Research on the ontogenetic development of brain networks using resting state has shown to be useful for understanding age-associated changes in brain connectivity. This work aimed to analyze the relationship between brain connectivity, age and intelligence.
MethodsA sample of 26 children and adolescents between 6 and 18 years of both sexes underwent a resting-state functional magnetic resonance imaging study. We estimated the values of fractional Amplitude low-frequency fluctuations (fALFF) and the values of Regional homogeneity (ReHo) in a voxelwise analysis to later correlate them with age and intelligence quotient (IQ).
ResultsNo significant correlations were found with IQ, but it was found that the fALFF values of the left precentral cortex (premotor cortex and supplementary motor area), as well as the ReHo values of the medial frontal gyrus, and the precentral cortex of the left hemisphere, correlate with age. Conclusions: Hubs related to various “task positive” networks closely related to cognitive functioning would present a development more related to age and relatively independent of individual differences in intelligence. These findings suggest that the premotor cortex and supplementary motor cortex could be a cortical hub that develops earlier than previously reported and that it would be more related to age than to intelligence level.
The human brain is organized thanks to a set of functional networks that interact with each other to articulate the different aspects of human behavior (Fair et al., 2009; Van Den Heuvel & Hulshoff Pol, 2010). These brain networks and their development can be studied using functional magnetic resonance imaging (fMRI) (Biswal et al., 1995; Cole et al., 2010; Sporns et al., 2004). Some of these networks are known as “task positive” because their activity usually increases during performance on cognitive tasks (Fox et al., 2005). Among these, there is the fronto-parietal network (FPN), which has been implicated in the control of executive functions and intelligence (Engelhardt et al., 2019; Sherman et al., 2014). This network is comprised of the dorsolateral prefrontal cortex (DLPFC), the precentral cortex, and the posterior parietal cortex (Vincent et al., 2008; Takeuchi et al., 2018). Another important “task positive” network is the dorsal attention network (DAN), which is responsible for attentional functions and is formed by the dorsolateral prefrontal cortex (DLPFC), the frontal eye fields, the inferior precentral sulcus, the superior occipital gyrus, parts of the medial temporal cortex and the superior parietal lobe (Fox et al., 2005). The Salience Network is fundamentally formed by the frontal operculum, the insula and the anterior cingulate cortex, related to the maintenance of tonic alertness, it focuses on multiple stimuli that compete for attention, identifying the most relevant ones, and acts as a switch of transition or bridge between networks (Sadagiani & D'esposito, 2015, Uddín, 2017). Complementarily, the Default Mode Network (DMN) is a “task Negative” network that increases its activity during undirected thinking (mind wandering) or autobiographical thinking and tends to disconnect during task performance (Harrison et al., 2008). Task positive networks and the DMN are anticorrelated so that the increase in activity of the former correlates with a decrease in the activity of the DMN and vice versa (Van Den Heuvel & Hulshoff Pol, 2010).
Using the fMRI brain signal, it is possible to study the ontogenetic development of brain networks: the relationships between age and the brain connectivity of these networks can be studied in various cognitive tasks (Crone et al., 2006; Engelhardt et al.., 2019 and Houdé et al., 2010) and can also be studied using resting state approach (Cao et al., 2016; Fair et al., 2009; DeSerisy et al., 2021). Various studies show that during ontogenetic development there is a certain decrease in functional segregation, that is, a decrease in the vibration force between nearby areas, and at the same time there is an increase in functional connectivity between distant brain areas, that is, an increase in functional integration (Fair et al., 2009; Fair, 2014; Cao et al., 2016). The brain hubs are present at birth, and its functional integration and segregation properties increase during infancy (Cao et al., 2016). Likewise, during childhood and adolescence, there is a strengthening of the cortical Hubs, that is, centres with a high density of connections with a wide distribution and containing highly connected areas (Cao et al., 2016).
When reviewing specific connectivity networks, some studies show that healthy children aged 7 to 12 years activate areas of the frontoparietal network (FPN) in cognitive control tasks (Koyama et al., 2013; Margolis et al., 2020), suggesting that the main hubs of this network are in place in infancy and its basic structures do not change from infancy to adolescence. A recent work using MEG did not find differences in age groups in the maturation of alpha and theta oscillations in the FPN in children ages 9 to 14. (Solis et al., 2021). However, some controversy exists since other authors found that connections in this network were inversely associated with age in participants from 7 to 25 years old and this decrease in connectivity will be an indicator of the network development (DeSerisy et al., 2021).
It has been suggested that the DAN develops its functional connectivity relatively early in infancy. Some authors found DAN functional connectivity and connectivity between the DAN and the rest of the brain in children from 4 to 7 years old (Rohr et al., 2016). In addition, the performance in selective attention tasks has been associated with the connectivity between the intraparietal sulcus and the frontal eye fields of the DAN (Rohr et al., 2016). Other works show that functional connectivity within areas of the DAN in children aged 7 to 12 years is similar to that of adults (Farrant & Uddin, 2015). But other works suggests that the functional connectivity in this network still evolves after the infancy and the puberty. Some researcher proposed that the connectivity between brain areas of the DAN is greater in children than in young adults, suggesting that the more efficient connectivity observed in young adults compared to children is related to a decrease in connectivity within the network (Jolles et al., 2011; Farrant & Uddin, 2015).
