessment of disgust acquisition and extinction in healthy participants and clinical populations (see Björkstrand et al., 2022; McGregor et al., 2021; Purves et al., 2019). Here, we investigated whether extinction of disgust responses and retrieval of disgust responses can be modified by conscious top down processes. To this end, we used explicit instructions about the CS-UCS contingency prior to the extinction of disgust responses. Conditioning responses were measured during acquisition, extinction and retrieval by using different indices, CS-UCS contingency, disgust and valence ratings. Lastly, to examine the effects of explicit instructions prior to extinction on short and long-term changes of conditioned disgust responses, half of the participants performed the extinction retrieval test 1-2 days after extinction and the other half 7-8 days after.
MethodsParticipantsParticipants were recruited via the psychology faculty's website and through advertisements on social media. Participation was restricted to healthy participants who had no current mental or neurological diseases. Out of the original sample (n = 156), we excluded participants who did not complete the second part of the study within the allowed time frame (n = 8) and who did not learn the contingencies after disgust acquisition (n =33). Here, a participant who rated the CS- as more/equally likely to be paired with the UCS than/as the CS+ was characterized as a “non-learner”. The final sample, thus, comprised 115 participants, out of which 54 participants received CS-UCS contingency instructions prior to fear extinction learning, whereas 61 participants did not. In the sample, 68% of the participants were female and were aged between 18 and 64 years (M = 28.4; SD = 10.62). All participants provided their informed consent online and as participation compensation, students received research hours and other participants received an Amazon gift card (with a value of 15 Euro). All experimental procedures were approved by the local ethics committee of the Ruhr University Bochum (approval no. 772) and were carried out in accordance with the principles outlined by the Declaration of Helsinki.
Disgust conditioningThe disgust conditioning took place online on the platform Pavlovia (https://pavlovia.org/), which is based on the open-source package PsychoPy (Peirce et al., 2019). Two black geometric figures (square and rhombus) served as initially neutral stimuli for paired or unpaired conditioning (CS+ and CS−, respectively). The allocation of the geometric figures to serve as CS+ or CS- was quasi-randomized and counterbalanced across the participants. The CS+ and CS- were presented for 5 seconds on a grey background with an intertrial interval of 35 seconds. Eight pictures selected from the Disgust-Related-Images (DIRTI) database (Haberkamp et al., 2017) served as the UCS. Pictures covered the categories a) food (e.g. spoiled food), b) body products (e.g. feces), injuries/infections (e.g. skin rashes, lesions) and c) hygiene (e.g. dirty bathrooms). Based on the participants’ ranking in the study of Haberkamp and colleagues (Haberkamp et al., 2017) the two most disgust-evoking pictures from each category were chosen for the present study. The UCSs were presented immediately after the presentation of the paired but not the unpaired stimulus for 15 seconds (see Fig. 1B).
A. Disgust Conditioning Paradigm. The experiment started with a habituation phase, then the disgust acquisition took place. Half of the participants received contingency instructions prior to the extinction, whereas the other half did not. The second part of the study (retrieval) was performed within 1-2 days or one week (7-8 days) later. B. Example trials of CS+ and CS-.
Disgust conditioning comprised four phases: habituation (presentations of the 2x CS+, 2x CS−), acquisition (12x CS+, 12x CS−), extinction (6x CS+, 6x CS−), and retrieval (3x CS+, 3x CS−). During acquisition, the CS+ was paired with the UCS at a 66% rate. The intermittent CS-UCS pairings during acquisition are known to slow fear extinction which allows for examining differences in learning rates in a more detailed manner. During acquisition, the CS- was never paired with the UCS (adapted from Armstrong & Olatunji, 2017). After fear acquisition, the UCS was no longer applied. Overall, there were three different pseudo-randomized orders of presenting the stimuli in which no more than two consecutive presentations of the same CS were allowed. The orders were randomly assigned to the participants and had no effect on the CS differentiation in any of the conditioning measures during disgust acquisition (CS-UCS contingency: F(1,113) = 0.300, p = 0.585; CS valence: F(1,113) = 0.654, p = 0.420; disgust: F(1,113) = 0.349, p = 0.556; ANOVA).
Experimental manipulationPrior to the extinction phase, participants were randomly assigned to receive either CS-UCS contingency instructions (CI) or no information on whether the CS will be followed by the UCS or not (NCI). The CI group was informed that “the experiment will be continued but no real pictures will be shown anymore.”, whereas the NCI group was instructed that “the experiment will be continued and that they should keep looking at possible connections between stimuli and real pictures.”
