Depression is often present concurrently with coronary artery disease (CAD), a disease with which it shares many risk factors. However, the manner in which depression mediates and moderates the association between traits (including biomarkers, anthropometric indicators, lifestyle behaviors, etc.) and CAD is largely unknown.
MethodsIn our causal mediation analyses using two-step Mendelian randomization (MR), univariable MR was first used to investigate the causal effects of 108 traits on liability to depression and CAD. The traits with significant causal effects on both depression and CAD, but not causally modulated by depression, were selected for the second-step analyses. Multivariable MR was used to estimate the direct effects (independent of liability to depression) of these traits on CAD, and the indirect effects (mediated via liability to depression) were calculated. To investigate the moderating effect of depression on the association between 364 traits and CAD, a cross-sectional phenome-wide interaction study (PheWIS) was conducted in a study population from UK Biobank (UKBB) (N=275,257). Additionally, if the relationship between traits and CAD was moderated by both phenotypic and genetically predicted depression at a suggestive level of significance (Pinteraction≤0.05) in the PheWIS, the results were further verified by a cohort study using Cox proportional hazards regression.
ResultsUnivariable MR indicated that 10 of 108 traits under investigation were significantly associated with both depression and CAD, which showed a similar direct effect compared to the total effect for most traits. However, the traits “drive faster than speed limit” and “past tobacco smoking” were both exceptions, with the proportions mediated by depression at 24.6% and 7.2%, respectively. In the moderation analyses, suggestive evidence of several traits was found for moderating effects of phenotypic depression or susceptibility to depression, as estimated by polygenic risk score, including chest pain when hurrying, reason of smoking quitting and weight change. Consistent results were observed in survival analyses and Cox regression.
ConclusionThe independent role of traits in CAD pathogenesis regardless of depression was highlighted in our mediation analyses, and the moderating effects of depression observed in our study may be helpful for CAD risk stratification and optimized allocation of scarce medical resources.
Coronary artery disease (CAD), an atherosclerotic disease, is considered the leading cause of death in humans in both developed and developing countries (Quertermous & Ingelsson, 2016). Depression, a widespread mental illness and a major contributor to disability, is mainly manifested by feelings of sadness, diminished enjoyment in activities, sleep disturbances, alterations in both body weight and appetite, and even suicidal tendencies (Friedrich, 2017). Depression tends to occur concurrently with CAD (Carney & Freedland, 2017). For instance, 30-45% of persons with CAD experience clinical depression symptoms (Celano & Huffman, 2011). The prevalence of depression in patients with CAD vary from 14% to as high as 47% (Lett et al., 2004). Depression independently increases the risk of CAD and its complications, leading to a higher probability of all causes of cardiac mortality (Khawaja, Westermeyer, Gajwani, & Feinstein, 2009). In addition, depression and CAD have many risk factors in common, such as body mass index (BMI) (Torgersen et al., 2022) and triglyceride levels (Khandaker et al., 2020). This may explain the concurrence of CAD and depression as they may share some common pathogenic process, such as inflammation (Corrado et al., 2010; Furtado & Katzman, 2015), long-term exposure of the cardiovascular system to endocrine, and autonomic dysfunction (Grippo & Johnson, 2009). However, it is difficult to determine whether associations between risk factors and CAD are mediated/moderated by depression or act independently.
Mendelian randomization (MR) analyses can investigate the causal association between exposures and outcomes by using genetic variants as instrumental variables, which makes MR studies less affected by residual confounding and reverse causality than conventional observational studies. Two-step MR for mediation analysis, an extension of MR study, can quantify whether the causal association of a risk factor with disease outcome is mediated by other factors (Walker et al., 2022). The MR strategy has been used to infer the causal relationship between depression and CAD (de Geus, 2021), but no MR study to date has investigated whether the risk factors increase the risk of CAD through depression and assessed the extent to which the associations between the risk factors and CAD are causally mediated by depression. In addition to the mediating effect of depression on the associations between risk factors and CAD, depression can also moderate such associations. For example, the association between BMI and cardiovascular disease mortality was different in participants with or without depression in a U.S. population (Jia & Li, 2021). Nonetheless, no study has systematically examined how depression moderates the association between CAD and a range of traits at the phenome-wide level.
Thus, we hypothesize that the causal associations between various traits and CAD may be mediated or moderated by depression. Various methods including two-step MR, phenome-wide interaction study (PheWIS) and cohort study have been performed to investigate the potential effect of depression. This research is crucial for understanding the relationship between depression and CAD, helping to stratify disease risks and optimize the allocation of limited medical resources.
MethodsStudy design for causal mediation analysesOur causal mediation analyses were performed using two-step MR, in which numerous GWAS summary statistics datasets were used (Walker et al., 2022). Specifically, univariable MR was first used to investigate the causal effects of 108 traits (Supplementary Fig. 1) on liability to depression and CAD (first step). Moreover, the causal effects of depression on these traits were also tested because mediation analyses require no reciprocal causal association between the exposure and the mediator. The traits that showed significant causal effects on both depression and CAD but could not be causally modulated by depression were selected for the second-step analyses. Multivariable MR was performed with these traits as exposures and liability to depression as a covariable to estimate the direct effect of traits on CAD. The indirect effects of traits on CAD were calculated by multiplying the estimate of the effect of traits on the liability of depression obtained from univariable MR by the effect of liability of depression on CAD obtained from multivariable MR, where the trait was adjusted as a covariable. Thus, the total effect, direct effect (independent of liability to depression) and indirect effect (mediated via liability to depression) of the traits on CAD were estimated.