The DMN probably also develops importantly in early childhood since it has been proposed that its main areas and subsystems show notable development at age 5 (Xiao et al., 2016). Other works suggest that the core regions of the DMN, FPN, DAN, and the salience network are present in children aged from 11 to 13, and they do not differ between children and adults (Jolles et al., 2011). However, this same work also points out that there are important age-related differences in the strength of connectivity within network areas, and in the size of functional networks; thus, the functional connectivity of these networks in children is greater than in adolescents and adults, and these decreases in connectivity with age will be related to the refinement in the network's development (Jolles et al., 2011; DeSerisy et al., 2021).
The social brain network has been studied much less, but it is very relevant to development (McCormick et al., 2018). Various brain areas are related to social cognition (Frith & Frith, 2007). Recently, The work by McCormick et al. (2018) shows that there is a brain social network that includes frontostiatal regions, the amygdala, and the temporal pole, among other structures, and these brain areas show a high functional integration with structures not specialized in social aspects such are several HUBs of the DMN and the frontoparietal network. (McCormick et al., 2018). This network develops during childhood and does not show changes in its functional architecture from 8 to 16 years of age (McCormick et al., 2018).
Previously cited research shows that brain connectivity develops first through forming the network's core regions and establishing the network's connections and later through refining and tuning the network's connectivity. However, there is also an important variability in the developmental trajectories of these networks and some controversies about the ages at which they are fully developed.
Another factor closely linked to the development of brain connectivity is cognitive development. Task positive networks undergo significant development during early childhood (Dennis et al., 2013), and the main networks associated with cognitive control are well established by around 10 years of age (Engelhardt et al., 2019). However, these networks continue to develop in late childhood and adolescence, and their functional integration is associated with better performance in cognitive control tasks (Marek et al., 2015). Likewise, individual differences in intelligence of adolescents and adults are related to structural and functional variations in the DLPFC cortex, the ventrolateral prefrontal cortex (VLPFC) and the medial prefrontal cortex, including the supplementary premotor cortex among other structures (Jung & Haier, 2007; Basten et al., 2015); areas that correspond to “task positive” connectivity networks (Fox et al., 2005). It has been suggested that increased functional connectivity in FPN structures of the right hemisphere positively correlates with the manipulative IQ of children between 6 and 8 years of age (Langeslag et al., 2013). Some works suggest that from 10 to 13 years of age, connectivity in the FPN increases and that its integration positively correlates with IQ (Sherman et al., 2014). Other works showed that a more anticorrelated FPN-DMN connectivity between the frontal pole and the precentral gyrus was associated with a better IQ in a large sample of children and youth aged 7 to 25 (DeSerisy et al., 2021).
All the above-mentioned works demonstrate the relationship between age, cognitive functioning of attention, executive functions, social functioning, intelligence and the development of brain connectivity. However, brain connectivity in neurotypical development has been studied using seed-based functional analysis techniques, independent component analysis (ICA) or methods derived from complexity measures and graph theory. There are other techniques for analyzing brain connectivity such as the analysis of the fractional amplitude of low-frequency fluctuations (fALFF) and the regional homogeneity (ReHO) in voxelwise analysis, which can be used in combination to study alterations in connectivity in different conditions of pathological development (see An et al., 2013 for an example).
A combination of ALFF, ReHo, and ICA has recently been used to study the effects of combat sports on cerebellar connectivity in adolescents (Li et al., 2022). The work of Yang et al. (2015) used both techniques to study the brain connectivity of children and adolescents about to working memory. However, the combination of fALFF and ReHo has been used very little to study the functional connectivity of ontogenetic development. Both techniques are data-driven functional segregation analysis methods that provide information of a somewhat different nature and are often combined in the same work (Lv et al., 2018). fALFF is a technique derived from the measurements of Amplitude of low frequency that measure the fluctuations (ALFF), that measures the strength of the regional intensity of spontaneous fluctuations in the BOLD signal but has often been criticized because it could be sensitive to physiological noise (Lv et al., 2018). Therefore, Zou et al. (2008) developed the fractional amplitude of low-frequency fluctuations analysis (fALFF), which enhances the sensitivity and specificity of spontaneous brain activity detection. On the other hand, ReHo estimates the temporal homogeneity of the signal between a given voxel and neighbouring voxels (Zang et al., 2004). ReHo measures the similarity between the time series of a specific voxel with that of its neighbouring voxels, reflecting local functional connectivity (Zang et al., 2004). Both approaches are complementary, while fALFF is focused on measuring local spontaneous fluctuations in activity related to their distant connections (Zhou et al., 2008), and ReHo estimates local neural activity (Zang et al., 2004). Furthermore, both techniques have very good test-retest reliability (Zuo et al., 2013; Küblböck et al., 2014). Thus, combining ReHo and fALFF to assess spontaneous brain activity among the neurotypical developing population could provide more information about brain function than using only one of these methods (Lv et al., 2018).