Conditioning measuresThe CS valence, disgust and CS-UCS contingency ratings were collected repeatedly via visual analogue scales that have been presented on the screen, i.e. before, in the middle and after the acquisition and extinction phase, as well as before and after the retrieval session. For the CS valence, participants had to place a mark on a line anchored from 0 = “very pleasant” to 100 = “very unpleasant”. The disgust rating was scored between 0 = “the geometric figure evokes no disgust” and 100 = “the geometric figure evokes a lot of disgust”. For the CS-UCS contingency rating, participants rated how likely it is that the geometric figure is paired with a disgust-evoking picture on a scale from 0 = “very unlikely” to 100 = “very likely”.
QuestionnairesThe Intolerance of Uncertainty (IU) scale (Gerlach et al., 2008) contains 18 items and measures a person's degree of vigilance, burden and disturbed action ability when experiencing uncertain situations. The items are scored on a 5-point scale from 1 = “not at all characteristic of me’ to 5 = “entirely characteristic of me” and higher IU scale scores are related to higher intolerance of uncertainty. High IU supposedly plays an important role in maintaining fear and anxiety and is related to poorer fear extinction (Morriss et al., 2021). Its relation to disgust sensitivity or disgust extinction has not been shown yet.
The Obsessive-Compulsive Inventory-Revised (OCI-R; Gönner et al., 2007) consists of 18 items and measures the six major symptoms of an obsessive-compulsive disorder: checking, washing, ordering, hoarding, obsessing and neutralizing. The items are measured on a 5-point scale from 0 = “not at all” to 4 = “extremely”. Symptoms of OCD have been shown to be related to heightened disgust sensitivity (Knowles et al., 2018). Therefore, it might be related to increased disgust conditioning and impaired disgust extinction.
To measure symptoms of depression, anxiety and stress over the last week, we used the short version of the Depression, Anxiety and Stress Scale (DASS-21; Nilges & Essau, 2015). The DASS-21 contains 21 items that are scored on a 4-point scale from 0 = “did not apply to me at all” to 3 = “applied to me very much, or most of the time”. The questionnaire has the following cut-off values for the different domains: depression: 10, anxiety: 6 and stress: 10. These cut-off values are not serving as a diagnosis, they rather are an indication of a potential, although not verified, pathological condition.
Disgust sensitivity was measured by the Assessment of Disgust Sensitivity Questionnaire (Schienle et al., 2002). Heightened disgust sensitivity is defined by easily provoked, prolonged and intense disgust reactions. These reactions are measured on five dimensions: death, body secretions, hygiene, spoilage and oral rejection. The assessment describes 37 potentially disgusting situations and the participants rate their personal disgust reaction on a 5-point scale from 0 = “not disgusting” to 4 = “extremely disgusting”.
ProcedureThe study was performed in an online format and performed on two days (see Fig. 1A). The participants received information about the study and had the opportunity to ask questions about the experiment. On the first day, the participants gave consent and completed the questionnaires using Qualtrics software (Version May 2022; https://www.qualtrics.com). After that, participants were redirected to Pavlovia to complete the habituation, disgust acquisition and extinction phases of the experiment. In the instructions prior to the acquisition, the participants were informed that two geometric figures will be presented and that one of them may sometimes be paired with realistic pictures. The extinction phase followed directly after acquisition, which is in accordance with a previous study by Armstrong and Olatunji (2017). At the end of day one, the participants were divided into two groups, one group was invited to the second part of the study performed one day later and the other group was invited to perform the experiment one week later (some participants completed the second part one day after the invitation). In the second part of the study, participants completed the retrieval phase.
Statistical procedureStatistical analyses were carried out with RStudio (RStudio Team, 2022). The effect of the disgust conditioning paradigm and experimental manipulation during habituation, acquisition and extinction were examined using mixed ANOVAs with CS type (two levels: CS- vs. CS+) as within-subjects factor and group (four levels: CI group & 1 day later, CI group & 1 week later, NCI group & 1 day later, NCI group & 1 week later) as between-subjects factors. Post-hoc tests were performed if either significant main effects of CS type, group or significant CS type x group interactions were found. Within- and between group comparisons of CS-UCS contingency, valence and disgust ratings were performed with t-tests for dependent and independent samples. Paired t-tests between the difference scores of CS+ and CS- were used to investigate the changes across different experimental stages. Unpaired t-tests were performed to determine between-group effects during specific test phases. A mixed ANOVA main or interaction effect was considered significant when p-values smaller than 0.05 were obtained. For the post-hoc tests a Bonferroni correction was used for alpha-adjustment and results were considered to be significant when p-values smaller than p = 0.016 were found.