Data sources for causal mediation analysesUsing a published selection procedure (Supplementary Fig. 1) (Walker et al., 2022), we selected minimally adjusted GWAS summary statistics for the targeted traits that were derived from the largest population of European or mixed ancestry of both sexes in the IEU OpenGWAS database. The GWASs of the investigated traits were mainly from UK Biobank (UKBB), and the GWASs for liability to depression and CAD were obtained from Psychiatric Genomics Consortium (PGC) (Wray et al., 2018) and CARDIoGRAM consortium (Nikpay et al., 2015), respectively (Supplementary Table 1).
Statistical methods for causal mediation analysesIn the univariable MR, the instrumental variables (IVs) were selected based on the following criteria: (1) IVs showed genome-wide significant association with the traits or phenotypes (P < 5 × 10−8); (2) IVs were independent as clumped using a 10 Mb window and linkage disequilibrium (LD) of R2 < 0.001 with the European population in 1000 Genomes Project as reference panel; (3) the variants were biallelic variants and had minor allele frequency (MAF) > 0.01; and (4) each of the instruments consisted of ≥ 10 variants. For the IVs of depression, a relaxed threshold of P value (5 × 10–7) was used because only two single nucleotide polymorphisms (SNPs) had a genome-wide significant association with depression (P<5 × 10–8). The inverse-variance weighted method was employed as the main method in univariable MR analyses, and weighted median and MR-Egger methods were conducted as sensitivity analyses. Furthermore, horizontal pleiotropy was assessed using a MR-Egger intercept test. Power estimation was achieved using mRnd (Brion, Shakhbazov, & Visscher, 2013). A false discovery rate (FDR) threshold of 5% was used for multiple comparison correction. All the univariable MR analyses were conducted in R using the TwoSampleMR package. In the multivariable MR analyses, the IVs selection criteria were the same as those of univariable MR, except that clumping was performed against the trait or liability depression, whichever harbored the smaller number of IVs. The conditional F statistics were calculated to evaluate the instrument strength. The multivariable MR analyses were conducted in R using the MVMR package.
Study design for moderation analysesThe potential moderating effect of depression on the relationship between 364 traits and CAD was first tested using a cross-sectional PheWIS, in which both phenotypic and genetically predicted depression were used. Because statistical analyses inherently have poor power to examine interactions compared to that of main effects (Marshall, 2007), we used a cohort study to verify the results from the PheWIS if the relationship between traits and CAD can be moderated by both phenotypic and genetically predicted depression at a suggestive level of significance (Pinteraction≤0.05). In our cohort analyses, the multiplicative interaction effects of the phenotypic depression/depression-polygenic risk score (PRS) and traits on CAD were examined using Cox proportional hazards regression. Using this strategy, we estimated the moderating effect of depression on the association between traits and CAD in a cohort study.
Data sources for moderation analysesIndividual-level data from UKBB were used in the moderation analyses. Participants aged 40-69 years were enrolled in the UKBB cohort study from 2007 to 2010. Information on various questionnaires was collected at the assessment centers in the UK, and physical measurements and blood sample collection were performed at the baseline of the cohort.
Phenotypic depression was defined though numerous sources. Self-reported depression was evaluated with a cutoff score ≥3 from the Patient Health Questionnaire (PHQ-2) collected at baseline, a 2-item assessment tool specifically designed to screen for depression. Additionally, clinical depression occurring before the cohort start date was identified according to electronic health records (ICD-10 codes F32-F39), as reported previously (Dregan et al., 2020; Löwe, Kroenke, & Gräfe, 2005).
Polygenic risk score (PRS) is formed by combining the effects of numerous genetic variants into a score, which has been widely used to predict the risk of developing a certain disease over a person's lifetime (Figtree, Vernon, & Nicholls, 2022; Lambert, Abraham, & Inouye, 2019). The UKBB participants were genotyped on the BiLEVE Axiom chip or UK Biobank Axiom array, and subsequent phasing and imputation were performed (Bycroft et al., 2017). The imputed SNPs were used to calculate the PRS of depression using PRSice‐2 (S. W. Choi & O'Reilly, 2019) for each of the individuals based on large‐scale GWAS summary statistics from PGC (Wray et al., 2018), in which the P value threshold was set as identical to that of mediation analysis (P=5 × 10–7). Participants who were of non-European ancestry or had a high heterozygosity/missing rate, sex discrepancies, sex chromosome aneuploidy or a third-degree relatedness with other participants were excluded from the analysis.
CAD was defined by self-reported or electronic health records (ICD-9 codes 410-414 or ICD-10 codes I20-I25) according to a recent publication (Luo, Au Yeung, & Schooling, 2021). For the analyses of CAD incidence, adults having CAD before their first visit to the assessment center were excluded, leading to a study population of 275,257 adults. The person-time for each participant was censored at the first CAD occurrence, death date, or the last follow-up date (30 June 2020 in England, 31 October 2016 in Scotland and 29 February 2016 in Wales), whichever came first.
In the cohort study, participants who reported chest pain/discomfort but indicated no such pain when walking at a normal pace were asked the question “Do you get this pain or discomfort when you walk uphill or hurry?”, and the dichotomous trait “chest pain when hurrying” was defined as positive if participants gave a positive answer to this question. The former tobacco smokers in UKBB were asked "Why did you stop smoking?", and the dichotomous trait “Stop smoking because of health precaution” was defined as positive if reason was because of health precaution, whereas this trait was coded as negative for other reasons, such as financial reasons. The participants in UKBB were asked the question "Compared with one year ago, has your weight changed?", and dichotomous traits weight gain and weight loss were coded according to the answer to this question.