But this combination must be justified. Koyama et al. (2020) they agree that both ReHo and fALFF can successfully detect regions that are associated with individual differences in cognitive skills. In their study, they used ReHo and fALFF values to simultaneously examine reading and arithmetic achievement, and IQ measures in young adults, with the aim to identify neural correlates of their common factors. Moreover, both, fALFF and ReHo provide useful information about brain development, such as the strength and specificity of functional connections between brain regions and the development of neural networks (Song et al., 2023). These authors explain this issue as follows: if the interest is to analyze whether the relationship between an independent variable (such as age) and a dependent variable (such as IQ) is mediated by a third variable (such as brain function), then, fALFF and ReHo are useful to identify specific regions that could mediate this relationship.
For this reason, our objective is to carry out an exploratory voxelwise and data-driven study of the relationships between brain connectivity, age and intelligence in a sample of children and adolescents from 6 to 18 years old, using fALFF and ReHo analysis. Our general hypothesis is that we will find important correlations between age and intelligence, with various hubs of “task positive” brain networks and the DMN.
Material and methodsParticipantsSchool children and adolescents were invited to participate, recruitment was carried out through contact with different basic level educational institutions in the Valles region of the state of Jalisco (México). Sampling was opportunistic following: (a) right-handed children and adolescents between 6 and 18 years old with a letter of consent signed by their legal guardian and assent from the minor, (b) with schooling appropriate to their age, (c) without suspicion of cognitive or mental health impairment, (d) average IQ with a maximum of one standard deviation below the average (minimum 85). The exclusion criteria were as follows: (a) withdrawal from the study, (b) excessive movement during fMRI data acquisition that prevents data analysis.
The initial evaluation involved a total of 61 children of which 1 could not continue for having a history of neurological conditions, 5 for having materials not compatible with fMRI (brackets and metallic crowns, 3 for having a lower IQ score, 15 for suspected psychiatric diagnosis (8 with probable attention deficit hyperactivity disorder, 5 with anxiety and 2 with depression) and 11 did not want to continue with the study once they had done the simulation training according to the inclusion and exclusion criteria, formed a final sample of 26 children and adolescents between 6 and 18 years of age (M = 10.4 and SD = 4 0.5), 50 % were women (n = 13). In Table 1, some of the descriptive statistics of the sample are shown.
Basics descriptive statistics of age and IQ of the sample.
| Quantitative variables | Min. | Max. | x¯ (SD) | Md | Skewness (Std. Error) |
|---|---|---|---|---|---|
| Age | 6.00 | 18.00 | 10.46 (4.48) | 8.00 | 0.86 (0.45) |
| IQ | 85.00 | 132.00 | 104.92 (10.90) | 104.50 | 0.57 (0.46) |
Note: IQ: intelligence quotient, Min: minimum, Max: maximum, x¯: Mean, SD: Standard deviation, Md: median, Std. Error: Standard error.
In all cases, the following evaluation and measurement elements were administered:
- •
Brief clinical history to parents about their child, to identify the healthy and optimal state to participate in the study.
- •
Depending on the age of the minor, respectively, the Wechsler intelligence subscales, designed with cubes and vocabulary, were administered as a short way to obtain the estimated IQ (Cronbach's α = 0.90; Sattler & Ryan, 2009, Chen et al., 2021):
○ WISC-IV Wechsler Intelligence Scale for children and/or adolescents from 6 years 0 months to 16 years 11 months.
○ WAIS-IV Wechsler Intelligence Scale for Adults applied to adolescents and adults from 16 to 89 years old.
- •
Interview for Psychiatric Syndromes in Children and Adolescents ChIPS (Cronbach's α = 0.90; Fristad et al., 2001) answered by the minor to rule out participants with suspected psychiatric syndromes.
Parents of children and young people from 6 to 18 years of age were requested to participate through a visit to schools, through an information session coordinated with the person in charge of the institution, to publicizing what the study consisted of, the inclusion criteria to participate and the benefits they would obtain (healthy management of social networks workshop, structural brain images delivered to parents by email and results of the participant's neuropsychological evaluation).
Subsequently, those who agreed to participate by signing the informed consent were provided with the privacy notice and the participants were explained what the study would consist of and were asked for their consent by means of their signature or fingerprint, depending on their age. Subsequently, in a first session, neuropsychological tests were administered by previously trained psychologists began. The total time spent was approximately two hours in the first session. In a second moment, training was carried out that sought to simulate the resonance situation with the minors, to ensure that they understood the instructions and the procedure that would be carried out to reduce anxiety, uncertainty and/or fears. This activity lasted approximately 30 min in the neuropsychology laboratory of the Los Valles University Center of the University of Guadalajara.
The entire protocol described was approved by the Ethics Committee of the CUCBA Neurosciences Institute (04/28/2023) with registration number: ET022023–365 and also by the Scientific Research Ethics Committee of CUValles (05/24/2023), both from the University of Guadalajara.