ResultsParticipant characteristicsIn order to determine potential differences between the instruction groups that might be caused by the randomization procedure, we compared the participant characteristics. Age (NCI group: M = 28.25, SD = 9.92; CI group: M = 28.57, SD = 11.63, p = 0.870) and gender ratio (%female: NCI group: 62%; CI group: 76%, X2 (1, n = 115) = 3.01, p = 0.222) were both similar in the two groups. Furthermore, the DASS-21 subscales depression (NCI group: M = 3.9, SD = 4.15; CI group: M = 4.13, SD = 4, p = 0.763), anxiety (NCI group: M = 2.36, SD = 3.11; CI group: M = 2.5, SD = 3.13, p = 0.812) and stress (NCI group: M = 5.82, SD = 4.47; CI group: M = 5.57, SD = 4.11, p = 0.761) were comparable for the groups. Groups were also comparable with regard to disgust sensitivity (NCI group: M = 77.34, SD = 25.25; CI group: M = 83.04, SD = 23.24, p = 0.213), intolerance of uncertainty (NCI group: M = 48.64, SD = 13.58; CI group: M = 46.91, SD = 14.03, p = 0.503) and obsessive-compulsive disorder symptoms (NCI group: M = 13.39, SD = 11.06; CI group: M = 15.07, SD = 10.59, p = 0.408).
HabituationAfter habituation, the disgust ratings for CS+ and CS- did not differ for the NCI groups (1-2 days later: CS+: M = 4.167, SD = 10.202; CS-: M = 6.667, SD = 12.472 and 7-8 days later: CS+: M = 5.700, SD = 11.894; CS-: M = 4.767, SD = 11.717) and CI groups (1-2 days later: CS+: M = 8.963, SD = 20.148; CS-: M = 2.296, SD = 4.697 and 7-8 days later: CS+: M = 6.481, SD = 14.805; CS-: M = 2.259, SD = 6.407; F(3,110) = 0.078, p = 0.972; ANOVA). For the valence ratings, there were group differences in the ratings for CS+ and CS- (group x CS type interaction: F(3,110) = 7.269, p < 0.001; ANOVA). The CI group that performed the retrieval after 7-8 days rated the valence of the CSs differently (CS+: M = 39.222, SD = 23.712; CS-: M = 17.037, SD = 16.681), whereas the other groups did not (NCI group 1-2 days later: CS+: M = 34.300, SD = 23.612; CS-: M = 37.500, SD = 22.833 and 7-8 days later: CS+: M = 31.800, SD = 21.011; CS-: M = 32.267, SD = 17.605; CI group 1-2 days later: CS+: M = 37.556, SD = 24.499; CS-: M = 27.111, SD = 16.752).
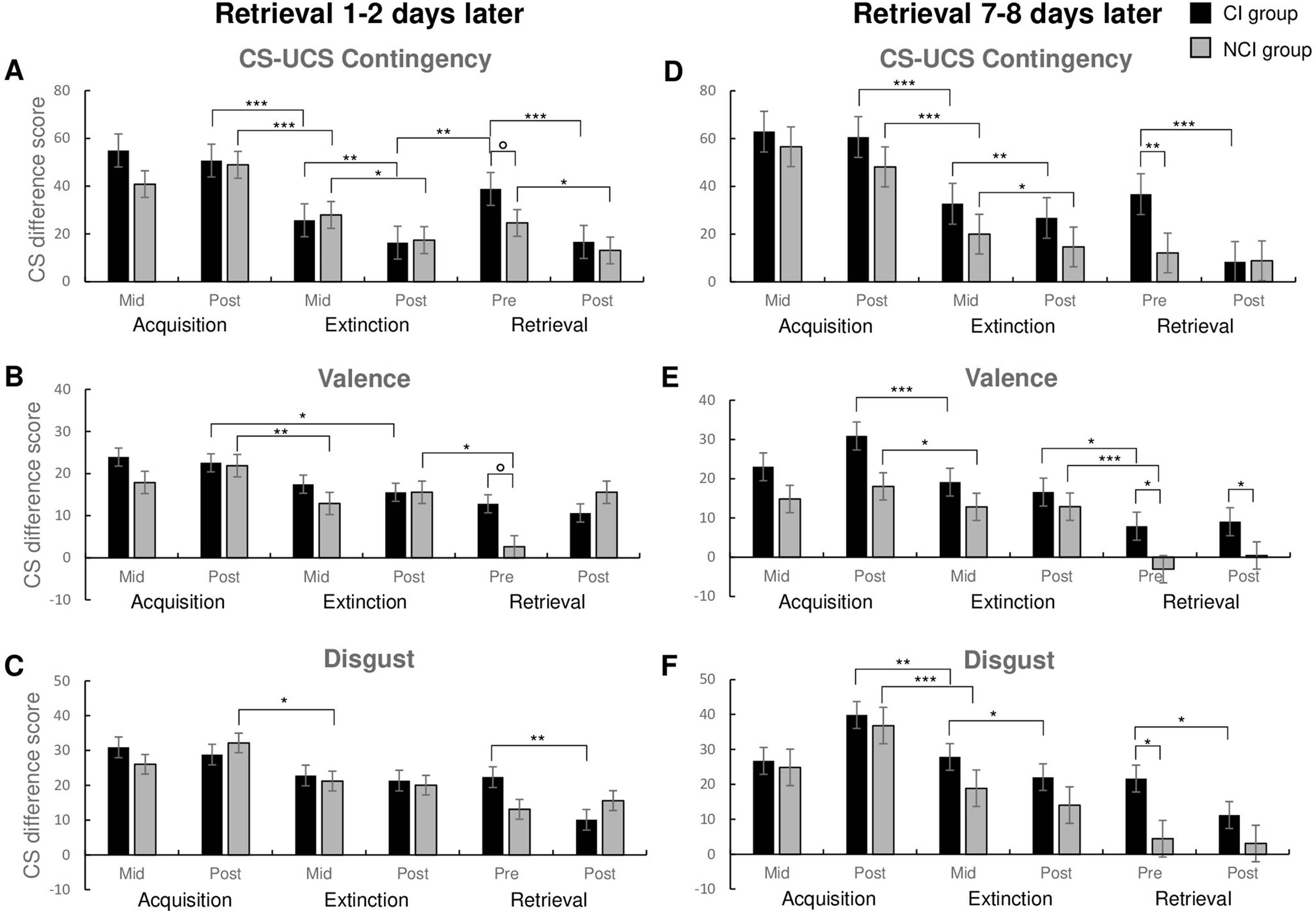
Disgust acquisitionAn overview of the discrimination performance during disgust acquisition, extinction and retrieval is presented in Fig. 2 and Table 1. After the first half of the conditioning trials, the participants indicated that the presentation of the CS+ was more likely to be followed by the UCS as compared to the presentation of the CS- (Mid, main effect of CS type: F(1,111) = 243.078, p < 0.001; ANOVA; Fig. 2A & D). The CS+ was also rated as being less pleasant (Mid, main effect of CS type: F(1,110) = 64.133, p < 0.001; Fig. 2B & E), and more disgusting as compared to the CS- (Mid, main effect of CS type: F(1,106) = 96.697, p < 0.001; Fig. 2C & F). This negative evaluation of the CS+ persisted until the end of the acquisition training (Post, main effects of CS type: CS-UCS contingency ratings: F(1,111) = 348.415, p < 0.001; Fig. 2A & D; valence ratings: F(1,111) = 84.624, p < 0.001; Fig. 2B & E; disgust ratings: F(1,110) = 118.984, p < 0.001; Fig. 2C & F). Overall, there were no significant between-group differences during the acquisition stage of the experiment.