Statistical methods for moderation analysesA cross-sectional screening of the multiplicative interaction effect of phenotypic/genetic predicted depression and CAD on 364 traits was performed using phenome scan analysis tool (PHESANT), an opensource tool that can test the association between exposure (the multiplicative interaction term in our case) and a large number of outcomes in UKBB (Millard, Davies, Gaunt, Davey Smith, & Tilling, 2018; Millard et al., 2019). Continuous variables were inverse normal rank transformed to ensure their normality, and age, sex and the first ten principal components (accounting for the population structure) were adjusted as covariables in the analyses. In the analyses of CAD incidence using Cox regression, the proportional hazards assumption was examined using the Kolmogorov-type supremum test. Additionally, SAS 9.4 software (SAS Institute Inc., Cary, NC) was used to perform the Cox regression, and the survival curves were generated using R.
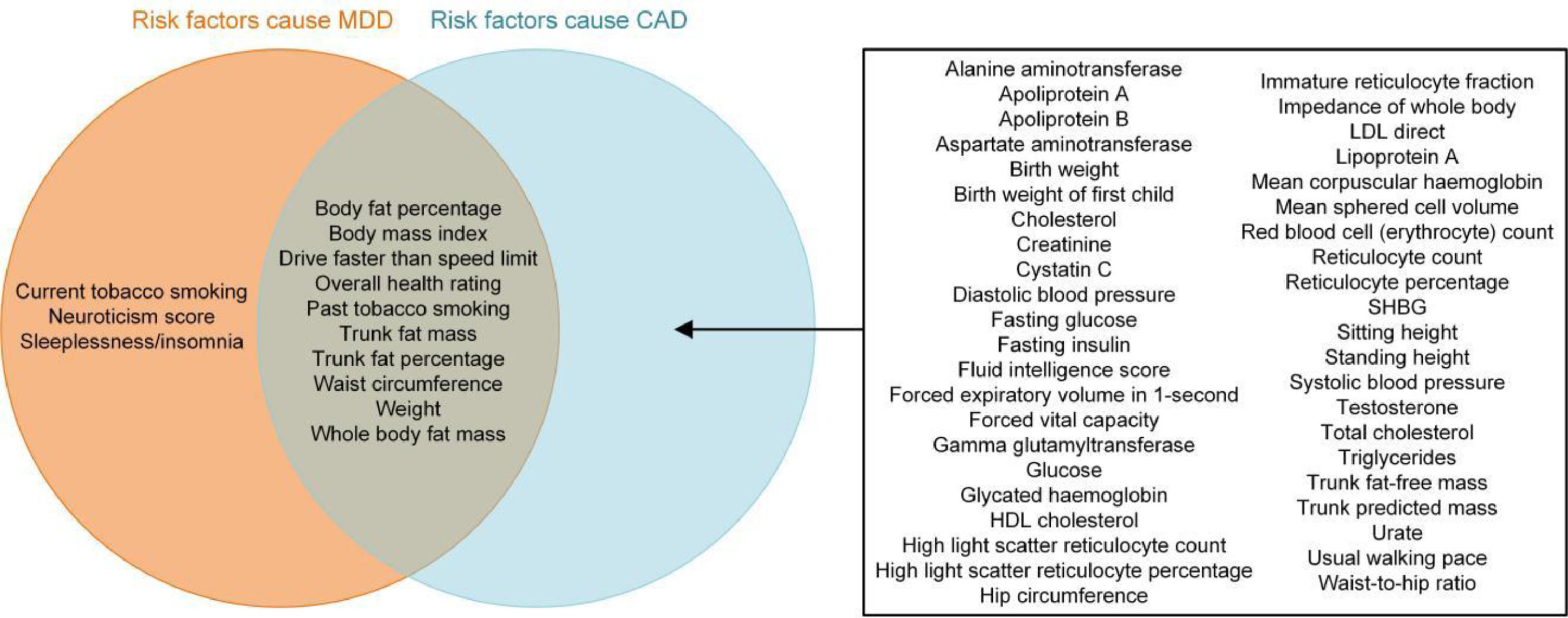
ResultsCausal mediation analysesAccording to the exclusion criteria in Supplementary Fig. 1, 108 traits were included in our causal mediation analyses based on two-step MR. Detailed information about the trait GWAS summary datasets is presented in Supplementary Table 1. Univariable MR using the IVW method indicated that depression was causally associated with CAD, with an odds ratio (OR) (95% confidence intervals; CIs) of 1.17 (1.05-1.31) (Supplementary Table 2). Univariable MR also indicated that 13 and 53 of 108 traits were significantly associated with depression and CAD, respectively (Supplementary Figs. 2 and 3, Supplementary Table 2 and 3). Moreover, the causal associations of 10 traits with both depression and CAD were identified (Fig. 1), and bidirectional univariable MR suggested that none of these 10 traits could be causally induced by depression (the only trait showing bidirectional causal association with depression was neuroticism/neuroticism score). In addition, power estimation indicated that these MR analyses had sufficient statistical power (Supplementary Table 4). Thus, these 10 traits were moved on to the second step of our causal mediation analyses (Supplementary Table 5). Multivariable MR analyses revealed that the direct effects of most traits were similar to their total effects on CAD, whereas the traits “drive faster than speed limit” and “past tobacco smoking” were no longer associated with CAD after adjusting for depression as a covariable (reflected by the direct effects of these two traits) (Fig. 2 and Supplementary Table 6). The proportion of the partially medicating effect by depression for these two traits was 24.6% and 7.2%, respectively (Supplementary Table 7). The range of the conditional F statistics of these traits in multivariable MR was 14.6-34.0, suggesting good instrument strength. In sum, our mediation analyses indicated that the causal effects of the investigated traits on CAD were independent of the effect of depression.
