Background/Objective: Almost no attention has been paid to depression in Friedreich ataxia (FRDA), a highly disabling cerebellar degenerative disease. Our aim was to study the presence and the profile of depressive symptoms in FRDA and their relationship with demographic-disease variables and cognitive processing speed. Method: The study groups consisted of 57 patients with a diagnosis of FRDA. The Beck Depression Inventory-II was used to assess symptoms of depression. Speed of information processing was measured with a Choice Reaction time task. Results: The mean BDI score for patients was significantly higher than the mean score in the general population. Twenty one percent of participants scored in the moderate/severe range. A Cognitive-Affective score and a Somatic-Motivational score was calculated for each patient. Patients’ scores in both dimensions were significantly higher than the scores in the general population. Demographic and disease variables were not related with symptoms of depression, except for severity of ataxia. Depressive symptoms predict cognitive reaction times. The greater proportion of variance was explained by the Cognitive-Affective dimension. Conclusions: Our data show that both somatic-motivational and cognitive affective symptoms of depression are frequent in individuals with FRDA. In addition, depressive symptoms may influence cognition, especially, the cognitive and affective symptoms.
Antecedentes/Objetivo: La depresión en la ataxia de Friedreich (FRDA), una enfermedad degenerativa cerebelosa altamente incapacitante, ha recibido poca atención. Nuestro objetivo es evaluar la presencia y el perfil de los síntomas depresivos en FRDA y su relación con variables clínico-demográficas y la velocidad de procesamiento cognitivo. Método: Se estudiaron 57 pacientes con diagnóstico de FRDA. Se usó el Inventario de Depresión de Beck-II para evaluar los síntomas de depresión. La velocidad de procesamiento se midió con una tarea de tiempos de reacción. Resultados: La puntuación media de los pacientes en el BDI fue significativamente mayor que en la población general. El 21% de los participantes obtuvo puntuaciones en el rango moderado/grave. Se calculó una puntuación cognitiva-afectiva y una puntuación somática-motivacional para cada paciente. Las puntuaciones en ambas dimensiones fueron significativamente mayores que en la población general. Las variables clínico-demográficas no estaban relacionadas con los síntomas de depresión, a excepción de la gravedad de la ataxia. Los síntomas depresivos predicen los tiempos de reacción cognitivos. Conclusiones: Nuestros datos muestran que en la FRDA son frecuentes los síntomas de depresión, tanto los síntomas somático-motivacionales como los cognitivo-afectivos. Además, los síntomas de depresión pueden influir en la cognición, especialmente, los de tipo cognitivo-afectivo.
The presence of comorbid depression in neurodegenerative disorders is a well recognized fact. Depression has been intensively studied in disorders such as Multiple Sclerosis or Parkinson's disease. Regarding cerebellar degenerative ataxias, several studies have focused on depressive disorders in dominant spinocerebellar ataxias (e.g., Lo et al., 2016; Schmitz-Hübsch et al., 2011). However, almost no attention has been paid to depression in Friedreich ataxia (FRDA), the most frequent inherited ataxia.
Friedreich ataxia is a progressive neurodegenerative disorder that classically appears around puberty, rarely in early childhood, and in some cases later in life. The disease occurs mainly in Caucasians. The prevalence in Europe shows large regional differences ranging from 1:20.000 in the south-west to 1:250.000 or lower in the north and east (Vankan, 2013). FRDA is a multisystem autosomal recessive disease. The gene associated with the disease has been mapped to chromosome 9q13-q21.1 and encodes a small mitochondrial protein called frataxin (Campuzano et al., 1996). Most patients carry two expanded GAA alleles, which lead to a reduced expression of the frataxin. A small number of FRDA patients (1-4%) are compound heterozygous with a GAA mutation in one allele and a micromutation in the other. Frataxin deficiency leads to mitochondrial iron overload, defective energy supply and generation of reactive oxygen species (Bürk, 2017). The major lesions in FRDA are located in the spinal cord and the dentate nucleus of the cerebellum (Koeppen & Mazurkiewicz, 2013).
