Background/Objective:To study pain-brain morphometry associations as a function of post-surgery stages (anesthesia, pain and analgesia) in an acute pain model. Method:Impacted mandible third molar were extracted. Before surgery, an anatomical T1 scan was obtained. Regional brain volumen and subcortical nuclei shapes were obtained. Statistical analyses were done using multiple regression, being pain scores the predictors and voxel volumes, subcortical nuclei volumes and subcortical nuclei shapes, the outcomes. Results:Pain was significantly larger at pain than at anesthesia and analgesia stages, and was higher during anesthesia than during analgesia. Pain intensity was related to grey matter in several cortical (Insula, Mid Frontal and Temporal Gyruses, Precuneus, Anterior Cingulate), and subcortical nuclei (Hippocampus, Thalamus, Putamen, Amygdala), depending of the post-surgical stage. A larger number of brain areas showed significance at pain that at anesthesia and analgesia stages. Conclusions:The relationships of regional brain volumes and subcortical nuclei shapes with pain scores seemed to be unsteady, as they changed with the patient's actual pain stage.
Antecedentes/Objetivo:Se trata de determinar la asociación entre dolor percibido y morfometría cerebral en tres etapas postquirúrgicas (anestesia, dolor y analgesia), en un modelo de dolor agudo. Método:Se obtuvo una imagen cerebral estructural de alta resolución y posteriormente se extrajeron los terceros molares mandibulares impactados. Se realizó un análisis morfométrico para determinar volumen cerebral y forma de núcleos subcorticales. Se realizaron análisis de regresión múltiple, siendo la intensidad del dolor el predictor, y el volumen y la forma de los núcleos subcorticales, medidos pre-cirugía, las variables dependientes. Resultados:El dolor experimentado fue mayor en la etapa de dolor que en las de anestesia y analgesia, y mayor en anestesia que en analgesia. El dolor se asoció con el volumen de materia gris en áreas corticales (insula, giros frontal medial y temporal, precuneus y cingulado anterior) y subcorticales (hipocampo, tálamo, putamen y amígdala). El número de áreas asociadas al dolor experimentado fue mayor en la etapa de dolor que en las de anestesia y analgesia. Conclusiones:La relación entre volumen cerebral regional y forma de núcleos subcorticales con la intensidad del dolor no es fijo, sino que varía en función de la etapa post-quirúrgica (magnitud del dolor).
Over the last decade, magnetic resonance imaging studies have explored the associations between brain volume and pain. These studies, mostly done with chronic pain patients, suggest that pain is associated to structural changes in a set of brain areas, including insula, cingulate, prefrontal, somatosensory and cuneus, and that some subcortical structures, as amygdale or thalamus, are also involved in pain perception (Apkarian et al., 2004; Baliki et al., 2012; Cauda et al., 2014; Geha et al., 2008; Kuchinad et al., 2007; Mansour et al., 2013; Schmidt-Wilcke et al., 2006; Valet et al., 2009). Structural brain abnormalities have also been associated to pain in the orofacial region, including spinal trigeminal nucleus, sensomotor, ventrolateral prefontal cortex, or hippocampus (Avivi-Arber, Lee, & Sessle, 2015; Khan, Keaser, Meiller, & Seminowicz, 2014; Moayedi et al., 2011; Moayedi et al., 2012; Wilcox et al., 2015). Despite the consistency of the findings within chronic pain literature, scarce research has addressed whether acute, transitory, pain is actually associated to structural brain features, and more concretely whether they are associated to the intensity of acute pain. Answering this question will help us to disentangle whether changes in brain structure, as those observed when the pain is cured (Coppieters et al., 2016), are actually linked to chronic pain itself.
Structural brain changes related to pain are commonly associated to neuronal atrophy (Apkarian et al., 2004; Kuchinad et al., 2007). This idea is reinforced by the fact that the longer the duration of pain, the larger the cortical reorganization and the loss of grey matter (Maihofner, Handwerker, Neundorfer, & Birklein, 2003; Tracey & Bushnell, 2009). There is also some evidence that brain alterations can disappear when pain reverses (Rodriguez-Raecke, Niemeier, Ihle, Ruether, & May, 2013; Seminowicz et al., 2011) and that can be produced in a very short time window (days). A recent study (Teutsch, Herken, Bingel, Schoell, & May, 2008) demonstrated structural changes in midcingulate and somatosensory cortex after 8 days of 20minutes sessions of pain stimulation. These changes receded after removing the stimulation. Similarly, Seminowicz et al. (2013) have observed that a short cognitive behavioral therapy produced significant increase in several cortical areas, and, importantly that decreased catastrophizing, was associated to increased gray matter volumes in pain processing related areas. Thus, pain can produce short- and long-term changes in brain structure and function (Lazaridou et al., 2017). However, this research does not provide information about the relationships between brain structure and perceived acute pain. Recently, Emerson et al. (2014) investigated the relationship between grey matter density and sensitivity to pain using a heat-evoked pain protocol. They observed an inverse relationship between pain sensitivity and grey matter density in a set of areas, including precuneus, posterior cingulate, inferior parietal, intraparietal sulcus and SI. Interestingly, the pain scores were below the middle of the pain scale, and no association was found with areas in the anterior part of the brain, as anterior cingulate, insula or orbitofrontal cortex (Erpelding, Moayedi, & Davis, 2012). It was also found that acute pain is negatively associated to cortical thickness in posterior regions including the left superior temporal and the left inferior parietal region. Thus, gray matter density/ thickness underlies the level of perceived acute pain.