Magnetic resonance imaging acquisition and preprocessingBrain images were acquired on an SIEMENS MAGNETOM 3.0T Scanner system located in Guadalajara Jalisco. All participants underwent an fMRI recording sequence in the following order: T1, T2, FLAIR, and T2* at rest, taking a total of 16 min to complete these sequences. A T1-weighted turbo field echo (TFE) structural image was obtained for each subject with a three-dimensional protocol (repetition time (TR) = 2300 ms, echo time (TE) = 2980 ms, 240 slices and field of view (FOV) = 240 × 240 × 170). Image acquisition was in the sagittal plane. Subsequently, the T2 sequence called Fluid Attenuated Inversion Recovery (FLAIR) and finally the resting state sequence were obtained. For these functional images, a T2*-weighted (BOLD) image was obtained (TR = 2000 ms, TE = 30 ms, FOV = 230 × 230 × 160, and voxel size = 3 × 3 × 3 mm, with 29 slices). Image acquisition was in the transverse plane.
For the resting-state sequence, all participants were asked, during signal recording, to remain quiet without moving and in a supine, awake position with their eyes open. Communication was maintained at all times through a headset and microphone system that had been placed on them when entering the scanner. From the beginning, they were told that if they felt uncomfortable they could notify them at any time and the study would be stopped. The total time of this sequence was 6 min.
Image preprocessing was performed using the Data Processing Assistant for Resting-State fMRI57 (DPARSF; http://rfmri.org/DPARSF). It is based on MATLAB, SPM12 (http://www.fl. ion.ucl.ac.uk/spm) and DPABI58. The first 10 functional images were removed to allow magnetization equilibration and to allow participants to adapt to the scanner. Then, a correction was made for the remaining functional images for slice acquisition timing difference and head motion. Nuisance signals were regressed out, including white matter signals, cerebrospinal fluid signals, linear trends and signals associated with the 24 Friston head-motion parameters. The derived functional images were coregistered with the corresponding structural images, which were segmented and normalized to Montreal Neurological Institute (MNI) space using diffeomorphic anatomical registration through exponentiated lie algebra (DARTEL). The functional images were normalized to MNI space with warped parameters and resampled to 3 mm cubic voxels. Regarding the ReHo analysis, the normalized functional images were then bandpass filtered (0.01–0.1 Hz). Given that participants ‘mean movement was 0.02766 on Jenkinson's FD, no person was eliminated for movement.
Voxel-based morphometryThe T1w-structural images were automatically processed with DPABI (Yan et al., 2016). The images were reoriented and individually checked for quality control. Afterwards, reoriented T1 images were segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF; Ashburner & Friston, 2005). Finally, the DPABI module uses the Diffeomorphic Anatomical Registration Through Exponentiated Lie algebra (DARTEL) tool (Ashburner, 2007) to compute transformations from individual native space to MNI space. Finally, grey matter segmentations were resliced and smoothed to match the parameters with the functional images. Additionally, total grey matter volumes and parcellation volumes were calculated using SPM121 and SPM12-based scripts (Maldjian et al., 2003, 2004).
Estimation of ALFF and ReHo valuesDPABI was used to estimate the fALFF and ReHo values (Yan et al., 2016). Specifically, in the case of ALFF, spatial smoothing was performed with a Kernell Gaussian of 4 mm full width at half (FWHM). To calculate the power spectrum, the time series of each voxel was transformed into the frequency domain using a fast Fourier transform (FFT). This power spectrum, which has a frequency range of 0–0.25 Hz, was rounded squared at each frequency and then averaged across 0.01–0.08 Hz at each voxel, which was taken as ALFF. To obtain fALFF, the ALFF values were divided by the entire frequency range observed in the signal (0–0.25 Hz) (Zou et al., 2008).
Regarding ReHo estimation, Kendall's concordance coefficient (KCC) was calculated from the time series of all voxels and their neighbors (Zang et al., 2015). All ReHo maps were smoothed with a four mm FWHM Gaussian kernel. Finally, individual fALFF and ReHo maps were standardized into z-score maps by subtracting the mean and dividing by the standard deviation.