FRDA is a highly disabling disease. Neurological features characteristically include progressive gait and limb ataxia, dysarthria, weakness, ocular fixation instability and reflex and deep sensory loss. Non-neurological involvement includes hypertrophic cardiomyopathy, diabetes mellitus and skeletal deformations such as kyphoscoliosis, pes cavus and pes equinovarus. All patients ultimately require wheel-chairs. The mean duration from age at onset to age to wheelchair-bound is between 11 and 15 years (Parkinson, Boesch, Nachbauer, Mariotti, & Giunti, 2013). The average age of death is in the 5th decade (Manto & Lorivel, 2011).
Depression has a negative impact on quality of life and may have a possible influence on the disease course itself. However, there are very few studies that have examined depression in patients with genetically defined FRDA and most of them have screened patients for symptoms of depression as a variable to controlling in cognitive studies. Using the Beck Depression Inventory, Corben and collaborators found mean scores in BDI in 10-15 individuals with FRDA ranging from 8 to 9.8 (Corben, Delatycki, Bradshaw, Churchyard & Georgiou-Karistianis, 2011; Corben et al., 2010; Corben, Georgiou-Karistianis, Bradshaw, Hocking, Churchyard & Delatycki, 2011). Applying cut-off scores suggested for BDI, mean scores in patients groups were in the range of “normality” (0-9) but they were significantly greater than scores of control participants without FRDA in two of these studies. Depression was also assessed in previous studies by our group. The mean BDI score of thirty-six FRDA patients (M=12.4; SD=10.27), was significantly greater than control participants’ scores. No significant differences were observed between patients with a typical and a late disease onset (Nieto, Correia, de Nóbrega, Montón & Barroso, 2013; Nieto et al., 2012). In the study of Da Silva et al. (2013) the mean BDI score of twenty-two FRDA patients was 9.63 and 36.3% fulfilled the criteria for major depression.
In sum, although mean scores reported in the aforementioned studies are not in the range of moderate or severe depression, available data show that a certain percentage of individuals with FRDA may suffer from depression. This fact has been recognized with the inclusion of mental health issues as a topic in the recent Consensus Clinical Management Guidelines for Friedreich ataxia (Corben, Lynch, Pandolfo, Schulz & Delatycki, 2014). Despite the above, there are no studies specifically addressed at examining depressive symptomatology in FRDA. Our aim is to study the presence and the profile of depressive symptoms in FRDA and their relationship with demographic, clinical and genetic variables. In addition, we aim to study the relationship between depressive symptoms and the slowing of information processing speed, probably the most consistent impairment reported in cognitive studies on FRDA (Nachbauer et al., 2014; Nieto et al., 2012, 2013).
MethodParticipantsA descriptive observational study was conducted. Fifty-seven individuals, homozygous for a GAA expansion in intron 1 of the FXN gene participated in the study. Patients were consecutively recruited from three Spanish hospitals: Hospital Universitario Nuestra Señora Candelaria (Santa Cruz de Tenerife), Hospital Marqués de Valdecillas (Cantabria), and Hospital La Paz (Madrid). Patients with a history of alcohol or drug dependence, major psychiatric illness (other than depression) or neurological disease (other than FRDA) were excluded.
InstrumentsSeverity of ataxia was quantified with the Scale for the Assessment and Rating of Ataxia (SARA) scoring from 0 to 40, higher scores reflecting a greater disease severity (Schmitz-Hübsch et al., 2006). The presence and severity of depressive symptoms were evaluated with the Spanish adaptation of the Beck Depression Inventory-II (BDI-II; Beck, Steer and Brown, 2011), which is an instrument that has been used in numerous previous studies with medical samples (e.g., Heldner et al., 2017; Ichikura et al., 2017; Morys, Pąchalska, Bellwon & Gruchała, 2016; Smeltere, Kuznecovs, & Erts, 2017). BDI is a 21 item self-rating questionnaire, known for its practicality, reliability, and accuracy (Sanz, Izquierdo & García-Vera, 2013). Scores on each item can range from zero (symptom absent) to 3 (pronounced presence of symptom), yielding a potential range of scores from 0 to 63. A score of 0-13 is classified as minimal depressive symptoms, 14-19 as mild, 20-28 as moderate, and 29-63 as severe.
