Fear acquisition and fear extinction are the most widely used experimental models to study anxiety related disorders, with the medial prefrontal cortex (mPFC) playing an important role in this process. Previous research suggests that trait self-compassion is associated with lower anxiety, but the neural mechanisms underlying this relationship remain unclear. Women generally exhibit lower self-compassion than men, making them more vulnerable to fear and anxiety. In this study, female participants were divided into two groups - high and low trait self-compassion, based on their scores on the Self-Compassion Scale-Short Form (SCS-SF). Both groups completed fear acquisition and fear extinction tasks, during which conditioned responses (CRs) were measured using self-reported unconditioned stimulus (US) expectancy ratings, skin conductance response (SCR), and functional near-infrared spectroscopy (fNIRS). The results showed that in the fear acquisition phase, all participants successfully acquired fear, showing greater responses to threat stimuli than safety stimuli. However, participants with high trait self-compassion exhibited lower SCR than those with low trait self-compassion. In the fear extinction phase, compared to individuals with low trait self-compassion, individuals with high trait self-compassion exhibited more effective fear extinction learning, characterized by lower US expectancy ratings, lower SCR, and higher mPFC activation. Moreover, trait self-compassion was significantly correlated with the behavioral extinction ability and the mPFC activation during the late phase of fear extinction, and behavioral extinction ability was significantly correlated with mPFC activation. The findings of this study suggest individuals with high trait self-compassion have better physiological flexibility during fear acquisition and fear extinction, and may through enhancing mPFC activation to facilitate fear extinction. The results provide new insights into the pathological mechanisms of anxiety.
Anxiety is a common negative emotion experienced in daily life, manifested as persistent fear, high arousal, and continuous vigilance (Tovote, Fadok & Lüthi, 2015). While moderate levels of anxiety can be adaptive, helping individuals recognize threats and respond to them (Kenwood, Kalin & Barbas, 2022; Saviola et al., 2020), prolonged and excessive anxiety can significantly impair a person's ability to study, work, and live effectively. Pavlovian fear conditioning is the most widely used experimental model for understanding anxiety-related disorders. This model allows researchers to examine how fear responses are acquired and subsequently attenuated or eliminated through controlled experimental settings. The process consists of two primary phases: fear acquisition and fear extinction. During fear acquisition, a neutral conditioned stimulus (CS+) is repeatedly paired with an aversive unconditioned stimulus (US), which evokes a fear-related conditioned response (CR) such as increased heart rate, blood pressure, or sweating when the CS is presented alone (Beckers et al., 2023). The other stimuli that are never accompanied by the US are the safety stimuli (CS−). Fear extinction occurs when the CS is presented repeatedly without the US, leading to a gradual reduction in the fear response (Herry et al., 2010). Research has consistently shown that exaggerated fear acquisition (Barrett & Armony, 2009; Dibbets, van den Broek & Evers, 2015; Greco & Liberzon, 2016) and impaired fear extinction are hallmarks of anxiety disorders and contribute to the persistence of anxiety over time (Abend et al., 2020; Barrett & Armony, 2009; Giustino & Maren, 2015; Treanor, Rosenberg & Craske, 2021; Vriends et al., 2011).
Self-compassion (SC) is strongly associated with well-being, with greater self-compassion associated with positive mental health outcomes and reduced psychopathology (MacBeth & Gumley, 2012; Muris & Petrocchi, 2017). Self-compassion refers to how individuals treat themselves in the face of failure, pain, or personal struggles (Neff, 2003a). Self-compassion involves accepting and integrating negative emotions with self-kindness, common humanity, and mindfulness, which may facilitate cognitive reappraisal and support for flexible emotional regulation (Allen & Leary, 2010; Breines & Chen, 2012; Neff & Dahm, 2015). Studies have consistently shown that individuals with high trait self-compassion are better able to reduce negative self-related feelings and emotions after stressful and anxiety events, demonstrating greater psychological flexibility in the face of negative life events (Leary, Tate, Adams, Batts Allen & Hancock, 2007; Luo, Qiao & Che, 2018). Moreover, individuals with high trait self-compassion exhibit better physiological stress responses. For instance, they tend to have higher vagally mediated heart rate variability (vmHRV) during both stress and anxiety situations (Breines et al., 2015; Luo et al., 2018; Svendsen et al., 2016, 2020). They also produce lower levels of salivary alpha-amylase, a marker of stress, in stressful situations (Breines et al., 2015). These studies reveal that individuals with high trait self-compassion can cognitively reappraise stress, anxiety, and pressure, allowing them to flexibly regulate their psychological and physiological responses. This highlights the important role of self-compassion as a trait that can promote emotion regulation through cognitive reappraisal.
The medial prefrontal cortex (mPFC) is one of the crucial brain regions involved in fear acquisition and fear extinction (Maier et al., 2012). It shows stronger activation to CS+ than to CS− (Fullana Rivas et al., 2016), indicating its role in threat evaluation, while its activation during fear extinction reflects fear suppression (Giustino & Maren, 2015). For example, the mPFC is highly active in fear extinction (Laurent & Westbrook, 2009), with strong activation in the ventral mPFC during late extinction (Milad et al., 2007) and a correlation between dorsal anterior cingulate activation and behavioral outcomes (Kruse, Tapia León, Stark & Klucken, 2017). The mPFC also plays a critical role in emotion regulation, with impairments in regulating emotions and contributing to anxiety disorders (Malik & Perveen, 2023; Suzuki & Tanaka, 2021; Van der Horn, Liemburg, Aleman, Spikman & van der Naalt, 2016; Wang et al., 2018).