Mean difference scores of the CS-UCS contingency, valence and disgust ratings at the middle and end of disgust acquisition and extinction and before and after retrieval in the CI and NI group one or two days and one week (7-8 days) later. p < 0.1. *p < .05. ** p < .01. ***p < .001. Error bars represent standard error.
Means (SDs) for conditioning responses to the CSs on a 100-point scale.
In the middle of disgust extinction, the two CSs were still differentiated based on the CS-UCS contingency ratings (main effect of CS type: F(1,111) = 107.036, p < 0.001; ANOVA; Fig. 2A & D), as well as valence (main effect of CS type: F(1,111) = 65.820, p < 0.001; Fig. 2B & E) and disgust ratings (main effect of CS type: F(1,110) = 82.233, p < 0.001; Fig. 2C & F). Thereafter, CS+ vs. CS- discrimination slightly decreased from mid to post extinction in terms of CS-UCS contingency ratings (as can be inferred from the decline in the difference scores; Fig. 2), but CS+ vs. CS- discrimination still remained significant for CS-UCS contingency (F(1,111) = 63.100, p < 0.001; Fig. 2A & D), valence (F(1,111) = 61.081, p < 0.001; Fig. 2B & E) and disgust ratings (F(1,111) = 73.767, p < 0.001; Fig. 2C & F). Comparison of the difference scores (CS+ rating minus CS- rating) between the mid acquisition and extinction stage in the CI group revealed a significant decrease in CS type discrimination performance for CS-UCS contingency ratings (t(53) = 4.327, p < 0.001), whereas no significant decline was found in the valence and disgust ratings (valence: t(53) = 1.670, p = 0.101; disgust: t(53) = .988, p < 0.328). In the NCI group, there was a significant decrease in all conditioning measures between the mid acquisition and extinction stage (t(60) = 5.570, p < 0.001; valence: t(60) = 1.391, p < 0.169; disgust: t(60) = 2.591, p = 0.012). However, a comparison of the difference scores between the post acquisition and extinction stage in both groups revealed a significant decrease in CS type discrimination performance for all conditioning measures (CI group: CS-UCS contingency: t(53) = 7.984, p < 0.001; valence: t(53) = 3.748, p < 0.001; disgust: t(53) = 3.264, p = 0.002; T-test for dependent samples. NCI group: CS-UCS contingency: t(60) = 8.293, p < 0.001; valence: t(60) = 2.591, p < 0.012; disgust: t(60) = 4.885, p < 0.001; T-test for dependent samples; Fig. 2). Furthermore, no significant effects of the instruction condition was found during the extinction stage of the experiment (CI vs. NCI: Mid ratings, CS-UCS contingency: t(113) = -1.009, p = 0.315; valence: t(113) = -1.438, p = 0.153; disgust: t(113) = -1.064, p = 0.289; Post ratings, CS-UCS contingency: t(113) = -1.170, p = 0.245; valence: t(113) = -0.969, p = 0.335; disgust: t(113) = -1.030, p = 0.305; T-test for independent samples).