Choice Reaction time task of the Reaction Unit/Vienna System (RT) was used to assess speed of information processing (Schuhfried, 1992). This system permits the dissociation of the cognitive component (Decision time) and the motor component (Motor time). A red light appeared randomly in a background of distractor stimuli at which time the subject was instructed to remove his/her index finger of the dominant hand from a rest button and press another key as quickly as possible. Decision time is the time interval between the appearance of the stimuli and release of the finger. Motor Time is the time interval between release of the finger and depression of the second key. Decision time is a cognitive measure of information processing speed, whereas Motor time reflects motor and coordination deficit (Botez-Marquard & Botez, 1993).
ProcedureParticipants were informed about the aim of the investigation and participated voluntarily. All subjects gave their informed consent and were not provided with any incentives to participate. All procedures were in accordance with the Helsinki Declaration for human research and were approved by the ethics committee of University of La Laguna. Demographic (age, gender, education), clinical (age at disease onset, duration of disease) and genetic data (length of expanded repeat in both shorter, GAA1, and longer, GAA2, alleles) were recorded. Each patient underwent a neurological examination. The questionnaires were completed and collected from patients after they agreed to participate. The Choice Reaction time task was administered by an experienced neuropsychologist.
Data analysisFirstly, descriptive statistics were calculated in order to know the demographic and disease characteristics of the sample. The possible sex differences in demographic and clinical parameters were studied using the one-way ANOVA method. Secondly, descriptive statistics of BDI-II measures of the sample were calculated and compared with norms of the Spanish population with the Student's t test for one sample and controlling Type I errors with the False Discovery Rate method (Benjamini & Hochberg, 1995). The effect sizes of comparisons were estimated with Cohen's d. The prevalence with which each individual item was positively endorsed (i.e., a non-zero response) was calculated and compared with the norms of the Spanish population using the Chi-square test. Thirdly, scores for the Cognitive-Affective and Somatic-Motivational factors of BDI were calculated averaging the scores of the items from each dimension. Mean scores of factors were compared to each other and with population scores using the Student's t test. The effect sizes of comparisons were estimated by the Cohen's d. Fourthly, correlation analyses between BDI-II scores and demographic-disease variables were carried out. Finally, the lineal regression model was used to determine the influence of depressive symptoms on cognitive reaction times adjusting for severity of ataxia (the only clinic-demographic variable with significant correlation with the BDI-II and factors’ scores). The percentage of explained variance for each factor was calculated by four measures of variance decomposition (Bi, 2012): (1) The LMG measure is an average of R2 for all possible order permutations for each predictive variable of lineal regression; (2) the PMVD measure is an analogue method to the LMG, but keeping in mind the weight of each predictive variable. This method is appropriate for controlling the effect of collinearity because it eliminates the possibility of cooperative suppression; (3) the First measure indicates the explained variance for each predictive variable when it is entered in the model in first place; (4) the Last measure indicates the explained variance for each predictive variable when it is entered in the model in last place. Analyses were performed using the ULLRtoolbox.v.1.0.R for R (Hernández, 2017), except the analysis of PMVD that was conducted with the non-US version of the relaimpo package for R (Grömping, 2006), downloaded from https://prof.beuth-hochschule.de/groemping/software/relaimpo-relative-importance-of-regressors/download-package-non-us-version/.
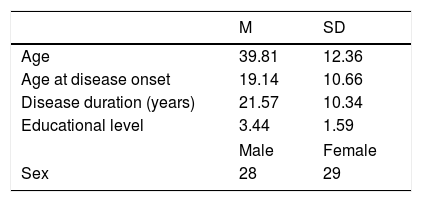
ResultsDemographic and clinical data are shown in Table 1. The age range of the participants was 19-66 years, with a disease duration range of 1-49 years. The age at onset was<25 years in the majority of patients (68%). The age range of disease onset was 3-43 years. Educational level was classified according to International Standard Classification of Education criteria (ISCED; UNESCO, 2011). Most of the patients had not attended university and were below level five of ISCED (67%).
Disease severity as measured by the SARA shows that the sample included people with a range of disease severity from 6 to 37. The sample had relatively equal gender groups (51% Females). One-way ANOVA showed that there were no differences in demographic and clinical parameters between gender groups.