Skin Conductance Response (SCR) is a psychological measure that is commonly used in research to assess fear conditioned responses (Alexandra Kredlow et al., 2017). The increased level of SCR during fear acquisition reflects the physiological arousal induced by the occurrence of CS-US (Lipp, 2006). In the process of fear extinction, individuals’ physiological arousal gradually decreases. Studies have found that higher self-compassion is associated with reduced activation of the threat response system (Creaser, Storr & Karl, 2022). In addition, cultivating self-compassion can also result in significant physiological changes, including increased heart rate variability and reduced heart rate and skin conductance levels. These physiological changes are specific to self-compassion (Kirschner et al., 2019; Kirschner, Kuyken & Karl, 2022), representing decreased threat sensitivity and improved emotional regulation (Thayer & Lane, 2000).
Self-compassion might also affect neural responses to threat and emotion regulation. A functional near-infrared spectroscopy (fNIRS) study found increased mPFC oxyhemoglobin during self-compassion thinking compared to stressful memory recall (Dos Santos et al., 2022). Moreover, higher mPFC activation was found in self-compassion than self-criticism in chronic pain patients (Lutz et al., 2020). This may reflect the cognitive reappraisal processes of the mPFC and other brain regions in self-compassion. Additionally, self-compassion was linked to weaker connectivity between ventral mPFC and the amygdala during negative feedback, suggesting reduced sensitivity to negative emotions (Parrish et al., 2018). Individuals with high trait self-compassion reappraise emotions more flexibly, reducing anxiety and enhancing mPFC activation (Eichholz et al., 2020; Ochsner, Bunge, Gross & Gabrieli, 2002).
Self-compassion can influence fear extinction. Our previous study found that self-compassion writing training after fear acquisition effectively reduces fear ratings during the fear extinction phase in healthy participants (Mei et al., 2023). However, the neural mechanisms underlying self-compassion's effects on fear acquisition and fear extinction remain unclear. Therefore, understanding how trait self-compassion influences mPFC activity during fear acquisition and fear extinction may provide insights into neural mechanisms of emotion regulation and resilience to anxiety disorders.
This study investigates how trait self-compassion influences US expectancy ratings, SCR, and mPFC activation intensity in females during fear acquisition and fear extinction. Research shows that females typically have lower self-compassion and higher self-criticism than males, making them more vulnerable to fear and anxiety (Neff, 2003b; Yarnell et al., 2015). In this study, self-compassion is considered a trait that promotes implicit (unconscious, autonomous) emotion regulation, helping individuals become more accepting when facing stimuli. We hypothesized that individuals with higher trait self-compassion will show lower US expectancy ratings, lower SCR and greater mPFC activation during fear acquisition and fear extinction, indicating more effective fear regulation and extinction learning.
MethodsParticipantsA total of 357 female college students from Sichuan Normal University were investigated by the Self-Compassion Scale-Short Form (SCS-SF). According to the SCS-SF score, the top and bottom 25% of the total sample scores were taken as the standard (Zhang, Wu, Chen and Liu, 2014). A total of 64 participants were screened out in the experiment, of which 3 participants could not stand the screaming, 1 participant had a headache and withdrew during the experiment, and 4 participants failed to acquire fear in the fear acquisition phase. Finally, 56 female participants were analyzed in the subsequent experiment. Among them, there were 28 female participants in the high trait self-compassion (HSC) group and 28 female participants in the low trait self-compassion (LSC) group (Table 1). All participants were right-handed, without color blindness, color weakness, with normal hearing, normal vision or corrected vision, and had no mental diseases. Before the formal experiment, the participants were required to carefully read and sign the informed consent, and corresponding remuneration was provided after the completion of the experiment. The study plan was approved by the Ethical Committee of the Institute of Brain and Psychological Sciences, Sichuan Normal University, and followed the latest version of the Declaration of Helsinki.
Demographics characteristics across samples.
| HSC(n = 28) | LSC(n = 28) | ||||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Significance a |
| Age | 19.66 | 2.29 | 19.39 | 1.59 | p = 0.614 |
| SCS-SF (1) | 49.82 | 3.56 | 30.25 | 2.93 | p < 0.001 |
| SCS-SF (2) | 47.18 | 5.26 | 32.71 | 4.78 | p < 0.001 |
| SAI | 32.07 | 5.75 | 45.39 | 9.33 | p < 0.001 |
| TAI | 37.18 | 6.18 | 51.25 | 11.03 | p < 0.001 |
| STAI | 69.25 | 11.03 | 96.64 | 16.63 | p < 0.001 |
Two-tailed p values reflect the significance of group differences derived from independent samples t-tests for all variables. SCS-SF (1) = Initial trait self-compassion scores used for grouping. SCS-SF (2) = Trait self-compassion scores after arriving at the lab. SAI = State anxiety subscale of the State Trait Anxiety Inventory. TAI = Trait anxiety subscale of the State Trait Anxiety Inventory. STAI = Total score on the State Trait Anxiety Inventory.