There is evidence that the hippocampus mediated the emergence of chronic pain (Apkarian et al., 2015; Ezzati et al., 2014; Khan et al., 2014; Vachon-Presseau et al., 2013), suggesting that the stress response of individuals with smaller hippocampus can be at the base of persistent pain states. Striatum appears to make a contribution to the subjective experience of pain likely influencing reward and pain-related areas in the cerebral cortex (Barad, Ueno, Younger, Chatterjee, & Mackey, 2014; Martikainen et al., 2015; Starr et al., 2011).
Almost all the above-cited studies used cross-sectional designs to compare a pain condition with a no-pain control group. The underlying assumption is that, all the other things been equal, any brain structure-pain association is static, and brain abnormalities are likely produced by pain. Furthermore, in experimental pain, ethics considerations prevent the use of intense pain stimulations, so that near-real pain stimulations are used. Here, near-real indicate that participants know that pain cannot be very intense and that it will finish if they decide to do so.
In this research, we ought to test whether relationships between brain gray matter structure and pain are static using a real pain situation (participants cannot control neither intensity nor duration of pain): the extraction of mandibular impacted third molar. This pain model (Moore et al., 2015) allows studying whether perceived pain and brain structure associations changed along the three main post-surgical stages: anesthesia, pain and analgesia, which will provide information on the strength and direction of the pain-brain relationships. We used a single structural pre-surgical scan to assess whether the relationships between subjective pain scores and gray matter are constant at the post-surgical stages or, as we hypothesized, they will be a function of the pain level. It is important to note that any changes in brain-pain association through the three post-surgical stages cannot be attributed to fast changes in brain structure, as a single brain scanning was done before the surgery, but must be attributed to the unsteady nature of the brain-pain associations, that is, have to the linked to the intensity of pain.
MethodParticipantsThis experimental study (Ramos-Álvarez, Moreno-Fernández, Valdés-Conroy, & Catena, 2008) was conducted after approval by the University of Granada Ethics Committee on Human Research (n° 877) and according to the Declaration of Helsinki for treatment of experimental human subjects. Subjects received detailed information about the procedure and provided written informed consent. A total of 30 participants (23 women) were recruited, with an average age of 21,83 years (range 18–32 years). Participants were right-handed, healthy, and were included only if they had an asymptomatic mandibular impacted third molar which needs surgical procedure to be removed. Half of the participants had a left-sided impacted third molar. Participants were recruited from the pool of patients of the School of Dentistry of the University of Granada.
InstrumentsMRI acquisition and analysisPreviously to the third molar surgery, all participants underwent a single magnetic resonance imaging session using a 3-T Siemens Magnetom Trio (Erlangen, Germany), located at the Mind, Brain and Behavior Research Center, University of Granada, using a 32-channel head coil. Head motion was controlled using a head restraint system and foam padding around the subject's head. T1 weighted anatomical volumes were obtained using a MPRAGE sequence (TR = 1900ms; TE = 2.52ms; flip angle = 9°, voxel size = 1 x 1 x 1mm3; FOV = 256 x 256mm2; matrix size = 256 x 256, 176 slices). The average interval between MRI session and third molar surgery was 23.6 (±42.41) days. The DARTEL (Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) algorithm implemented in SPM12 (Welcome Trust Center for Neuroimaging, London, UK; http://www.fil.ion.ucl.ac.uk/spm/) was used for Voxel-Based Morphometric analysis (VBM).
DARTEL improves accuracy between participants’ alignment of gray matter images modeling the shape of each brain (Ashburner, 2007). The analysis protocol was as follows (Catena et al., 2017; Megías et al., 2018). (1) T1-weighted images were segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) by the new segmentation algorithm implemented in SPM12. (2) Estimation of the DARTEL template and deformation flow fields for each participant that best align all the images from the GM and WM maps. (3) GM maps of each subject were spatially normalized to the MNI space by warping them to the template. (4) GM and WM images were smoothed with a 8mm width at half maximum (FWHM) Gaussian kernel. The total volumes of grey (GMV) and white matter (WMV) were also estimated, and the total intracraneal volume (TIV) was computed as GMV + WMV + CSF.