The mean BDI score for FRDA patients was 13.62 (SD=9.52). This score is significantly higher than the mean score in the general population (t(56)=3.15, p <.01), according to populations norms (Beck et al., 2011). According to the cut-off scores proposed by the manual text, 59.6% of participants scored in the “minimal” range (0–13), 19.3% of participants scored in the “mild” range (14–19), 17.5% scored in the “moderate” range (20–28), and 3.5% scored in the “severe” range (28–63).
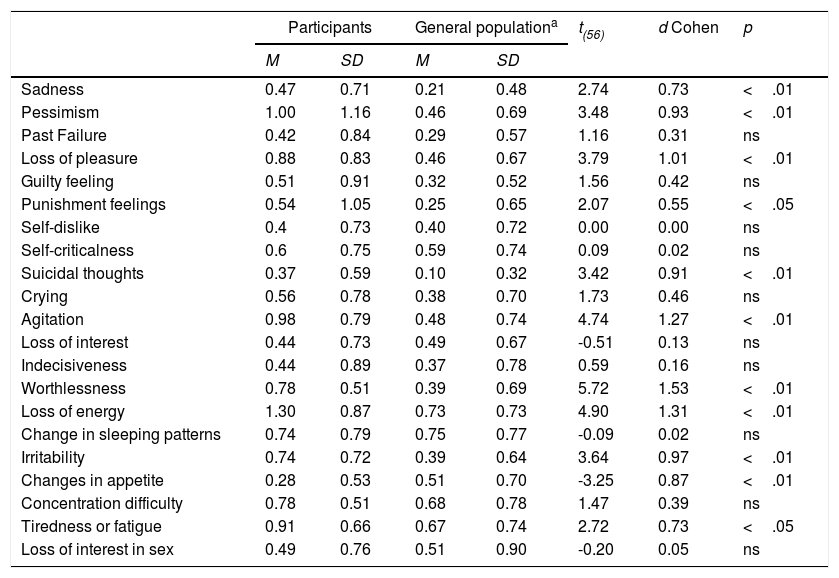
Table 2 shows results at item level. Means ranged from 0.28 to item 20, Changes in appetite to 1.3 to item 17, Loss of Energy. Patients’ means scores were compared to general population norms by the one-sample t-test with corrections for multiple comparisons. Patients’ scores were significantly higher than the values for the general population in Sadness (I1), Pessimism (I2); Loss of Pleasure (I4), Punishment Feelings (I6), Suicidal thoughts (I9), Agitation (I11), Worthlessness(I14), Loss of Energy (I15) Irritability (I17). Patients scored significantly less in comparison to the general population in Changes in Appetite (I18). Effect sizes were large for the majority of significant comparisons (Table 3).
Descriptive statistics of items and t-test results.
| Participants | General populationa | t(56) | d Cohen | p | |||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| Sadness | 0.47 | 0.71 | 0.21 | 0.48 | 2.74 | 0.73 | <.01 |
| Pessimism | 1.00 | 1.16 | 0.46 | 0.69 | 3.48 | 0.93 | <.01 |
| Past Failure | 0.42 | 0.84 | 0.29 | 0.57 | 1.16 | 0.31 | ns |
| Loss of pleasure | 0.88 | 0.83 | 0.46 | 0.67 | 3.79 | 1.01 | <.01 |
| Guilty feeling | 0.51 | 0.91 | 0.32 | 0.52 | 1.56 | 0.42 | ns |
| Punishment feelings | 0.54 | 1.05 | 0.25 | 0.65 | 2.07 | 0.55 | <.05 |
| Self-dislike | 0.4 | 0.73 | 0.40 | 0.72 | 0.00 | 0.00 | ns |
| Self-criticalness | 0.6 | 0.75 | 0.59 | 0.74 | 0.09 | 0.02 | ns |
| Suicidal thoughts | 0.37 | 0.59 | 0.10 | 0.32 | 3.42 | 0.91 | <.01 |
| Crying | 0.56 | 0.78 | 0.38 | 0.70 | 1.73 | 0.46 | ns |
| Agitation | 0.98 | 0.79 | 0.48 | 0.74 | 4.74 | 1.27 | <.01 |
| Loss of interest | 0.44 | 0.73 | 0.49 | 0.67 | -0.51 | 0.13 | ns |
| Indecisiveness | 0.44 | 0.89 | 0.37 | 0.78 | 0.59 | 0.16 | ns |
| Worthlessness | 0.78 | 0.51 | 0.39 | 0.69 | 5.72 | 1.53 | <.01 |
| Loss of energy | 1.30 | 0.87 | 0.73 | 0.73 | 4.90 | 1.31 | <.01 |
| Change in sleeping patterns | 0.74 | 0.79 | 0.75 | 0.77 | -0.09 | 0.02 | ns |
| Irritability | 0.74 | 0.72 | 0.39 | 0.64 | 3.64 | 0.97 | <.01 |
| Changes in appetite | 0.28 | 0.53 | 0.51 | 0.70 | -3.25 | 0.87 | <.01 |
| Concentration difficulty | 0.78 | 0.51 | 0.68 | 0.78 | 1.47 | 0.39 | ns |
| Tiredness or fatigue | 0.91 | 0.66 | 0.67 | 0.74 | 2.72 | 0.73 | <.05 |
| Loss of interest in sex | 0.49 | 0.76 | 0.51 | 0.90 | -0.20 | 0.05 | ns |
Normative data from Spanish adaptation of the Beck Depression Inventory-II (BDI-II; Beck et al., 2011).