Retrieval 1-2 days laterAn overview of the discrimination performance during the retrieval stage is presented in Fig. 2. In the CI group, the pre-retrieval CS-UCS contingency ratings were significantly higher as compared to the CS-UCS contingency ratings that have been collected immediately after the completion of the extinction stage (t(26) = -3.547, p = 0.002; T-test for dependent samples; Fig. 2A). In contrast, the pre-retrieval CS-UCS contingency ratings were not significantly different from the post-extinction ratings in the NCI group (t(30) = -1.476, p = 0.150; T-test for dependent samples; Fig. 2A). However, the within-group comparison of the CS-UCS contingency ratings for the CS+ vs. the CS- stimuli was significantly different in both the CI and NCI groups (NCI group: t(30) = 4.021, p < 0.001; CI group: t(52) = 6.768, p < 0.001; t-test for dependent samples; Fig. 2A).
After completion of the retrieval stage, the within-group comparison of the CS+ and the CS- contingency ratings was significant in the CI (t(26) = 4.195, p < 0.001; t-test for dependent samples; Fig. 2A), but not in the NCI group, for which only a trend for a significant difference was observed (t(30) = 1.902, p = 0.067; t-test for dependent samples; Fig. 2A).
A within-group comparison of the NCI participants revealed significantly lower valence rating CS difference scores during the pre-retrieval stage as compared to the post-extinction stage (t(30) = -2.154, p = 0.039; t-test for dependent samples; Fig. 2B). No such effect was found for the CI group (t(26) = 0.897, p = 0.378; t-test for dependent samples; Fig. 2B). A between-group comparison of the valence rating CS difference scores during the pre-retrieval stage suggested a trend for a higher CS type discrimination in the CI as compared to the NCI group (t(56) = -1.969, p = 0.054; T-test for independent samples; Fig. 2B), which however failed to reach the predetermined level of statistical significance.
Furthermore, in the CI group the CS+ vs. CS- valence ratings were significantly different before and after the retrieval phase (pre-retrieval: t(26) = 3.935, p < 0.001; post-retrieval: t(26) = 3.111, p = 0.004; t-test for dependent samples; Fig. 2B), while no such differences were evident in the NCI group (pre-retrieval: t(30) = 1.743, p = 0.092; post-retrieval: t(30) = 1.852, p = 0.074; t-test for dependent samples (Fig. 2B). Both groups rated the CS+ as being more disgusting, as compared to the CS-, both before (NCI group: t(30) = 2.628, p = 0.013; CI group: t(30) = 5.018, p < 0.001; t-test for dependent samples; Fig. 2C) and after the retrieval session (NCI group: t(30) = 3.129, p = 0.004; CI group: t(30) = 2.74, p = 0.011; Fig. 2C). However, only the CI group exhibited a significant decrease in CS-type difference scores from pre- to post-retrieval disgust ratings (CI: t(30) = -3.700, p = 0.001; NCI: t(30): -0.724, p = 0.475; Fig. 2C).
Retrieval 7-8 days later7-8 days after extinction, during the pre-retrieval stage, the CS-UCS contingency rating difference scores of the CI group were significantly higher as compared to the NCI group (t(55): -2.783, p = 0.007; t-test for independent samples, Fig. 2D). Within-group comparisons of the CS-UCS contingency ratings from the pre-retrieval to the post-retrieval stage indicated a significant decline in the CI (t(26): 5.122, p < 0.001; t-test for dependent samples; Fig. 2D), but not in the NCI group (t(29): 0.740, p = 0.465; t-test for dependent samples; Fig. 2D). The CS-UCS contingency rating difference scores of the NCI remained at a very low level. Additionally, within-group comparisons of the CS-UCS contingency ratings for the CS+ vs. the CS- during the pre-retrieval stage were only significantly different in the CI group (t(25): 4.851, p < 0.001; t-test for dependent samples; Fig. 2D), but not in the NCI group (t(29): 2.277, p = 0.030; Fig. 2D). After the completion of the retrieval trials the CS-UCS contingency ratings for the CS+ vs. the CS- stimuli were no longer significantly different neither in the CI nor in the NCI group (CI group: t(25): 1.768, p = 0.089; NCI group: t(29): 2.038, p = 0.051; Fig. 2D).