The fear conditioning stimuli consisted of two gray circles with identical brightness on a black background. The large circle had a diameter of 11.94 cm, and the small circle had a diameter of 5.08 cm. The size of stimuli was counterbalanced between participants. For half of the participants, the large circle was used as CS+ and the small circle was used as CS−, and vice versa for the other half. The experimental procedures were presented on a Dell computer LCD display. The participants were required to keep their bodies upright and perform the experiment at a distance of 60 cm from the computer. The US was a 95 dB female scream (Bradley & Lang, 1999) delivered through a headset (PHILIPS, TAH4105).
ProcedureAfter arriving at the laboratory, the participants were asked to fill in the SCS-SF again to ensure accurate grouping, went through habituation, fear acquisition, and fear extinction tasks. The habituation was followed by fear acquisition and fear extinction occurred immediately after fear acquisition.
The habituation phase consisted of 4 presentations of CS+ and CS−, respectively, and none of the CSs was accompanied by the scream. Participants were tasked with becoming familiar with the stimuli and judging the number of graphic categories (Milad et al., 2006; Vervliet, Lange & Milad, 2017). During the fear acquisition phase, participants were presented with CS+ and CS− in 15 trials each, with 12 of the CS+ trials accompanied by the scream (reinforcement rate: 80%) (Michalska et al., 2017). In the fear extinction phase, participants were presented with CS+ and CS− in 18 trials each. None of the CSs was accompanied by the scream. SCR and fNIRS data were recorded throughout the fear acquisition and fear extinction phases.
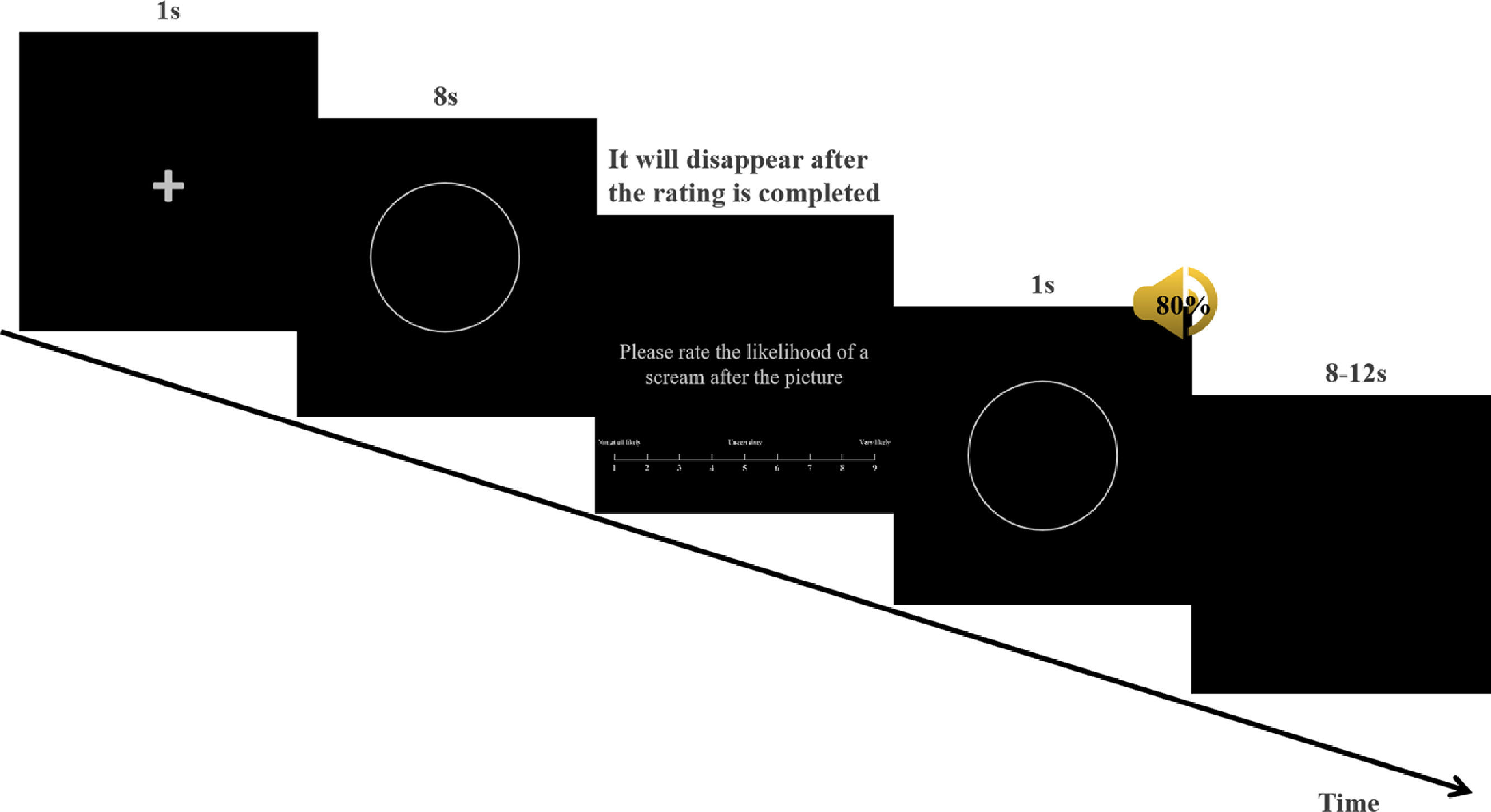
In the experiment, E-Prime 2.0 was used to present conditioned stimulus pictures and screams and record behavioral data. At the beginning of each trial, a fixation cross (+) appeared in the center of the screen for 1 s. Subsequently, CS+ or CS− was presented, and each stimulus was presented on a computer monitor for 8 s. Afterwards, the participants were asked: "Please rate the likelihood of a scream after the picture." Participants were required to give a quick rating based on their immediate experience using the computer keyboard (1 = Not at all likely, 5 = Uncertain, 9 = Very likely). This rating was called the US expectancy rating and represents the behavioral data. The US was presented for 1 s and terminated concurrently with the CS offset. All stimuli were presented in pseudo-random order, and each stimulus was presented no more than twice, with an inter-trial interval (ITI) ranging from 8 to 12 s (Fig. 1).