The results of two-step MR showing the total, direct (independent of liability to depression) and indirect (mediated by liability to depression) effects of the traits on liability to CAD. MDD, major depressive disorder; CAD, coronary artery disease; OR, odds ratio; CI, confidence interval.
The individual-level data from UKBB were used to study the moderating effect of depression on the relationship between traits and CAD (Supplementary Fig. 4), and the descriptive characteristics indicated that CAD patients were more likely to be older, males, overweight, smokers, nondrinkers, less educated, and from areas with a less negative Townsend deprivation index (TDI) (Table 1). Consistent with the results of univariable MR in causal mediation analyses, depression was correlated with CAD (Table 1).
Descriptive characteristics of the study population (N=275,257).
| Overall | CAD | |||
|---|---|---|---|---|
| Characteristics | Yes | No | P value | |
| Age (years), Mean (SD) | 56.3 (8.0) | 60.3 (6.7) | 56.1 (8.0) | <0.01 |
| Sex (female), N (%) | 150,614 (54.7) | 6,001 (35.6) | 144,613 (56.0) | <0.01 |
| TDI, Mean (SD) | -1.6 (2.9) | -1.3 (3.1) | -1.6 (2.9) | <0.01 |
| Education (have a degree), N (%) | 99,584 (36.2) | 4,695 (27.8) | 94,889 (36.7) | <0.01 |
| BMI (≥25), N (%) | 179,167 (65.1) | 13,008 (77.1) | 166,159 (64.3) | <0.01 |
| Smoking (yes), N (%) | 123,316 (44.8) | 9,297 (55.1) | 114,019 (44.1) | <0.01 |
| Drinking (yes), N (%) | 200,014 (72.7) | 11,736 (69.5) | 188,278 (72.9) | <0.01 |
| Depression (yes), N (%) | 26,675 (9.7) | 2,087 (12.4) | 24,588 (9.5) | <0.01 |
Abbreviation: TDI: townsend deprivation index; CAD: coronary artery disease; BMI: body mass index; N: number; SD: standard deviation.
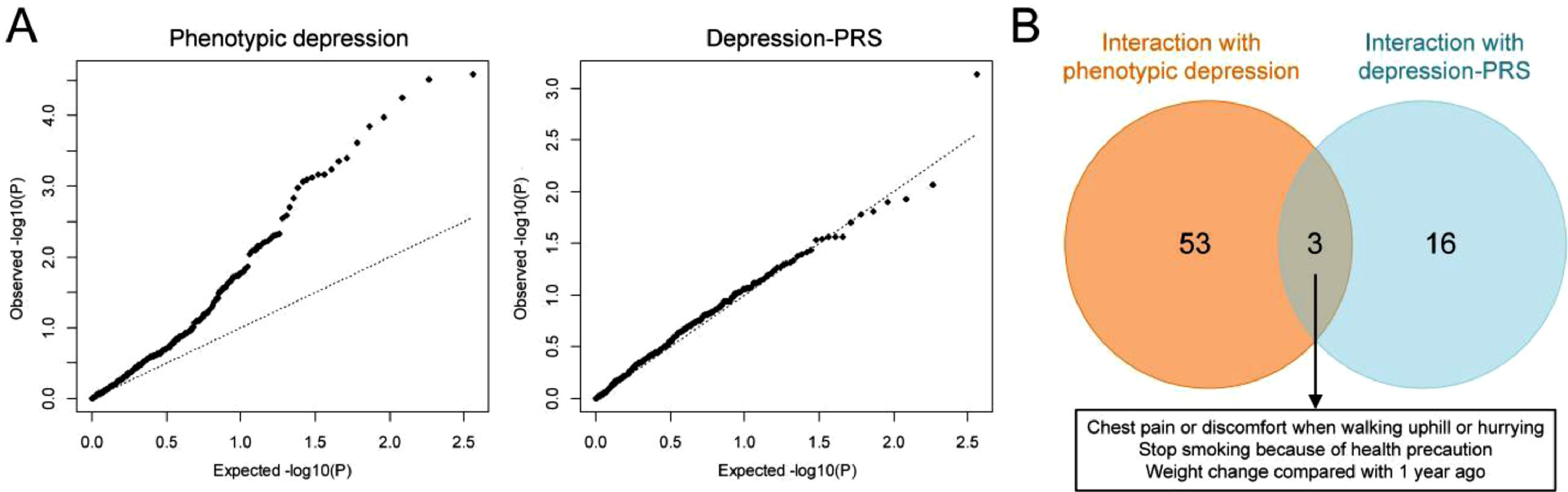
According to the statuses of depression risk alleles of UKBB participants, the PRS of depression was calculated for each individual, which was externally weighted by the effect size estimate of a depression GWAS that did not overlap with the UKBB population. A substantial increase in depression risk was observed in participants with elevated depression-PRS (Supplementary Fig. 5). A cross-sectional PheWIS was then conducted to investigate the moderating effect of depression on the relationship between 364 traits and CAD (Fig. 3A). The results indicated that the association between 17 of 364 traits and CAD could be moderated by phenotypic depression because the testing of interaction terms survived 5% FDR correction (Supplementary Table 8). Notably, an additional 28 traits showed significant results when increasing the FDR threshold to 20% (Supplementary Table 8), a method used in interaction analyses (Hall, Moorman, Millikan, & Newman, 2005; Sansbury et al., 2005). However, no interaction testing of depression-PRS survived FDR correction (Supplementary Table 9).