In the NCI group, a significant decrease in CS-type difference scores for the valence ratings was found during the pre-retrieval stage as compared to the post-extinction stage (t(29): 3.949, p < 0.001; t-test for dependent samples; Fig. 2E), whereas no difference was found in the CI group (t(26): 2.182, p = 0.038; Fig. 2E). Both groups exhibited similar CS-type difference scores during the pre-and post-retrieval stage (Pre: t(42.141): -2.148, p = 0.037; Post: t(55): -2.320, p = 0.024; t-test for independent samples; Fig. 2E).
Furthermore, within-group comparisons revealed that the CI group exhibited higher negative valence ratings for the CS+ as compared to the CS- during the pre-retrieval stage (t(25): 3.141, p = 0.004; Fig 2E), whereas the NCI group showed no CS differentiation (t(29): 0.980, p = 0.335; Fig. 2E). Neither CI nor the NCI group, displayed higher negative valence ratings for the CS+ as compared to the CS- during the post-retrieval stage (CI group: t(25): 1.886, p = 0.071; NCI group: t(25): 0.404, p = 0.689; Fig. 2E).
During the pre-retrieval stage, participants of the CI group rated the CS+ as being more disgusting than the CS- (t(25): 3.773, p < 0.001; T-test for dependent samples; Fig. 2F), whereas the NCI group did not differentiate between the CSs (t(29): 1.075, p = 0.291; Fig. 2F). The CI group also showed significantly higher CS-type difference scores for the disgust ratings during the pre-retrieval stage as compared to the NCI group (t(25): -2.575, p = 0.013; T-test for independent samples; Fig. 2F). The CS-type difference scores of both groups did not change from the pre- to the post-retrieval stage (CI group: t(26): 2.173, p = 0.039; NCI group: t(29): 0.773, p = 0.446; T-test for dependent samples; Fig. 2F). During the post-retrieval stage the CS+ was no longer rated as being more disgusting than the CS- in the NCI group (t(29): 0.921, p = 0.365; T-test for dependent samples; Fig. 2F), whereas in the CI group the CS+ was still rated as being more disgusting than the CS- (t(25): -2.575, p = 0.004; Fig. 2F).
DiscussionSummary of findings and general implicationsIn the present study, a novel de novo conditioning paradigm was developed for the measurement of conditioned disgust responses in an online setting. Our results indicate that our task is suitable to induce de novo disgust conditioning in a remote online paradigm. The results are comparable to findings from existing online fear conditioning (e.g. Björkstrand et al., 2022; McGregor et al., 2021; Purves et al., 2019) or threat conditioning tasks (Zlomuzica et al., 2022). To our best knowledge, no online studies on disgust conditioning exist so far. During the acquisition phase, participants discriminated between CS+ and CS- throughout all conditioning measures: CS-UCS expectancy, valence and disgust. In particular, the CS+ was rated more likely to be paired with a UCS, more unpleasant and disgusting than the CS- after the disgust acquisition, which suggests that the disgust conditioning was successful. Subjective valence and disgust ratings (expressed as CS difference scores) of the participants suggest that a significant and stable conditioned response is measurable already after 6 CS-UCS pairings (mid acquisition stage) and that there is no further increase in the conditioned response after 6 more CS-UCS parings (post acquisition stage). However, a small proportion of participants did not detect the contingencies between the CSs and the UCS during acquisition. Future studies should therefore test to which extent differences in reinforcement rate or frequency of CS presentation influence the performance in our newly developed task.