Example of fear acquisition. At the beginning of each trial, a fixation cross (+) was displayed in the center of the screen for 1 s. Subsequently, the CS was presented for 8 s, after which participants were required to assess the likelihood of a scream after the picture. Participants were asked to quickly rate their immediate feelings. Ratings were given on a nine-option forced-choice scale (1 = Not at all likely, 5 = Uncertain, 9 = Very likely) using a computer keyboard. Subsequently, the CS+ and US were presented for 1 s and terminated simultaneously with the offset of the CS. All stimuli were presented in a quasi-random order. The inter-trial interval (ITI) was 8 to 12 s.
The trait self-compassion was measured online using the Self-Compassion Scale-Short Form (SCS-SF; (Gong, Jia, Guo & Zou, 2014), Cronbach's alpha = 0.77) Chinese version. The scale is widely used not only in adolescents, but also in college students. The SCS-SF consists of 12 items and contains three subscales, namely self-kindness, common humanity, and mindfulness. Example items are: “I'm disapproving and judgmental about my own flaws and inadequacies” and “I try to see my failings as part of the human condition.” Responses are indicated with a 5-point Likert scale ranging from 1 (Almost Never) to 5 (Almost Always). A total trait self-compassion score was computed by reverse scoring negatively worded items and then summing all 12 items. The total score range is 12–60, with a higher score indicating greater trait self-compassion. In this study, the Cronbach's alpha coefficient of this scale was 0.911.
The State-Trait Anxiety Inventory (STAI) was used to measure participants' anxiety levels (Spielberger, 1970). The inventory was divided into State Anxiety Inventory (used to assess participants' "present" emotional experience) and Trait Anxiety Inventory (used to assess participants' "usual" emotional experience), each with 20 items. The two inventories could be measured separately or together. In this study, anxiety levels were measured separately, and the total anxiety score was also recorded. The inventory contains 40 items, such as "I feel calm," with responses expressed on a Likert 4-point scale ranging from 1 (not at all) to 4 (very strong). The overall score ranges from 40 to 160, with higher scores indicating greater anxiety levels. In this study, the Cronbach's alpha coefficient of this scale was 0.956.
Skin conductance response (SCR)SCR was measured using a BIOPAC MP160 system with EDA 100C Isotonic Gel Electrodes. We recorded and analyzed the SCR data with AcqKnowledge 5.0 software (http://www.biopac.com/product/acqknowledge-software). The sample rate in our experiment was 2000 Hz. The Ag/AgCl electrodes with gel were placed on the middle and index fingers of the participants’ left hands. Before the experiment, the participants’ SCR sensitivity was tested. The participants were asked to take slow, deep breathe to induce an increase in SCR. The participants whose SCR increases were lower than 0.02 μS during the deep breathing were excluded (Boucsein et al., 2012; Hornstein, Fanselow & Eisenberger, 2016). SCR is calculated as the maximum value within 0–8 s after the CS appears minus the average value within 2 s before the CS appears. Data less than zero are recorded as zero, and then converted to normalized data by square root transformation (Klein, Berger, Vervliet & Shechner, 2021).
Functional near infrared spectroscopy (fNIRS)A 48-channel (15 emitters and 16 receivers) Nirscan-8000A device (Danyang HuiChuang Medical Equipment Co., Ltd., Beijing China) was used to collect the cerebral blood oxygen signals. The system uses a light-emitting diode (LED) as a near-infrared light emitter and an avalanche photodiode (APD) as a near-infrared light detector. The light source operates at 730 nm and 850 nm, and the device sampling rate is 11 Hz. The optical probe arrangement covers the medial prefrontal cortex and bilateral lateral prefrontal cortex, and the average distance between the transmitter and detector is 3 cm. The localization of the channels first refers to the international 10/20 coordinate system (Jasper, 1958) to determine the corresponding scalp coordinate position of the channel, based on previous studies ((Dos Santos et al., 2022; Ochsner et al., 2002)). In this study, the mPFC was selected as the Region of Interest (ROI), and the mPFC region coordinates were found in the adult Brodmann Talairach brain phantom (Lancaster et al., 2000). The mPFC corresponding channels were then confirmed based on channel coordinates, specifically channels 6, 7, 8, 9, 10, 11, and 25 (Fig. 2).