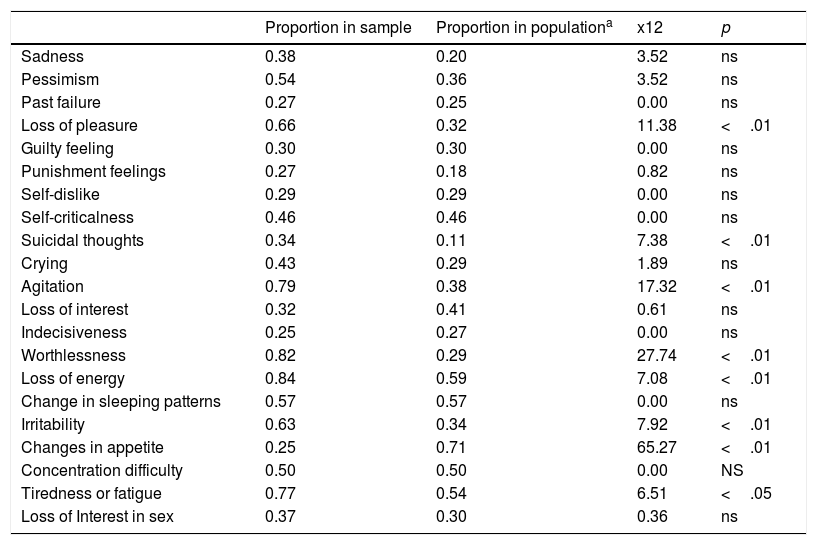
Proportion of response choices greater than zero for each item and Chi-Square test results.
| Proportion in sample | Proportion in populationa | x12 | p | |
|---|---|---|---|---|
| Sadness | 0.38 | 0.20 | 3.52 | ns |
| Pessimism | 0.54 | 0.36 | 3.52 | ns |
| Past failure | 0.27 | 0.25 | 0.00 | ns |
| Loss of pleasure | 0.66 | 0.32 | 11.38 | <.01 |
| Guilty feeling | 0.30 | 0.30 | 0.00 | ns |
| Punishment feelings | 0.27 | 0.18 | 0.82 | ns |
| Self-dislike | 0.29 | 0.29 | 0.00 | ns |
| Self-criticalness | 0.46 | 0.46 | 0.00 | ns |
| Suicidal thoughts | 0.34 | 0.11 | 7.38 | <.01 |
| Crying | 0.43 | 0.29 | 1.89 | ns |
| Agitation | 0.79 | 0.38 | 17.32 | <.01 |
| Loss of interest | 0.32 | 0.41 | 0.61 | ns |
| Indecisiveness | 0.25 | 0.27 | 0.00 | ns |
| Worthlessness | 0.82 | 0.29 | 27.74 | <.01 |
| Loss of energy | 0.84 | 0.59 | 7.08 | <.01 |
| Change in sleeping patterns | 0.57 | 0.57 | 0.00 | ns |
| Irritability | 0.63 | 0.34 | 7.92 | <.01 |
| Changes in appetite | 0.25 | 0.71 | 65.27 | <.01 |
| Concentration difficulty | 0.50 | 0.50 | 0.00 | NS |
| Tiredness or fatigue | 0.77 | 0.54 | 6.51 | <.05 |
| Loss of Interest in sex | 0.37 | 0.30 | 0.36 | ns |
Normative data from Spanish adaptation of the Beck Depression Inventory-II (BDI-II; Beck et al., 2011).