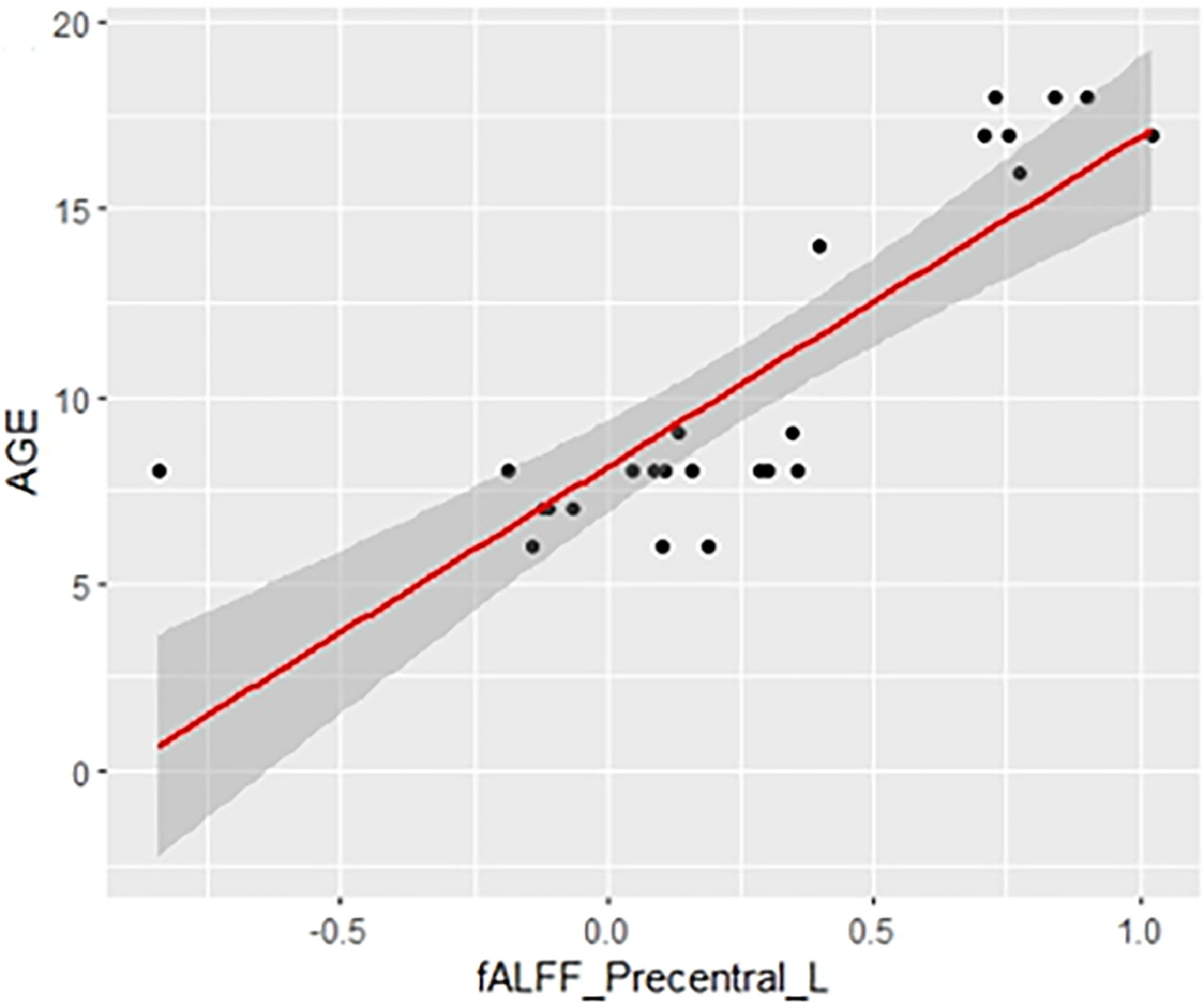
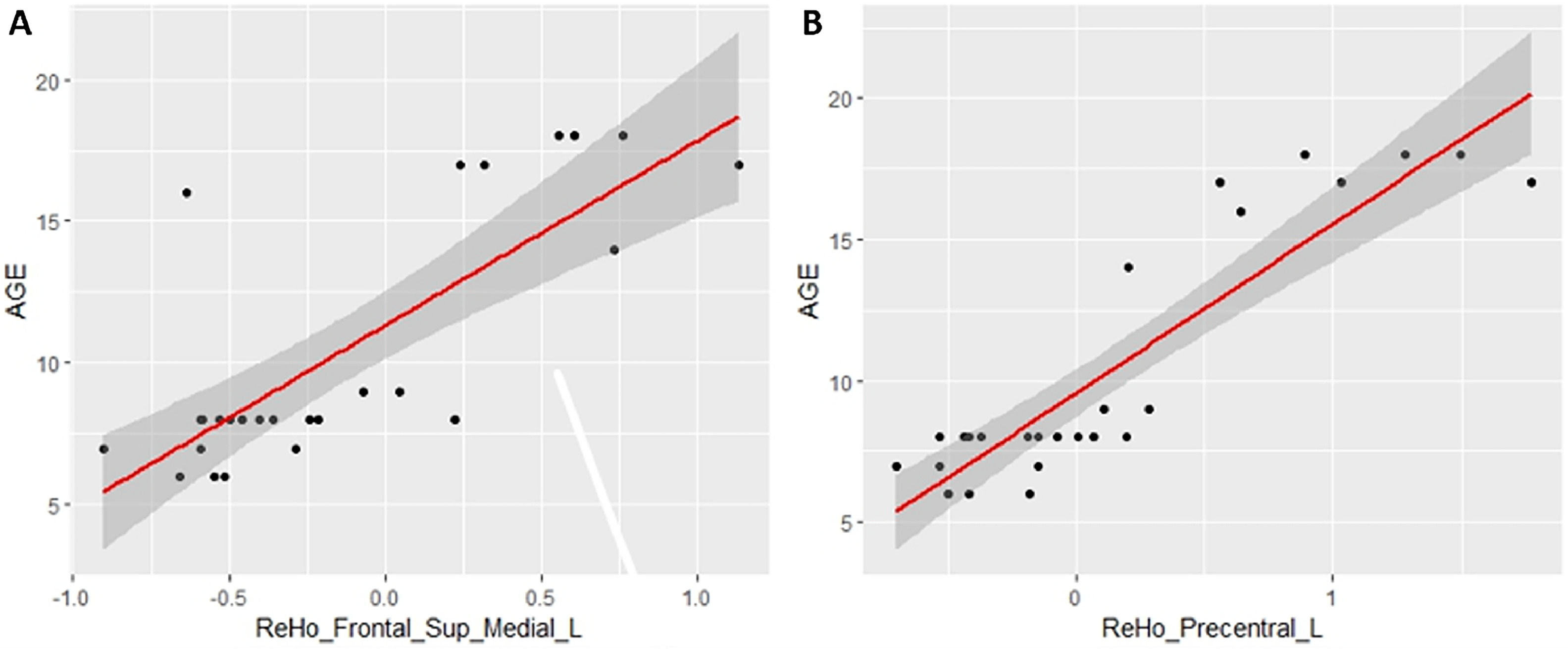
Data analysisFor the statistical analysis in fALFF and ReHo, DPABI was used and the estimation of the Pearson correlation with age and with the estimated IQ was calculated. As mentioned above, when talking about developmental stages, total grey matter volume was included as a covariate in all analyses. Significant differences in the study were reported using the Gaussian Random Field (GRF) multiple comparisons criterion (Eklund et al., 2016) with a cluster p-value of 0.001 and a cluster threshold of p = 0.05. An additional threshold with a minimum extent of 30 voxels for ReHo and 10 voxels for fALFF was set to exclude very small clusters, although they passed the strict permutation test with GRF correction. Finally, to improve the visualization of the correlation values and to increase the comprehension of the correlation direction, the significant values of fALFF and ReHo were extracted and were represented through a scatter plot together with the age of all individuals. It is important to highlight