NirSpark software 1.8.1 was used for data preprocessing. First, motion artifacts were detected by sliding window. Sliding window signals with more than 6 standard deviations were considered as motion artifacts, and a spline interpolation function was used to correct motion artifacts (Molavi & Dumont, 2012). Then the data were band-pass filtered using a filter of 0.01–0.2 Hz to filter noise such as heartbeat and respiration (Dou, Lei, Cheng, Wang & Leppänen, 2020; Gervain et al., 2011; Piper et al., 2014). Finally, the filtered optical density signal was converted into HbO and HbR concentration change signals, Δ[HbO] and Δ[HbR] based on the modified Beer-Lambert law. Previous studies have shown that Δ[HbO] has a higher signal-to-noise ratio than Δ[HbR] (Tong, Hocke & Frederick, 2011), so Δ[HbO] data were used in subsequent analyses.
In this study, an event-related design was used, and the period of the CS presentation (8 s) in fear acquisition and fear extinction tasks was used as a regression term. After convolution with the hemodynamic response function (HRF), it was included in the general linear model (GLM), and the activation strength of the corresponding channel region was measured by calculating the β value of the preprocessed fNIRS signal (Lei, Bi, Mo, Yu, & Zhang, 2021). Trial-stacking averaged β values for each channel within the mPFC were calculated at the time of analysis, and subsequent group-level statistical analyses were performed.
Statistical analysesAll statistical analyses were conducted using IBM SPSS 26 (IBM Corporation, Armonk, NY, USA). An independent sample t-test was used to evaluate the differences in age, SCS-SF, SAI, TAI, and STAI score between the two groups.
In the fear acquisition phase, the Group (HSC, LSC) was the between-subject factor, and the Stimulus Type (CS+, CS−) was the within-subject factor. Mixed ANOVA was performed for US expectancy ratings, SCR, and mPFC activated β values, to examine the mechanism of trait self-compassion on fear acquisition. In the fear extinction phase, the first half of the total number of trials was defined as the early phase of fear extinction, and the second half was defined as the late phase of fear extinction (Guhn et al., 2012). Group (HSC, LSC) was the between-subject factor, and the Stimulus Type (CS+, CS−) was the within-subject factor. Mixed ANOVA was performed for US expectancy ratings, SCR, and mPFC activated β values in the early and late phases of fear extinction, respectively, to examine the mechanism of trait self-compassion in the fear extinction phase. The significance level was set at 0.05, and partial η2 was used as the effect size index.
Finally, we conducted an exploratory correlation analysis to examine the relationship between trait self-compassion and the change in US expectancy ratings to CS+ during fear acquisition phase to the late phase of fear extinction (ΔUS expectancy = US expectancy ratings for CS+ during fear acquisition – US expectancy ratings for CS+ during the late phase of fear extinction), the change in SCR to CS+ during fear acquisition phase to the late phase of fear extinction (ΔSCR = SCR for CS+ during fear acquisition – SCR for CS+ during the late phase of fear extinction), and the β values of mPFC activation in response to CS+ during the early and late phases of fear extinction. The results section reported p values and r values, and the significance level was set at 0.05. In this study, ΔUS expectancy and ΔSCR were defined as participants’ ability to realize both behaviorally and physiologically that the CS+ is no longer threatening. Larger values indicate better fear extinction ability, while smaller values suggest poorer fear extinction ability.
ResultsUS expectancy ratings resultsDuring fear acquisition, the 2 × 2 mixed ANOVA analysis of US expectancy results revealed significant main effect of Stimulus Type (F(1, 54) = 630.871, p < 0.001, partial η2 = 0.921). The US expectancy of CS+ was significantly higher than CS− within both groups. However, the main effect of Group (F(1, 54) = 0.453, p = 0.504, partial η2 = 0.008) and the Stimulus Type × Group interaction (F(1, 54) = 0.115, p = 0.736, partial η2 = 0.002) did not reach significance (Fig. 3A).
US expectancy and SCR results for high and low trait self-compassion groups across the fear acquisition and fear extinction phase. (A), (B), and (C) show the US expectancy during the fear acquisition phase, early phase of fear extinction, and late phase of fear extinction, respectively. (D), (E), and (F) show the SCR results during the fear acquisition phase, early phase of fear extinction, and late phase of fear extinction, respectively. The error bars in the figure represent the standard error of the mean, *: p < 0.05, **: p < 0.01, ***: p < 0.001.