The most frequently endorsed item (response choices greater than zero) was Loss of Energy which was endorsed by 82.45% of the patients, while the less frequently endorsed items were Indecisiveness and Changes in appetite (24.56% in both cases). The percentage of patients reporting any problem was significantly higher than that of the general population in several items: Loss of Pleasure (4), Suicidal thoughts (I9), Agitation (I11) Worthlessness (I14), Loss of Energy (I15) Irritability (I17) and Fatigue (I20). In the case of Changes in appetite, the proportion of patients who endorsed this item was lower than that of the population norms.
BDI-II appears to represent two factors measuring cognitive-affective and somatic-motivational symptoms (BDI-II; Beck et al., 2011). Based on these two symptom groups, a Cognitive-Affective score and a Somatic-Motivational score was calculated for each patient (mean of the item scores in each dimension). Items included in the Cognitive-Affective dimension were Sadness (1), Past Failure (3), Guilty Feeling (5), Punishment Feelings (6), Self-Dislike (7), Self-Criticalness (8), Suicidal thoughts (9), Crying (10) and Irritability (17). Items included in the Somatic-Motivational score were Loss of Pleasure (4), Loss of interest (12), Worthlessness (14), Loss of Energy (15), Concentration Difficulty (19), Tiredness or Fatigue (20) and Loss of Interest in Sex (21). The mean score in the Somatic-Motivational dimension was significantly higher than the mean in the Cognitive-Affective dimension (t (56)=-4.34, p <.01, d=1.16). Patients’ scores in both dimensions were significantly higher than the scores in the general population (Cognitive-Affective: t (56)=2.51, p=0.00, d=0.67; Somatic-Motivational:t (56)=3.91, p <.01, d=1.05), with a greater effect size in the Somatic-Motivational dimension.
A significant positive correlation was found between total BDI-II scores and SARA scores (r=.28, p <.05). No significant correlations were found for age, education, age at onset of disease, duration and GAA repeats. When scores in each dimension were considered instead of the global BDI-II, the association between depressive symptoms and SARA was only observed for the Somatic-Motivational dimension (r=.38, p <.01).
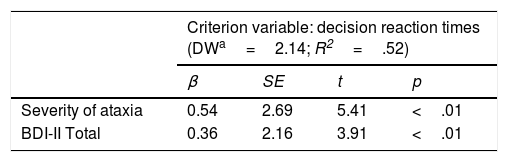
Lineal regression analyses were carried out to determine the influence of depressive symptoms on cognitive reaction times adjusting for severity of ataxia (Table 4). The regression model explained 52% of decision reaction time variability (F (2, 49)=7.18, p <.01). BDI scores explained 22% of the variance of decision reaction times (Table 5). In order to determine whether this predictive capability is also observed when factors other than BDI global scores are considered, lineal regression analyses were performed with Cognitive-Affective scores and Somatic-Motivational scores as predictor variables, also adjusting for severity of ataxia (Table 4). The regression model explained 55% of decision reaction time variability (F (3, 48)=5.20, p <.01). Severity of ataxia explained 40% of this variance and Cognitive-Affective and Somatic-Motivational factors explained 15% jointly. Considering each factor separately, only Cognitive-Affective factor was a significant predictor (Table 4). Measures of variance decomposition were calculated to determine the proportion of variance corresponding to each factor (Table 5). The Cognitive-Affective factor explained between 7-15% of variance, while somatic-Motivational factor explained between 1-8%.