The results of the phenome-wide interaction study (PheWIS). (A) QQ plot of PheWIS. (B) Venn diagram showing the moderating effect of phenotypic/genetic predicted depression on the relationship between traits and CAD at a suggestive level of significance (Pinteraction≤0.05). CAD, coronary artery disease.
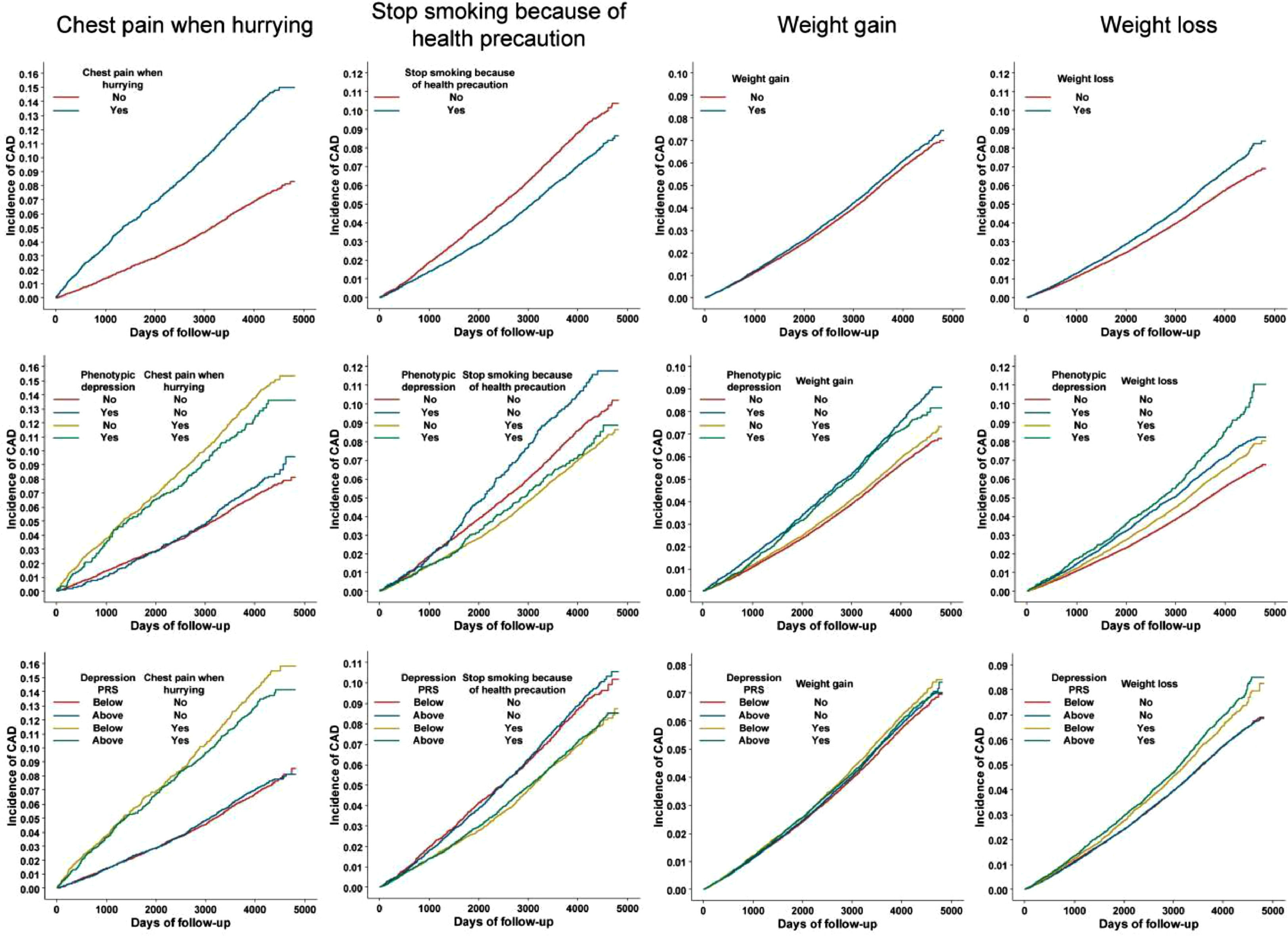
Because of the inherent poor statistical power in assessing interaction effects, we used a cohort design to verify the results of the traits showing suggestive significance (Pinteraction≤0.05) in the PheWIS of both phenotypic and genetically predicted depression (i.e., the three traits in Fig. 3B). A post-hoc power analysis was also performed for the cohort study (Supplementary Table 10). Cox regression revealed that the trait “chest pain when hurrying” was associated with increased risk of CAD with hazard ratio (HR) (95% CIs) of 2.05 (1.89-2.23), and this elevation of CAD risk was diminished in participants with phenotypic depression or higher susceptibility to depression (Pinteraction for phenotypic depression and depression-PRS was 0.06 and 0.03, respectively) (Table 2). In addition, the trait “stop smoking because of health precaution” was associated with a decreased risk of CAD, and this trait had a strong effect in depressive individuals (Pinteraction=0.06) (Table 2). The cohort study also indicated an increased CAD risk in participants with weight gain compared to one year ago, but the effect was not significant in participants with depression or higher liability to depression (Pinteraction for both phenotypic depression and depression-PRS was 0.03) (Table 2). Moreover, weight loss led to a higher CAD incidence, and this effect was further enhanced in individuals with higher susceptibility to depression (Pinteraction=0.01) (Table 2). We also found that the trends of HR differences between depressive and non-depressive individuals were generally the same in the sex-stratified population compared to that of the overall population (Supplementary Table 11). The moderating effects of phenotypic/genetic predicted depression on the relationships between these traits and CAD incidence were also observed in survival analyses (Fig. 4 and Supplementary Table 12). Interestingly, depression decreased the CAD incidence in people who had chest pain or discomfort when walking uphill or hurrying according to the survival curves (Fig. 4).