In the early phase of fear extinction, the 2 × 2 mixed ANOVA analysis of US expectancy results revealed significant main effects of Stimulus Type (F(1, 54) = 65.369, p < 0.001, partial η2 = 0.548) and Group (F(1, 54) = 6.788, p = 0.012, partial η2 = 0.112). The Stimulus Type × Group interaction (F(1, 54) = 4.737, p = 0.034, partial η2 = 0.081) was also significant. The simple effects analysis showed that in both the HSC and LSC groups, the US expectancy of CS+ was higher than that of CS− (p < 0.001). However, the LSC group had a significantly higher US expectancy than the HSC group under the CS+ condition (p = 0.002), but there was no significant difference between the two groups under the CS− condition (p = 0.349). This indicated that in the early phase of fear extinction, compared with the LSC group, the HSC group had begun to show a more obvious extinction of fear response (Fig. 3B). In the late phase of fear extinction, the 2 × 2 mixed ANOVA analysis of US expectancy results revealed significant main effects of Stimulus Type (F(1, 54) = 13.96, p < 0.001, partial η2 = 0.205) and Group (F(1, 54) = 8.137, p = 0.006, partial η2 = 0.131). The Stimulus Type × Group interaction (F(1, 54) = 12.825, p = 0.001, partial η2 = 0.192) was also significant. The simple effects analysis found that the US expectancy of the LSC group was significantly higher than that of the HSC group for both CS+ (p = 0.001) and CS− (p = 0.012). However, in the HSC group, there was no significant difference between CS+ and CS− (p = 0.535), while in the LSC group, CS+ had a higher US expectancy than CS− (p < 0.001). The results indicated that the HSC group had nearly completely extinguished fear by the late phase of fear extinction, while the LSC group had not completely extinguished fear (Fig. 3C).
SCR resultsIn the fear acquisition phase, the 2 × 2 mixed ANOVA analysis of SCR results revealed significant main effect of Stimulus Type (F(1, 54) = 16.450, p < 0.001, partial η2 = 0.233), the SCR for the CS+ was significantly higher than those for CS− in both groups. The main effect of Group (F(1, 54) = 7.031, p = 0.010, partial η2 = 0.115) was significant, the SCR of the LSC group was significantly higher compared to the HSC group. However, the Stimulus Type × Group interaction (F(1, 54) = 2.223, p = 0.142, partial η2 = 0.040) did not reach significance. (Fig. 3D).
In addition, we examined differences in SCR between the two groups during the early and late phase of fear extinction. In the early phase of fear extinction, the 2 × 2 mixed ANOVA analysis of SCR revealed that the main effect of Stimulus Type (F(1, 54) = 0.035, p = 0.852, partial η2 = 0.001) and the Stimulus Type × Group interaction (F(1, 54) = 2.058, p = 0.157, partial η2 = 0.037) were not significant. However, the main effect of Group was significant (F(1, 54) = 4.868, p = 0.032, partial η2 = 0.083). The SCR of the LSC group was significantly higher than that of the HSC group (Fig. 3E). In the late phase of fear extinction, the 2 × 2 mixed ANOVA analysis of SCR revealed that the main effect of Stimulus Type (F(1, 54) = 0.540, p = 0.466, partial η2 = 0.010) and the Stimulus Type × Group interaction (F(1, 54) = 0.270, p = 0.606, partial η2 = 0.005) were not significant. However, the main effect of Group (F(1, 54) = 5.243, p = 0.026, partial η2 = 0.089) was significant. The SCR of the LSC group was significantly higher than that of the HSC group. This result indicated that at the late phase of fear extinction, the HSC group still showed stronger fear extinction than the LSC group (Fig. 3F).
fNIRS resultsThe final analyses focused on mPFC activation. In the fear acquisition phase, the 2 × 2 mixed ANOVA analysis of mPFC activation revealed significant main effect of Stimulus Type (F(1, 54) = 16.906, p < 0.001, partial η2 = 0.238). The mPFC activation for the CS+ was significantly higher than that for CS− in both groups. However, the main effect of Group (F(1, 54) = 0.014, p = 0.906, partial η2 < 0.001) and the Stimulus Type × Group interaction (F(1, 54) = 0.426, p = 0.517, partial η2 = 0.008) did not reach significance. (Fig. 4A,4D).
Functional NIRS results of mPFC neural activity for both high and low trait self-compassion groups during fear acquisition and fear extinction phase. (A), (B), and (C) show the results of mPFC neural activity during fear acquisition phase, early phase of fear extinction, and late phase of fear extinction, respectively. (D), (E), and (F) represent the mPFC brain activation maps corresponding to the CS+ and CS− during the fear acquisition phase, early phase of fear extinction, and late phase of fear extinction, respectively. Color bars indicate activation levels. Error bars represent the standard error of the mean, *: p < 0.05, **: p < 0.01, ***: p < 0.001, ns: not significant.
In addition, we examined differences in mPFC activation between the two groups during the early and late phase of fear extinction. In the early phase of fear extinction, the 2 × 2 mixed ANOVA analysis of mPFC activation revealed that the main effect of Stimulus Type (F(1, 54) = 0.565, p = 0.455, partial η2 = 0.010), the main effect of Group (F(1, 54) = 2.057, p = 0.157, partial η2 = 0.037) and the Stimulus Type × Group interaction (F(1, 54) = 0.508, p = 0.479, partial η2 = 0.009) were not significant. This result indicated that there was no significant difference in mPFC between the HSC group and the LSC group in the early phase of fear extinction (Fig. 4B,4E). In the late phase of fear extinction, the 2 × 2 mixed ANOVA analysis of mPFC activation revealed that the main effect of Stimulus Type (F(1, 54) = 3.101, p = 0.084, partial η2 = 0.054) and the Stimulus Type × Group interaction (F(1, 54) = 0.333, p = 0.233, partial η2 = 0.006) were not significant. However, the main effect of Group (F(1, 54) = 9.158, p = 0.004, partial η2 = 0.145) was significant. The β value of the HSC group was higher than that of the LSC group. This result suggests that differences in activity in the mPFC begin to emerge between the LSC and HSC groups in the late phase of fear extinction (Fig. 4C,4F).