Lineal regression analyses to determine the influence of each predictor adjusting for severity of ataxia.
| Criterion variable: decision reaction times (DWa=2.14; R2=.52) | ||||
|---|---|---|---|---|
| β | SE | t | p | |
| Severity of ataxia | 0.54 | 2.69 | 5.41 | <.01 |
| BDI-II Total | 0.36 | 2.16 | 3.91 | <.01 |
| Criterion variable: decision reaction times (DW=2.08; R2=.55) | ||||
|---|---|---|---|---|
| β | SE | t | p | |
| Severity of ataxia | 0.56 | 2.73 | 5.43 | <.01 |
| Cognitive-affective factor | 0.35 | 50.73 | 2.76 | <.01 |
| Somatic-Motivational factor | 0.06 | 49.73 | 0.45 | > .05 |
Proportion of variance corresponding to each predictor.
| First | Last | LMGc | PMVDd | |
|---|---|---|---|---|
| Severity of ataxia | .40 | |||
| Cognitive-affective factore | .15 | .07 | .11 | .14 |
| Somatic-Motivational factore | .08 | .01 | .04 | .01 |
First measure informs on the explained variance value when the variable is the first to be entered in the model.
Last measure informs on the explained variance value when the variable is the last to be entered in the model.
LMG measure decomposes R2 into nonnegative contributions based on semipartial correlation coefficients that automatically sum to the total R2.
Few studies have focused on depression in FRDA despite being the most frequent of cerebellar degenerative ataxia. The fact that depression is not considered a hallmark clinical feature of FRDA could be the reason for this negligence. However, depression has been included as a topic in the recent clinical management guidelines for FRDA (Corben et al., 2014). This inclusion recognizes that depression may have a major negative impact on the health and well-being of people with FRDA.
In the present study, the mean score of FRDA patients in BDI-II is significantly higher than that expected in the general population (Beck et al., 2011), but it is in the range considered as minimal/mild severity of depression. This result is similar to the ones reported in other studies of FRDA patients with previous versions of BDI (Corben, Delatcky et al., 2011; Corben et al., 2010; Corben, Georgiou-Karistianis et al., 2011; Da Silva et al., 2013; Nieto et al., 2012, 2013). When patients were classified using categories of severity, most of them (59.5%) were in the range of minimal severity, but 21% of FRDA patients were classified as showing moderate to grave symptoms of depression, a higher percentage than that expected in the general population. This result is comparable with that obtained in samples of dominant spinocerebellar ataxias (Lo et al., 2016; Schmitz-Hübsch et al., 2011) and slightly lower than the one reported by Da Silva et al. (2013) for FRDA patients based on a clinical examination. The presence of clinically relevant depressive symptoms in 21% of patients is an important result that should be taken into account in the planning of the clinical management and intervention for FRDA patients.
It could be argued that this prevalence may be overestimated because some symptoms of depression overlap those of FRDA. An analysis of profile of symptoms suggests that this is not the case. Compared to the general population, the severity and/or prevalence was higher for symptoms of sadness, pessimism, loss of pleasure, punishment feelings, suicidal thoughts, agitation, worthlessness, loss of energy, irritability and fatigue. Some of these symptoms, such as loss of energy or fatigue, may represent a physical manifestation of FRDA, but other items have a clear cognitive or affective content. In order to better define the profile of depressive symptoms, the different clinical aspects assessed in the BDI-II according to factorial analyses were considered separately: a Somatic-Motivational dimension and a Cognitive –Affective dimensions (Sanz, Perdigón & Vázquez, 2003). Both dimensions were significantly higher than the scores in the general population. These results indicate that the symptoms of depression in FRDA are not solely attributable to the physical manifestation of the disease.
BDI-II items examine all the symptomatic diagnostic criteria of DSM-V of depressive disorders. Although the patients were not clinically examined for a depression diagnostic, the analysis of the self-reported symptoms shows six of the nine symptoms of diagnostic criteria for major depressive disorders have a significant intensity and prevalence in the FRDA sample, including depressed mood and loss of pleasure. It is noteworthy that suicidal thoughts is one of these symptoms. This result underlines the relevance of considering the risk of suicide in individuals with FRDA and managing it proactively (Corben et al., 2014).
Regarding demographic and disease parameters, age, education, age at onset, duration and GAA repeats were not related with symptoms of depression. The only parameter related with the BDI-II scores was the severity of ataxia. Thus, demographic and disease variables do not seem to play an important role in the appearance of depressive symptoms, except for severity of ataxia. The lack of association of depressive symptoms with age, age at onset and duration of illness is in agreement with the results reported by Da Silva et al. (2013) although the severity of ataxia in that study was not related with BDI scores either.