Hazard ratio for CAD incidence by statuses of phenotypic/genetic predicted depression and investigated traits.
| N | HR (95% CI) | Pinteraction | ||
|---|---|---|---|---|
| Chest pain when hurrying | ||||
| Overall | No | 24636 | Ref | |
| Yes | 6835 | 2.05 (1.89-2.23) | ||
| Phenotypic depression | 0.06 | |||
| Yes | No | 3275 | Ref | |
| Yes | 1268 | 1.72 (1.41-2.11) | ||
| No | No | 21361 | Ref | |
| Yes | 5567 | 2.12 (1.94-2.32) | ||
| Depression-PRS | 0.03 | |||
| Above the median | No | 12323 | Ref | |
| Yes | 3412 | 1.92 (1.71-2.16) | ||
| Below the median | No | 12313 | Ref | |
| Yes | 3423 | 2.19 (1.95-2.45) | ||
| Stop smoking because of health precaution | ||||
| Overall | No | 26347 | Ref | |
| Yes | 38805 | 0.79 (0.75-0.84) | ||
| Phenotypic depression | 0.06 | |||
| Yes | No | 3016 | Ref | |
| Yes | 3452 | 0.68 (0.58-0.81) | ||
| No | No | 23331 | Ref | |
| Yes | 35353 | 0.81 (0.76-0.86) | ||
| Depression-PRS | 0.14 | |||
| Above the median | No | 13044 | Ref | |
| Yes | 19532 | 0.79 (0.73-0.85) | ||
| Below the median | No | 13303 | Ref | |
| Yes | 19273 | 0.80 (0.73-0.86) | ||
| Weight gain | ||||
| Overall | No | 197313 | ref | |
| Yes | 74076 | 1.05 (1.01-1.09) | ||
| Phenotypic depression | 0.03 | |||
| Yes | No | 16396 | Ref | |
| Yes | 9791 | 0.94 (0.86-1.03) | ||
| No | No | 180917 | Ref | |
| Yes | 64285 | 1.05 (1.01-1.09) | ||
| Depression-PRS | 0.03 | |||
| Above the median | No | 98675 | Ref | |
| Yes | 37021 | 1.02 (0.97-1.07) | ||
| Below the median | No | 98638 | Ref | |
| Yes | 37055 | 1.08 (1.03-1.14) | ||
| Weight loss | ||||
| Overall | No | 230638 | Ref | |
| Yes | 40751 | 1.19 (1.14-1.24) | ||
| Phenotypic depression | 0.77 | |||
| Yes | No | 21401 | Ref | |
| Yes | 4786 | 1.20 (1.07-1.34) | ||
| No | No | 209237 | Ref | |
| Yes | 35965 | 1.18 (1.13-1.23) | ||
| Depression-PRS | 0.01 | |||
| Above the median | No | 115229 | Ref | |
| Yes | 20467 | 1.23 (1.16-1.30) | ||
| Below the median | No | 115409 | Ref | |
| Yes | 20284 | 1.15 (1.09-1.23) |
Abbreviation: CAD: coronary artery disease; HR: Hazard ratio; N: number; CI: confidence interval; Ref: reference; PRS: polygenic risk score.
In this study, we investigated the mediating and moderating effects of depression on the association between numerous traits and CAD using a variety of methods, including two-step MR, PheWIS and cohort studies. The mediation analyses using GWAS summary datasets indicated that most traits acted on CAD independent of depression, whereas partial mediation effects of several traits, such as “drive faster than speed limit”, were identified. The moderation analyses using individual-level data from UKBB suggested that depression moderated the relationships between CAD and several traits, including the status of chest pain when hurrying, smoking cessation due to health precautions and weight change.
Bidirectional causal association between neuroticism and depression revealed by Mendelian randomization analysesUnivariable MR from step one of the two-step MR analyses for mediation revealed a bidirectional causal association between neuroticism and depression. This is consistent with the notions that neuroticism, featured by negative emotions, is a risk factor for depression (Speed, Hemani, Speed, Børglum, & Østergaard, 2019), and the therapeutic role of selective serotonin reuptake inhibitors in depression treatment is through reducing neuroticism (Quilty, Meusel, & Bagby, 2008). In addition, depression increases neuroticism scores (Karsten et al., 2012). Thus, neuroticism was not selected as the trait in mediation analyses because of its bidirectional association with depression.