Correlation analysis resultsPearson correlation analysis was used to examine the relationship between trait self-compassion, ΔUS expectancy, ΔSCR, and the β values of mPFC activation in response to CS+ during the early and late phases of fear extinction (Table 2). The findings revealed significant positive correlations between trait self-compassion and ΔUS expectancy (r = 0.398, p = 0.002), trait self-compassion was positively correlated with the β values of mPFC activation during the late phase of fear extinction (r = 0.397, p = 0.002), and ΔUS expectancy was positively correlated with the β values of mPFC activation during the late phase of fear extinction (r = 0.351, p = 0.008). However, the correlation between other variables was not significant. This indicates that the higher the trait self-compassion, the greater the behavioral extinction ability and the greater the mPFC activation during the late phase of fear extinction. Additionally, the greater the behavioral extinction ability, the greater the mPFC activation during the late phase of fear extinction.
Pearson correlation results.
| Pearson Corre | SCS-SF | ΔUS expectancy | ΔSCR | β value (1) | β value (2) |
|---|---|---|---|---|---|
| SCS-SF | 1 | ||||
| ΔUS expectancy | .398** | 1 | |||
| ΔSCR | −0.150 | −0.122 | 1 | ||
| β value (1) | .210 | −0.042 | .205 | 1 | |
| β value (2) | .397** | .351** | −0.163 | .191 | 1 |
The relationship between trait self-compassion, behavioral and physiological fear extinction ability, and the β values of mPFC activation during the early and late phases of fear extinction. β value (1) = the β values of mPFC activation during the early phase of fear extinction. β value (2) = the β values of mPFC activation during the late phase of fear extinction. *: p < 0.05, **: p < 0.01, ***: p < 0.001.
The purpose of this study was to explore how different levels of trait self-compassion affect fear acquisition and fear extinction. Consistent with the hypothesis, the high trait self-compassion (HSC) group exhibited lower SCR during fear acquisition and more successful fear extinction compared to the low trait self-compassion (LSC) group, marked by lower US expectancy ratings, reduced SCR, and greater mPFC activation to both CS+ and CS−. Trait self-compassion was positively correlated with the behavioral extinction ability and mPFC activation during the late phase of fear extinction, and behavioral extinction ability was positively correlated with the mPFC activation. These findings suggest that individuals with higher trait self-compassion demonstrate better physiological flexibility during fear acquisition and extinction, as well as better fear extinction, which is associated with enhanced activation of the mPFC.
During the fear acquisition and fear extinction phase, individuals with high trait self-compassion exhibit lower SCR indicators in response to both CS+ and CS−, indicating a reduced level of physiological stress. However, trait self-compassion was not associated with the change in SCR (ΔSCR). This supports previous findings that higher levels of trait self-compassion predict a better ability to physiologically adapt emotional responses (Svendsen et al., 2016). Individuals with high trait self-compassion demonstrate flexible adjustment of physiological and psychological responses to stress (Luo et al., 2018). Our results support the idea that trait self-compassion exhibits better flexibility in physiological regulation. This suggests that trait self-compassion acts as a protective factor against greater physiological changes induced by stimuli. Moreover, the differences in SCR offer a potential marker for distinguishing individuals based on their physiological responses.
During the fear extinction phase, the HSC group was significantly different from the LSC group. In terms of US expectancy ratings, the HSC group showed faster fear extinction and finally successfully extinguished fear. Self-compassion involves accepting and integrating negative emotions with self-kindness, common humanity and mindfulness, which may facilitate reappraisal and support flexible emotional regulation (Allen & Leary, 2010; Breines & Chen, 2012; Neff & Dahm, 2015).Thus, in this study, the HSC group may have reappraised the association of CS-noUS to facilitate fear extinction through more acceptance and integration. However, the LSC group had difficulty with fear extinction, indicating that the LSC group believed that the stimulus was more likely to follow the threat stimulus, and found it difficult to update the association of CS-noUS. This suggests that the LSC group may have a harder time reappraising threat-related stimuli through more acceptance and integration.
Interestingly, there were no significant SCR differences between the two groups for CS+ and CS− during both the early and late phases of fear extinction. This is inconsistent with the US expectancy, which may be due to a weaker bonding response during this phase (Constantinou et al., 2021). Although individuals recognized the CS-US connection in terms of US expectancy, they were unable to elicit significant differences in SCR between CS+ and CS−.