that, as in the significant correlations (Tables 2 and 3), the results include the covariable of total grey matter volume, here no covariable was included for the scatter plot representation.
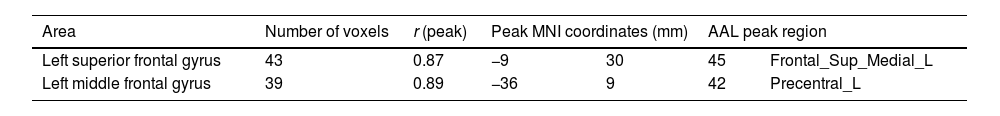
Significant correlations between ReHo maps and age.
| Area | Number of voxels | r (peak) | Peak MNI coordinates (mm) | AAL peak region | ||
|---|---|---|---|---|---|---|
| Left superior frontal gyrus | 43 | 0.87 | −9 | 30 | 45 | Frontal_Sup_Medial_L |
| Left middle frontal gyrus | 39 | 0.89 | −36 | 9 | 42 | Precentral_L |
MNI: Montreal Neurological Institute; AAL: Automatic Anatomical Labeling.
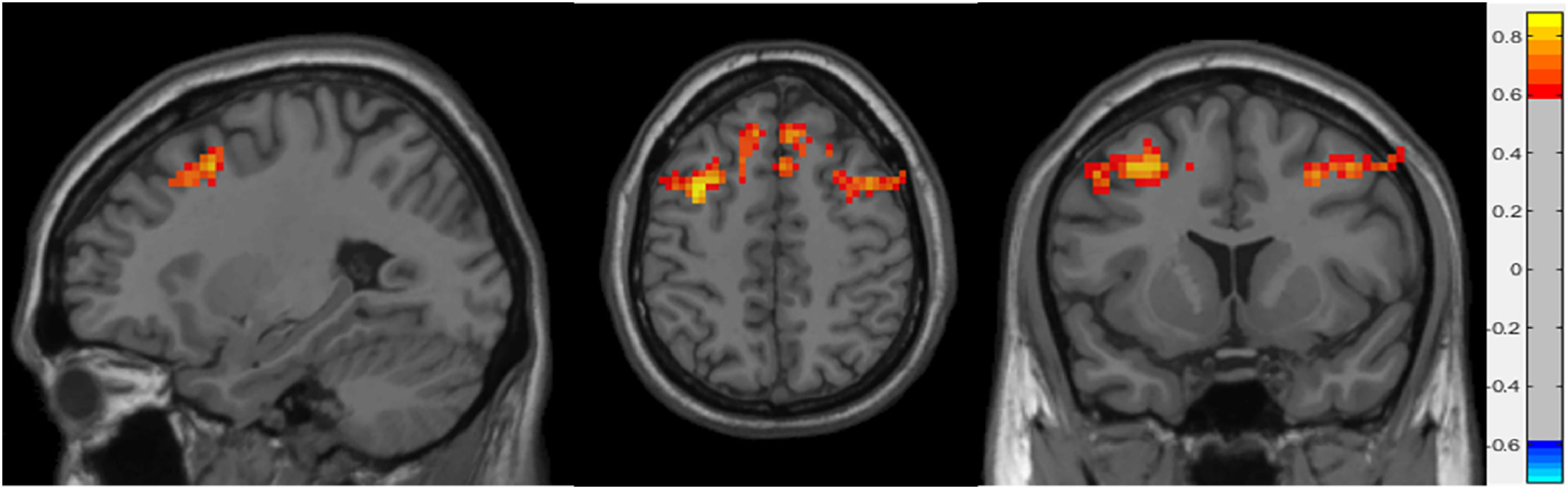
Table 2 shows the significant cluster between the fALFF values and age localized in MNI coordinates and the corresponding brain region defined by the Automatic Anatomical Labeling Atlas (AAL; Tzourio-Mazoyer et al., 2002). Fig. 1 shows the graphical representation of the correlation results visualized with DPARSF (Yan & Zang, 2010). Fig. 2 shows the graphical representation of the correlation using a scatter plot.