Partial mediation effect of depression on the causal association between “drive faster than speed limit” and CADForward univariable MR analysis showed the negative causal association of driving overspeed with depression. Driving faster than the speed limit is considered risky driving behavior, and individuals who tend to exceed the speed limit are often characterized as bold, impulsive and aggressive, which is largely opposite to the characteristics of depression. Thus, people who drive overspeed may have a lower likelihood of depressive symptoms, which is consistent with our findings.
Our reverse univariable MR analysis suggested that depression was not causally associated with driving overspeed. The current literature mainly focuses on studying driving performance in patients with depression, and the conclusion is still conflicting (Wickens, Smart, & Mann, 2014). Some epidemiological studies have found correlation between depression and aggressive driving (Scott-Parker, Hyde, Watson, & King, 2013; Scott-Parker, Watson, King, & Hyde, 2011; Wickens et al., 2014), whereas others have reached different conclusions. For example, people with depression are normally immersed in negative self-emotions such as melancholy and sadness, showing decreased attention and perception of the outside world, which could result in cognitive distraction and affect driving ability (Cunningham & Regan, 2016). The capability to divide attention among competing driving-related tasks, such as overspeed, overtaking and rapid line changes, was more challenging for drivers with depression (Bulmash et al., 2006). However, a study revealed that risky driving that includes actions such as speeding was unrelated to depression evaluated throughout adolescence (Vassallo et al., 2008).
As drive faster than speed limit was not causally modulated by depression, this trait was moved on to the second step of the two-step MR analyses for mediation. The results indicated that driving overspeed could lower the risk of CAD by lowering the risk of depression.
Moderation effect of depression on the association between “chest pain when hurrying” and CADChest pain is a common complaint, with at least 20% of the general population experiencing chest pain at some point in their lifetime (Wong et al., 2004). In a retrospective cohort study, the prevalence of chest pain in the emergency department was reported to be 16.4% (Pedersen et al., 2019). Chest pain can be found of cardiac and noncardiac origin, some of which are benign, while others are probably life-threatening (Haasenritter et al., 2015). In cases of cardiac origin, one of the reasons for chest pain in CAD is the mismatch between myocardial oxygen demand and supply, usually induced by vigorous exercise, such as walking uphill or hurrying. Whereas in cases of noncardiac origin, chest pain is usually not brought on by exercise (Cassar, Holmes, Rihal, & Gersh, 2009), it is instead caused by factors such as visceral hyperalgesia, esophageal issues, and psychiatric abnormalities (Fang & Bjorkman, 2001).
Our studies consistently indicated that among individuals who reported having chest pain, those with walking uphill/hurrying-induced chest pain were more likely to develop CAD than those whose chest pain could not be induced by walking uphill/hurrying. Indeed, intense outdoor activities and physical exertion, such as walking uphill/hurrying, promote acute changes in blood pressure and heart rate, resulting in major cardiovascular events like acute myocardial infarction (Mieda et al., 2021; Willich et al., 1993).
Interestingly, we found that in the population of people with walking uphill/hurrying-induced chest pain, participants with depression had a lower incidence of CAD than nondepressed individuals, suggesting the protective role of depression on CAD incidence in this specific population. One reason for this observation is that patients with depression tend to lose interest or pleasure in normal activities, including exercise (Clinic, 2018), leading to a reduced risk of heart events. Thus, attention should be given to people with exercise-induced chest pain, especially those who are willing to perform vigorous exercises.
Moderation effect of depression on the association between “reason of smoking quitting” and CADSmoking increases the risk of CAD by endothelial injury (Dikalov et al., 2019; Lavi et al., 2007), formation of atheroma (McEvoy et al., 2015), and arterial thrombosis (CDC, 2005). Therefore, smoking cessation is an important step in the prevention of CAD (Rigotti & Clair, 2013). However, different reasons to quit smoking may have different influences on the risk of CAD. People who quit smoking due to illness tend to have a higher prevalence of cardiovascular diseases and diabetes than those who quit smoking due to other reasons (Liu et al., 2018). People who give up smoking because of health precautions, on the other hand, tend to be more aware of their own health and adopt a healthier lifestyle, which has been proven to be associated with a significantly reduced risk of CVD (Anderson et al., 2017; Soares et al., 2014).
Our study consistently suggested that in the population of both depressed and nondepressed people, people who stopped smoking due to health precautions had a lower incidence of CAD than those who stopped smoking for other reasons. Interestingly, our study also showed that giving up smoking because of health precautions had a stronger effect in reducing CAD risk in people with depression than in those without depression. Thus, our results highlight the importance of health education to increase the awareness of disease prevention in depressive patients, which may lead to a high level of cardiovascular benefits.
Moderation effect of depression on the association between “weight gain” and CADWeight gain has been considered a well-known risk factor for CAD (Chei, Iso, Yamagishi, Inoue, & Tsugane, 2008; S. Choi et al., 2018; Katsoulis et al., 2021; Stevens, Erber, Truesdale, Wang, & Cai, 2013; Willett et al., 1995). In a Japanese prospective study, weight gain was significantly associated with a 40% increased risk of CAD (Chei et al., 2008). Another study also showed that long-term weight gain of over 2.7% was linked to an increased risk of CAD (Stevens et al., 2013). Our result is in accordance with previous studies, where people with weight gain were more likely to develop CAD than participants without weight increase in the overall population. However, depression-stratified analyses indicated that the effect of weight gain on increasing CAD risk was not significant in participants with depression.