In terms of neural activity, there was no significant difference between the two groups during the early phase of fear extinction. Combined with the behavioral results, we found that the LSC group showed fear extinction. It can be speculated that the extinction ability of the LSC group was not impaired. This indicates that early in the extinction process, both groups engage similar cognitive mechanisms, such as evaluating the changing threat-safety stimulus and updating their expectations. This is consistent with previous findings in healthy individuals that mPFC activation in response to CS+ and CS− was not significantly different during the early phase of fear extinction (Guhn et al., 2012). However, individuals with high trait self-compassion showed strong activity responses in the mPFC during the late phase of fear extinction, reflecting a continued engagement of top-down regulatory processes. Also, we found that trait self-compassion was significantly positively correlated with the mPFC activation in response to CS+ during the late phase of fear extinction. Previous studies found that the mPFC is known to be involved in emotion regulation and cognitive control (Etkin, Egner & Kalisch, 2011). Self-compassion shows greater mPFC activation during emotional regulation (Dos Santos et al., 2022; Lutz et al., 2020). During the fear extinction, the mPFC is highly active (Giustino & Maren, 2015), and stronger mPFC activation is associated with the inhibition of fear (Laurent & Westbrook, 2009). The stronger or prolonged activation in the HSC group could indicate that these individuals maintain a more persistent effort in reappraisal and emotional regulation as fear extinction progresses. This led to more effective inhibition of fear responses to threat-related stimuli.
Furthermore, it showed that behavioral extinction ability was positively correlated with the mPFC activation during the late phase of fear extinction. Consistent with a previous study, there was a correlation between dorsal anterior cingulate cortex (which corresponds to the mPFC region) activation and behavioral outcomes (Kruse et al., 2017). This was also demonstrated in our study. Individuals with higher trait self-compassion may use higher activity of the mPFC for reappraisal to update the association of CS-noUS, as reflected in their behavioral extinction ability. It is important to emphasize that the fear extinction process itself can reduce fear responses (McNally, 2007). Therefore, trait self-compassion may have only partially contributed to the effects of fear extinction.
This study reveals the cognitive neural mechanisms by which trait self-compassion facilitates fear extinction. It provides new insights into the pathological mechanisms of anxiety disorders. This finding is consistent with previous research suggesting that mPFC activation enhances emotional regulation and reduces anxiety symptoms (Abend et al., 2020; Jafari et al., 2021). These results reveal the potential of self-compassion in reducing anxiety symptoms, particularly through its regulation of neural mechanisms that can effectively alleviate the core pathological issues of anxiety disorders.
Previous intervention studies have suggested that promoting mPFC activation through pharmacological and physiological means as well as electrical stimulation can be a useful adjunct to promote fear memory extinction (Guhn et al., 2012; Vicario et al., 2020), and to promote the ability to regulate emotions (Abend et al., 2019), and treat mental disorders such as anxiety (Jafari et al., 2021). Self-compassion, as a non-invasive intervention, has the unique advantage of being mild and free of side effects, showing great potential for emotional regulation and the maintenance of mental health. Compared to invasive means such as medication or electrical stimulation, self-compassion provides a more natural way to address anxiety, stress, and other emotional problems by helping individuals develop acceptance and care for negative emotions (Neff & Germer, 2012). However, many current intervention studies do not measure mPFC activation as a relevant metric. Based on this, future studies could also use the activation level of the mPFC as a reference index for the effects of various non-intrusive cognitive behavioral interventions, including self-compassion.
The present study had several limitations. First, although fNIRS is considered safe, it is limited to capturing brain activity at the surface level (Pinti et al., 2020). Future research could benefit from using multimodal imaging techniques, such as combining fNIRS with functional magnetic resonance imaging (fMRI), to deepen our understanding of fear responses. fMRI, with its higher spatial resolution, can detect the involvement of deeper brain regions, such as the amygdala and hippocampus, which are closely associated with fear (Singh & Topolnik, 2023), providing more comprehensive neurophysiological data. Second, this study focused exclusively on female participants, limiting the generalizability of the findings. Recent studies have revealed gender-specific differences in brain activity related to emotional processing. Females tend to show heightened activation in the prefrontal cortex and amygdala, compared to males, especially when dealing with negative emotions, suggesting a stronger emotional response and greater effort in emotion regulation compared to males (Balada, Aluja, García, Aymamí, & García, 2024; Mcrae, Ochsner, Mauss, Gabrieli & Gross, 2008). Based on this gender difference, future research should explore whether similar neural mechanisms are observed in male populations or other demographic groups. Finally, self-compassion includes three sub-components: mindfulness, self-kindness, and common humanity. These three sub-components have been found to differ in specific brain activation (Guan et al., 2021). However, this study did not delve deeply into these differences. Future studies could further explore the relationship between these subcomponents and neural activation to better understand the neural basis by which trait self-compassion facilitates fear extinction.
ConclusionThis study investigated fear acquisition and fear extinction in female adults with different levels of trait self-compassion. It was found that individuals with higher trait self-compassion exhibited better physiological flexibility during fear acquisition and fear extinction, as well as better fear extinction, which is associated with enhanced activation of the mPFC. These findings deepen our understanding of how trait self-compassion modulates fear acquisition and fear extinction, providing new insights into the neurobiological mechanisms underlying emotional regulation. Our results suggest that self-compassion may be an important target for treating anxiety disorders.
Funding statementThis study was supported by the grants from STI 2030—Major Projects 2022ZD0210900; National Natural Science Foundation of China [NSFC32271142]; Guangdong Key Project in “Development of new tools for diagnosis and treatment of Autism” [2018B030335001], and the Ministry of Education Key Projects of Philosophy and Social Sciences Research [grant number 21JZD063].