Contingency ratings of the participants, included in our final analysis, suggested that they were aware of the specific relationship between CS+/CS- and UCS presentations. The speed of acquisition during disgust conditioning seems to be at least equal, if not superior, to online fear conditioning protocols (Björkstrand et al., 2022; McGregor et al., 2021). The strong disgust conditioning response established during the acquisition phase, apparently, also leads to a higher resistance to extinction (at least if the extinction phase immediately follows the acquisition phase and the memory trace strength for the CS-UCS association is still at its maximum peak). Although the valence and disgust ratings during the extinction stage were, in general, lower as compared to the acquisition stage, they nevertheless remain at a relatively high level with little change between early (mid) and late (post) extinction stages. In sum, these results suggest that the CS+ (as compared to the CS-) was persistently evaluated as highly unpleasant and disgusting. This is another similarity to online fear conditioning which is characterized by high resistance to extinction (Björkstrand et al., 2022; McGregor et al., 2021). Furthermore, there was a significant decline in the CS+ ratings one week (7-8 days) later after retrieval with no differentiation between the CSs anymore in all conditioning measures. This pattern of responses implies that extinction of subjective disgust is present, but might be delayed and therefore needs more time and trials to be fully extinguished. While the CI and NCI group did not differ during extinction learning, assessing the magnitude and time course of disgust extinction and retrieval in patients with anxiety disorders would be highly informative. It has previously been shown that poor fear extinction and retrieval is predictive of a less favorable therapy exposure accomplishment and efficacy in specific phobia (Ball et al., 2017; Forcadell et al., 2017; Mason & Richardson, 2010; Raeder et al., 2020). Studies exploring the functional relation between disgust extinction and exposure therapy outcome in patients with specific phobias do not exist. This is surprising given that disgust reactions are less well extinguished and processed during exposure therapy (e.g. Preusser et al., 2017).
Both, the acquisition and extinction of fear seem to rely on a number of cognitive top down processes (Hofmann, 2008). Contingency instructions, which might induce conscious processing of contingency information (Dere et al., 2020; Hütter et al., 2012; Luck & Lipp, 2016; Mertens & Engelhard, 2020), have been studied as a way of influencing fear acquisition, extinction and return of fear. In our study, the announcement that no disgust pictures (UCS) will be presented during the extinction phase had no significant effect on the contingency ratings in the extinction phase, since informed and non-informed groups showed similar contingency ratings. The instruction had also no significant effect on the valence and disgust ratings. However, the instruction had an effect on the contingency, valence and disgust ratings collected prior to the retrieval test performed one week later. Here, the informed group showed significantly higher contingency, valence and disgust ratings as compared to the non-informed group. This effect was also present, but somewhat weaker when the ratings were made 1-2 days after the acquisition stage. Interestingly, the non-informed group showed significant levels of extinction during the retrieval trial one week later as compared to the group that received the retrieval trial 1-2 days later. No such effect of delay between extinction and retrieval has been observed for the informed group. Thus, our results suggest that the instruction given prior to the extinction session (informing participants that in what follows no disgust pictures (UCS) will be presented) has an acute decelerating or inhibitory effect on the subsequent extinction process. Several explanations are conceivable for this phenomenon: The instruction either induces the immediate recall of the disgust pictures and stimulates the re-processing of the association of the relationship between CS+ and UCS. This could lead to additional consolidation and strengthening of the CS+-UCS memory trace, which consequently would increase the resistance to extinction. The instruction might also slow down extinction learning, block forgetting during the delay between extinction and retrieval trials or counteract the effects of post-acquisition latent extinction learning (Miller et al., 2022). The latter refers to the possibility that the conditioned response further weakens in-between extinction trials due to the replay of events in the brain structures that are part of the brain defence system (Chen et al., 2009; Fanselow, 1994; Kaefer et al., 2022).
Conscious vs. implicit fear extinction learningA recently formulated theory on conscious information processing, the platform theory, holds that the brain enters a conscious operation mode, whenever mental representations of stimuli (that includes spatio-temporal contingencies), associations, concepts, memories, and experiences are effortfully maintained (in working memory) and actively manipulated to generate an adaptive response to acute challenges in the environment (Dere et al., 2020; Zlomuzica & Dere, 2022). It is conceivable that the contingency instruction given prior to the extinction experience has induced a conscious information processing mode in the instructed group that re-activated the memories of the disgust pictures, as well as the learned CS-UCS contingencies, to be maintained in working memory and to be reprocessed in the light of the following experiences in the extinction session. The findings of the current study indicate that the extinction of disgust responses cannot be influenced by contingency instructions, which probably reactivate recent disgust experiences previously learned CS-UCS contingencies and stimulate conscious processing of contingency information. The group that gained explicit knowledge about the contingencies before extinction displayed the same conditioning ratings as the non-informed group and therefore did not show enhanced extinction learning. It seems that interventions that increase conscious information processing during extinction learning trials do not facilitate the extinction process and might on the contrary disturb this process as evidenced by the subjective ratings made one week after the extinction session.