The results of the present study indicate that depressive symptoms predict decision reaction times, after controlling for severity of disease. When the proportion of variance corresponding to each factor was studied, the greater proportion of variance was explained by the Cognitive-Affective dimension and the Somatic-Motivational dimension was not a significant predictor. The impairment in speed of processing is a consistent component of the cognitive profile of individuals with FRDA and it could also affect performance in other cognitive domains (Nachbauer et al., 2014; Nieto et al., 2012). Thus, this result indicates that depression may negatively influence the cognitive functioning of FRDA patients and reflects a greater relative importance for Cognitive-Affective symptoms.
The present study has several limitations. Although the sample size is larger than in previous studies, a greater sample may be necessary for a more precise estimation of the prevalence of depressive symptoms in FRDA. On the other hand, depressive symptoms were assessed with a self-reported questionnaire and the patients were not clinically examined for depression diagnostic. Future studies should include a clinical examination for depression.
In sum, this is the first study on the prevalence and profile of depressive symptoms in FRDA. The data show that both somatic-motivational and cognitive affective symptoms of depression are frequent in individuals with FRDA. In addition, depressive symptoms may influence cognition, especially, cognitive and affective symptoms.
Depression in FRDA could have an endogenous origin or could be an exogenous reaction to disability or a combination of the two. On the one hand, the cerebellum is not only involved in motor functions but also in cognitive and emotional processes. More specifically, the vermis and fastigial nucleus are considered as the limbic cerebellum and their lesions have been related with emotional dysregulation (Schmahmann, 2013). The major lesion of the central nervous system in FRDA is the atrophy of dentate nucleus and its efferent fibers, but imaging studies have also shown structural anomalies in other grey and white matter regions, with the vermis being one of these regions (Della Nave et al., 2008; França et al., 2009; Rizzo et al., 2011). Although vermis degeneration is not always observed (Dogan et al., 2016; Selvadurai et al., 2016), this damage in the limbic cerebellum could be linked to the occurrence of depression in FRDA. On the other hand, depression in FRDA could be considered as “reactive” and secondary to the psychosocial stress of a chronic disease with perspectives of a progressive deterioration, the associated disability and dependence and the absence of an effective treatment. The greater affectation of the Somatic-Motivational dimension observed in this study may be evidence in favour of this interpretation. Items included in this dimension such as loss of pleasure, loss of energy, worthlessness are characteristics of reactive depression and fit into the conceptual framework of learned helplessness well (Abramson, Seligman & Teasdale, 1978; Weiner, 1985). In addition, the association observed between the severity of ataxia and severity of depressive symptoms may be further evidence in favour of this proposal. However, it is necessary to point out that other disease variables, such as duration or GAA repeats, are not related with symptoms of depression. In the authors’opinion, there is not enough data at present to resolve the problem of the etiology of depression in FRDA and further research is needed. In any case the two proposals are not mutually exclusive.
Depression may probably be undiagnosed in these patients and, consequently, the treatment of the disorder is not initiated. Therefore, the present study suggests that FRDA patients should be regularly evaluated in terms of risks for developing depression so they can be offered appropriate support. Given the characteristics of FRDA, patients could benefit from psychological interventions aimed at improving their sense of control, self-efficacy and acceptance of the difficulties related to the disease. Cognitive behavioral therapy has been shown to be effective in reducing depressive symptoms in patients with multiple sclerosis or Parkinson's disease (Egan, Laidlaw & Starkstein, 2015; Hind et al., 2014). In addition, training in self-management is currently used, as well as psychological interventions based on mindfulness, to increase the sense of control and acceptance of the difficulties in these patients, with satisfactory results (Berzins et al., 2017; Fiest et al., 2016; Muñoz et al., 2016; Pickut et al., 2015; Sajatovic et al., 2017; Verkerke, Sepopo, Kilbourn & Lageman, 2016). FRDA patients may benefit from these therapeutic approaches to reduce their depressive symptoms.
FundingThis work was supported by the Ministerio de Economia y Competitividad (Spain) [grant number PSI 2015-67514-P].
The authors thank Dr. Berciano (Hospital Marqués de Valdecillas, Santander) and Dr. Arpa (Hospital La Paz, Madrid) for providing access to patients and for their helpful assistance.