Sagittal, axial and coronal planes representation of significant correlations between fALFF whole-brain maps and age of the participants. The colour bar indicates the value of the correlation, with 0.8 (yellow) representing direct correlations and –0.6 (light blue) indirect correlations.
Regarding the correlation analyses using the whole brain fALFF maps and the estimated intelligence scores, no significant correlations were found.
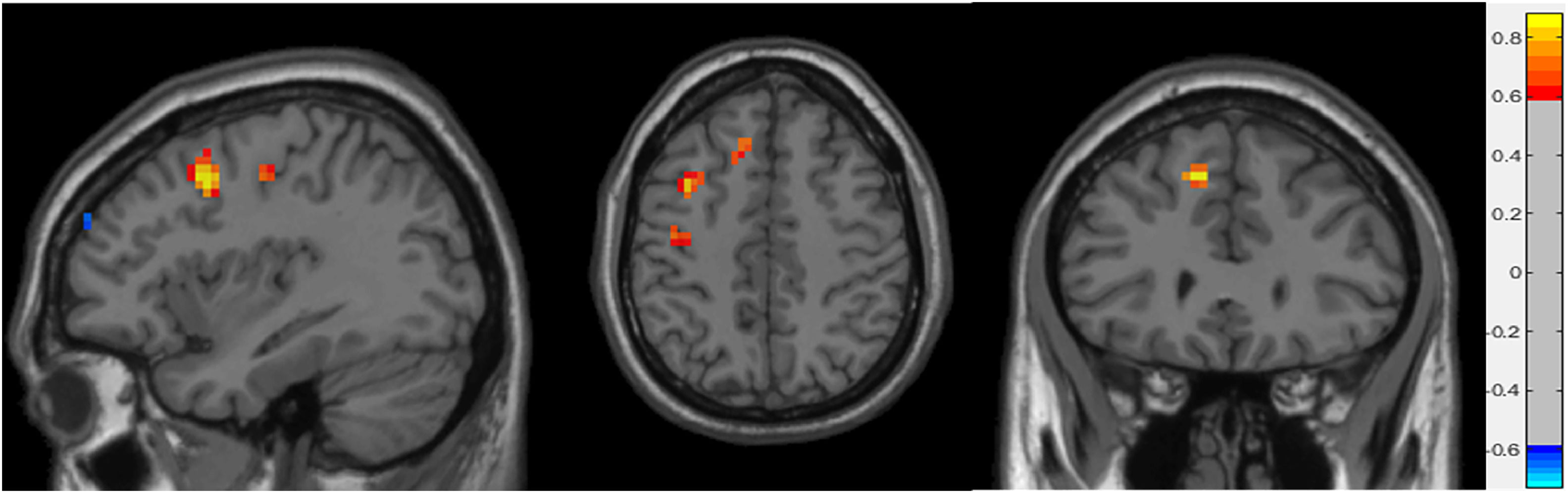
ReHo resultsTable 3 shows the significant cluster between the ReHo values and age localized in MNI coordinates and the corresponding brain region defined by the AAL. Fig. 2 shows the graphical representation of the correlation results visualized with DPARSF (Yan & Zang, 2010). Fig. 4 shows the graphical representation of the correlation using a scatter plot (Fig. 3).
Sagittal, axial and coronal planes representation of significant correlations between ReHo whole-brain maps and age of the participants. The colour bar indicates the value of the correlation, with 0.8 (yellow) representing direct correlations and –0.6 (light blue) indirect correlations.
Regarding the correlation analyses using the whole brain ReHo maps and the estimated intelligence scores, no significant correlations were found.
DiscussionIn this work we carried out an exploratory cross-sectional study of the relationships between brain connectivity, age and intelligence in a sample of children and adolescents from 6 to 18 years old, using fALFF and ReHo analysis. Our data suggest that areas of the left precentral cortex belonging to the premotor cortex and the supplementary motor area increase their connectivity in a manner closely correlated with age during ontogenetic development. Likewise, we also found that areas of the left premotor and supplementary motor cortex, and the left middle frontal gyrus corresponding to the frontal eye field, increase their regional homogeneity in a very closely related way with age during development from 6 to 18 years. In our study, we did not find correlations between the fALFF and ReHo maps with the intelligence of the children and adolescents in our sample.