Previously, it has been shown that the induction of awareness or insight into stimuli contingencies before fear extinction diminishes the discrimination between the CS+ and CS- (Hugdahl & Öhman, 1977; Javanbakht et al., 2021; Rowles et al., 2012) and accelerates the learning rate (Wendt et al., 2020). However, the studies are not directly comparable since different methods of extinction induction and measurement were used. Studies using valence ratings show that they do not reach full extinction nor do the instructions enhance the effectiveness of extinction (Luck & Lipp, 2015a; Luck & Lipp, 2015b; Wendt et al., 2020). On the other hand, it has been demonstrated that contingency instructions strengthen the discrimination between the fearfulness ratings towards the CSs (Duits et al., 2017) and the CS-UCS expectancy ratings (Morriss & van Reekum, 2019; Scheveneels et al., 2019). Interestingly, CS-UCS expectancy and electrodermal activity are influenced by contingency awareness, whereas fear-potentiated startle is not (Sevenster et al., 2012; Sevenster et al., 2014). Based on these findings, it seems also possible that evaluative measures, such as valence and disgust responses might be less modifiable during extinction by contingency instructions.
Unlike extinction, retrieval was affected by the contingency instructions in the present study. The CI group exhibited higher conditioned disgust responses towards the CS+ than the NCI group after one week. Only a few fear conditioning studies examined the effect of contingency instructions on the reinstatement of fear and revealed mixed findings depending on the timing of the retrieval test. Performing retrieval directly after extinction leads to smaller fear responding towards both CSs (Scheveneels et al., 2019), whereas performing it one day later, instructions have no effect (Javanbakht et al., 2021) or even increase fear when the threat of the UCS is present (e.g. by re-attaching the electrode; Wendt et al., 2020). Likewise, the current study showed no significant differences between the instruction groups after 1-2 days. However, retrieval one week later, revealed that receiving contingency instructions was associated with significantly higher conditioned disgust responses towards the CS+, indicating a preservation of the disgust response over the retention delay of one week at the level of the post-extinction stage. This is in accordance with a previous finding that safety signals given before the CS+ during extinction learning lead to a higher return or latent perseveration of fear when the CS+ was presented without the safety signal (Lovibond et al., 2000). Applying this to the present study, the contingency instructions prior to the disgust extinction might have acted as a safety signal and when it was not presented again, the participants became unsure of the contingencies. Therefore, they might have developed higher UCS expectancy and higher conditioned disgust responses. In this case, the instructions might serve as an occasion-setter and as more time passes the cognitive distinction between the occasions (extinction and retrieval) increases. In the clinical context, this could be the equivalent of safety behaviors that hinder exposure therapy success (Dunsmoor et al., 2015).
Conclusions and perspectivesWe have developed a remote online task for de novo conditioning of disgust responses. Our results confirm that disgust responses can be conditioned with neutral and disgust-irrelevant stimuli (see also Klucken et al., 2013). This is important because de novo conditioning can be used to reveal differences in general conditionability and the propensity for generalization in patients with high-trait anxiety, patients with different anxiety disorders or related disorders such as OCD (Blechert et al., 2007; Mosig et al., 2014; Olatunji et al., 2007; Orr et al., 2000). The remote online disgust conditioning paradigm might be further used to predict treatment outcomes based on individual disgust extinction performances (e.g. Forcadell et al., 2017; Raeder et al., 2020). Our findings indicate that the current protocol is suitable to detect acquisition of conditioned disgust responses. However, a small proportion of participants failed to learn the contingencies between the CSs and the UCS during acquisition, so more experimental work (including modifications of the original protocol) is warranted. Nevertheless, we found that contingency instructions had no effect on the extinction of disgust, but seemed to hamper the extinction retrieval. This was the first study that examined retrieval of disgust extinction after a longer time delay. Our findings generally support the conclusion that conditioned disgust responses can be retrieved 1-2 days and even 7-8 days later. Finally, in the present study, all readouts that have been used to evaluate the effect of the instruction on the disgust response used were based on explicit ratings. However, in order to generalize our findings to other implicit measures, future studies should test the same approach with additional psychophysiological measures, e.g. pupil dilatation and startle responses or electromyography (EMG). It has been shown that startle responses do not require contingency awareness during fear conditioning, whereas conditioned electrodermal responses critically rely on awareness of CS-UCS associations (Sevenster et al., 2014). Furthermore, while our results indicate that some (e.g. explicit and verbal) measures might be influenced by contingency instructions, this might be different for other measures (e.g. implicit measures). For example, skin conductance responding might be more influenced by instructions than subjective CS valence ratings (Luck & Lipp, 2015a; Wendt et al., 2020). In future studies, the use of additional readouts such as implicit measures and psychophysiological measures (i.e. EMG or startle) which are less sensitive to contingency information might help to decipher the role of contingency awareness on different levels (physiological, cognitive, emotional, and behavioral) of disgust extinction.
The study was funded by the Deutsche Forschungsgemeinschaft (DFG; German Research Foundation – project number: 316803389 - SFB1280; project A13 and projects ZL 59/4-1 and ZL 59/5-1). The funders had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
El conocimiento consciente de la información de contingencia CS-UCS afecta a la recuperación de la extinción de las respuestas de asco condicionadas: Resultados de una tarea de condicionamiento de asco online de novo