One explanation for this observation may be the function of leptin. Leptin, an adipocyte-derived hormone that controls energy homeostasis (Zhang & Chua, 2017), has been demonstrated to be a contributor to CAD by increasing vascular injury, oxidative stress and arterial pressure (Koh, Park, & Quon, 2008; Lu & Akanji, 2020). Previous studies have shown that leptin promotes atherosclerosis, which leads to CAD (Bodary et al., 2005; Chiba et al., 2008). Weight gain usually means more accumulation of fat and leptin. However, experimental results indicated that animals subjected to a stressful environment that mimicked depression exhibited lower levels of leptin than controls (Chuang et al., 2010; Lu, Kim, Frazer, & Zhang, 2006). Thus, the inverse relationship between depression and leptin may weaken the effect of weight gain on CAD risk in patients with depression.
In addition, weight gain increases total blood volume and cardiac output, thereby placing a great burden on the cardiovascular system, which may be further aggravated by exercise (Alpert, 2001). Because depressed individuals were less physically active, they were less likely to suffer from the elevated level of cardiovascular burden that leads to CAD. Therefore, screening for depression can help identify people with weight gain who have a higher risk of CAD incidence.
Moderation effect of depression on the association between “weight loss” and CADWeight loss and an underweight status have both been proposed as indicators for the deteriorating health condition (Gaddey & Holder, 2014; Gangadharan et al., 2017; Wang et al., 2021), with weight loss having been identified as a noticeable sign for worse clinical outcomes of CAD (Danaei et al., 2016; Kennedy et al., 2006; Lopez-Jimenez et al., 2008). The consequences of weight loss may differ according to the reasons for weight loss. Intentional weight loss was linked to favorable or neutral outcomes, whereas unintentional weight loss was linked to unfavorable outcomes (Harrington, Gibson, & Cottrell, 2009; Pack et al., 2014). However, even purposeful weight loss does not always result in improved outcomes (Pack et al., 2014). It has been reported that weight loss over a short period was linked to increased immediate CAD risk (HR: 1.46, 95% CI: 1.18,1.81) (Stevens et al., 2013).
Our study was in line with previous studies showing that weight loss compared to one year ago increased CAD incidence in the overall study population. The coexistence of depression and weight loss is very common (Weissenburger, Rush, Giles, & Stunkard, 1986). Less activity in certain brain regions prevent depressed individuals from feeling rewarded when eating, leading to appetite decrease and weight loss (Simmons et al., 2016). Moreover, some antidepressants might cause further weight loss due to side effects such as nausea and diarrhea (Arterburn et al., 2016). On the other hand, since food and high fat help relieve stress (Ulrich-Lai, Fulton, Wilson, Petrovich, & Rinaman, 2015), dramatically cutting calories and losing weight might put people at more risk of depression. Indeed, a study indicated that people with weight loss were more likely to experience depression (Jackson, Steptoe, Beeken, Kivimaki, & Wardle, 2014).
Both depression and weight loss can increase the risk of cardiovascular problems, and our study found that the effect of weight loss on increasing CAD risk was stronger in participants with higher depression susceptibility, as defined by the PRS of depression. However, phenotypic depression did not moderate the relationship between weight loss and CAD risk, and the moderating effect of depression on the association between weight change and CAD warrants further investigation.
Strength and limitationsThe major strength of this study is that multiple data sources (GWAS summary statistics and individual-level data from UKBB) and methods (e.g., two-step MR and PheWIS) were employed to disentangle the potential mediating and moderating effects of depression on the relationship between traits and CAD at a phenome-wide level.
The current study also has several limitations. First, the potential mediating effects could be underestimated, as the number of traits under investigation in our study increases the multiple testing burden. Moreover, multivariable MR requires more statistical power than univariable MR, which also decreased our ability to identify a mediating effect. Second, the estimates from the MR analyses using summary statistics of depression and CAD, both as binary variables, may be subject to the bias caused by the noncollapsibility of ORs. Third, horizontal pleiotropy in MR studies could not be completely ruled out. Fourth, some of the traits, such weight change compared to one year ago, were self-reported, and the extent to which the weight changes were not quantified. Fifth, the post-hoc power analysis, employed to estimate power for the cohort study, often yields a low post-hoc power for analyses with nonsignificant results, which can be mistakenly interpreted as indicating insufficient power for the analysis (Zhang et al., 2019).
ConclusionDepression was not considered a mediator of the effects of most eligible traits on liability to CAD, highlighting the independent role of these traits in CAD pathogenesis regardless of depression. The moderating effects of depression on the relationship of several traits (e.g., chest pain when hurrying, reason for smoking cessation and weight change) with CAD have been identified, which may be helpful for CAD risk stratification and optimized allocation of scarce medical resources.
FundingThis study is supported by the National Natural Science Foundation of China (grant no. 81973698 and 81703942), Young Elite Scientists Sponsorship Program by CACM (grant no. 2019-QNRC2-B08), BUCM Precision Cultivation Program (Grant No. JZPY-202205), Science Fund for Distinguished Young Scholars in BUCM (grant no. BUCM-2019-JCRC004) and the Key Research Projects of BUCM (grant no. 2020-JYB-ZDGG-113).
Ethics approval and consent to participateAll participants provided written informed consent, and the UK Biobank is ethically approved by the UK National Research Ethics Service (ref 11/NW/0382).
Authors’ contributionsSL designed the study. WXL, YND and TTY performed the statistical analyses. XYZ, XZH and WXL drafted the manuscript. XW critically reviewed the manuscript. All authors read and approved the final manuscript.
Not applicable.