The precentral cortex, including the premotor cortex and supplementary motor cortex, is part of the frontoparietal network that is related to executive functioning, spatial rotation and attentional tasks, as well as intelligence (Ptak et al., 2017; Takeuchi et al., 2017). al., 2018; Takeuchi et al., 2021). The more dorsal portion of this network which includes the precentral cortex is also involved in motor-type tasks and also in the cognitive substrate for motor actions (Ptak et al., 2017). Both the fALLF and ReHo connectivity maps of the precentral cortex of our sample that correlated positively with age also included areas of the left supplementary motor cortex. The supplementary motor area (SMA) is profusely connected with motor areas, sensory areas of the parietal lobe, the cerebellum, and areas more related to various cognitive functions such as the prefrontal cortex, the insula and the temporal cortex (Narayana et al., 2012). In this way, it would not only be a motor area, but a HUB related to various motor, perceptual, and cognitive networks (Narayana et al., 2012), also having a prominent role in working memory (Roth et al., 2014). Some works suggest that as children and adolescents grow, there is a strengthening of the cortical HUBs (Cao et al., 2016). The premotor cortex and the SMA would be a HUB related to an important diversity of brain functions that would probably develop at different time points. This would support our results, as it would explain at least in part why both fALFF maps and the ReHo map of the left precentral cortex showed such a high positive correlation with age in our study. However, we must remember that the work of Cao et al. (2016) suggests that this effect occurs in late childhood and adolescence, while our data suggest that at least for the HUBS of the precentral cortex this strengthening would occurs during a larger period of development time. Other works did not find a correlation between age and connectivity within the FPN, including the precentral cortex, in a sample of participants aged 7 to 25 years (DeSerisy et al., 2021).
The left frontal eye field (left FEF) showed increased ReHo with a very high and positive correlation with age. This brain area forms part of the DAN, which is classically attributed to the control of top-down visual attention, thus having an important role in controlling where attention is directed. Although it primarily receives visual information, the FEF also receives spatial information since it contains a topographic representation of the contralateral space and would be involved not only in the control of attention but also in oculomotor control and visuospatial working memory (Bedini & Baldauf, 2021). Some authors suggest that the frontal eye fields would also be part of the cingulum-opercular network in addition to the DAN (Ji et al., 2019). Thus, the left ocular field would be an important cortical HUB related to at least two “task positive” connectivity networks. Again, the increase in connectivity at the local level of this area in a highly correlated manner with age could be due to a progressive strengthening of the cortical HUBS (Cao et al., 2016); although we must also consider that in other works the development of the DAN and the cingulum-opercular network as a whole was not shown to be so linear with age (Jolles et al., 2011; Cao et al., 2016).
In our study, we did not find significant correlations between voxelwise connectivity measured by fALFF and ReHo and intelligence in our sample. Several studies suggest that the connectivity of task positive networks such as the FPN may be sensitive to individual differences in intelligence (Song et al., 2008; Takeuchi et al., 2018, 2021); and these differences could already be detected during ontogenetic development. Thus, some works suggest that the level of non-verbal intelligence positively correlates with right parietal-frontal connectivity, and with right parietal-cingulate connectivity in children aged 6 to 8 years (Langeslag et al., 2013). Other works suggest that the activity of the right fronto-parietal network is related to the level of intelligence in late childhood, while the activity of the left frontoparietal network is related to the level of intelligence in adolescents (Li & Tian, 2014). The work of DSerisy et al. (2021) Conducted in a large sample with an age range of 7 to 25 years, did not find correlations between intelligence and connectivity within the frontoparietal network, but it did find that young people with a greater anti-correlation between FPN and DMN had a higher intelligence quotient than those who had less mature connectivity between both networks. However, despite all these data, we did not find any relationship between brain connectivity measured by fALFF and ReHo and intelligence. Taken together, our results suggest that the development of the cortical HUBs corresponding to the left precentral cortex and the left frontal eye field that are closely related to cognitive functioning would present an age-related development and at least relatively independent of the difference between individuals in intelligence.
Our work has clear limitations. One of them is that we did not find connectivity correlated with age of other brain areas such as the superior parietal lobe, the precuneus, the cingulate or the temporal cortex among other brain areas, which are also considered very important HUBS of global connectivity (Van den Heuvel & Sporns, 2013).
The most important limitation of our work is its cross-sectional design since longitudinal approaches are always better used study the different aspects of ontogenetic development. This issue is what justifies our use of the term first study since we are aware of the need to generate the entire age range for a greater specification of the effects found in this paper. But, however, our work also has some notable qualities. The sample selection was very strict so we made sure that all participants were healthy through a very clinically rigorous evaluation. This is reflected in the large difference between the number of pre-selected participants in the initial sample and the people we ultimately resonated with.
ConclusionsFinally, as a main conclusion, we must highlight the very important correlations that we have found between the density of the connections of the left precentral cortex, the left frontal ocular field and the age of the participants. Another important conclusion is to promote the use of a voxelwise analysis of fALFF and ReHo, which have been used very little in the study of normal development. More longitudinal studies with larger sample sizes are needed to gain insight into the relationships between brain connectivity measured by fALFF and ReHo, ontogenetic development, and intelligence.