Subcortical nuclei were segmented using the FSL/FIRST software (Patenaude, Smith, Kennedy, & Jenkinson, 2011; http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIRST) to deeply explore associations of their volumes and shapes with pain scores. FIRST is a model-based registration tool that use a set of 15 subcortical structures obtained from 336 subjects ranging from 4.2 to 72 years. It allows the fine grain assessment of the relationships between subcortical structures and subjective pain. Subcortical nuclei volumes were adjusted using a regression approach: Subcortical Volume = Raw_Subcortical_Volume - b (TIV- average TIV), being b the slope of the regression of the Subcortical Volume on TIV (Kennedy et al., 2009; Ortega et al., 2017).
Shape analysis is based on the individual meshes composed by a large number of vertices and triangles. The number of triangles and vertices is the same for each nucleus, so that within and between participants comparison of each vertex can be performed. These comparisons are possible because all meshes are aligned to the Montreal Neurological Institute (MNI) space, and pose (rotation and translation) is removed (Patenaude et al., 2011). Shape analysis was performed using MNI maps of each nucleus for each participant. Each voxel in a map contain the value of the deformation of this point of the nucleus to the corresponding point in the standard map of this nucleus.
Surgical procedureThe third molars were classified on a panoramic radiograph by an expert oral surgeon (PGM). A standardized surgical procedure was performed by the same surgeon in all patients (PGM). A standard inferior alveolar and buccal nerve block was given using 1.8-mL carpule of 2% articaine with adrenaline 1:100,000. Surgical access was routinely achieved buccally through a triangular flap. Bone removal around the tooth was then performed with a round bur on a straight hand-piece under continuous irrigation. Sectioning of the crown and/or roots was performed as necessary. After extraction, the socket was inspected and the flap was sutured back by 2-4 interrupted stitches using a 3-0 silk suture. Gauze impregnated with either clorhexidine or hyaluronic acid was applied over the socket and the usual post extraction instructions were given written to the patient. All the patients received routinely the following postoperative medication: Amoxicilin/Clavulanic acid 2g two times a day and desketoprofen 25mg three times a day for 7 days; metamizole 565mg was given as a rescue analgesic. The duration of surgery (from the incision to the extraction) and the duration of the anesthetic agent in minutes, among others, were recorded.
Pain intensityAfter third molar extraction, patients were asked to report their pain using a visual analogue Likert-type scale (hereafter, subjective pain), in which 0 meant “no pain at all” and 10 meant “worst pain imaginable”. The first pain recording was taken 30minutes after the surgical procedure, when patients were still under the effects of the local anesthesia (Anesthesia stage). The second pain measure was taken either when local anesthetic ceased its function, as reported by the participant, or 3hours after the extraction (Pain stage). At this moment, patients were supposed to be in the pain peek, caused by the surgical trauma provoked by the dental surgery. The length of these two intervals (30 m. and 3hrs.) was selected according to Senes et al. (2015), who reported anesthesia durations around 3hrs.) Then, all participants were given an analgesic (Metamizole 565mg, Laboratorios Normon S.A, Spain). Finally, 30minutes after the analgesic administration, the last measure of pain was taken (Analgesia stage). This interval was based on metamizole onset time 10.9 (±5.8) min (Schmieder, Stankov, Zerle, Schinzel, & Brune, 1993).
Statistical methodsThe associations between pain sensitivity scores and grey and white matter volumes, estimated from the single pre-surgery MRI scan at the voxel level were done using a multiple regression approach, in which age, sex, time between anatomical scan and third molar surgery (surgery delay), and TIV were introduced in the regression equation as variables o no interest, the pain scores were the predictors of interest, and the voxel volumes were the dependents. In this analysis, we used the Alphasim simulator, as implemented by Song et al. (2011) to determine the corrected significance p-level. According to our simulation (1000 runs) significance threshold was set up at p<.001, and 234 voxels per cluster. We reported only cluster that survived the non-statationary Hayasaka extent correction (Hayasaka, Phan, Liberzon, Worsley, & Nichols, 2004). This is equivalent to a .05 corrected p-level. For the volumetric and shape analysis of subcortical nuclei we used the same multiple regression. According to Alphasim simulator, the significance level for the shape analysis was set up at a p-level of .02 and a minumun of 20 voxels per cluster. This is equivalent to a .05 corrected p-level. Statistical analyses were done in SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12/). The analysis of pain scores was done using SPSS v20.0 (IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp).
ResultsSubjective pain scoresThe pain score means for the post-surgery stages were significantly different, F(2, 58)=30.69, p<.001, R2=.51: Anesthesia: 4.3 (SD= 2.25), Pain: 5.5 (SD= 2.53), and Analgesia: 2.25 (SD= 1.51). Bonferroni corrected t-test showed that pain scores were significantly larger in pain stage than in anesthesia and analgesia stages, and were higher in anesthesia than in analgesia (all p<.001).
Voxel based morphometryTotal Grey and White matter volumes. No significant associations were found between total grey or white matter volumes and subjective pain scores.
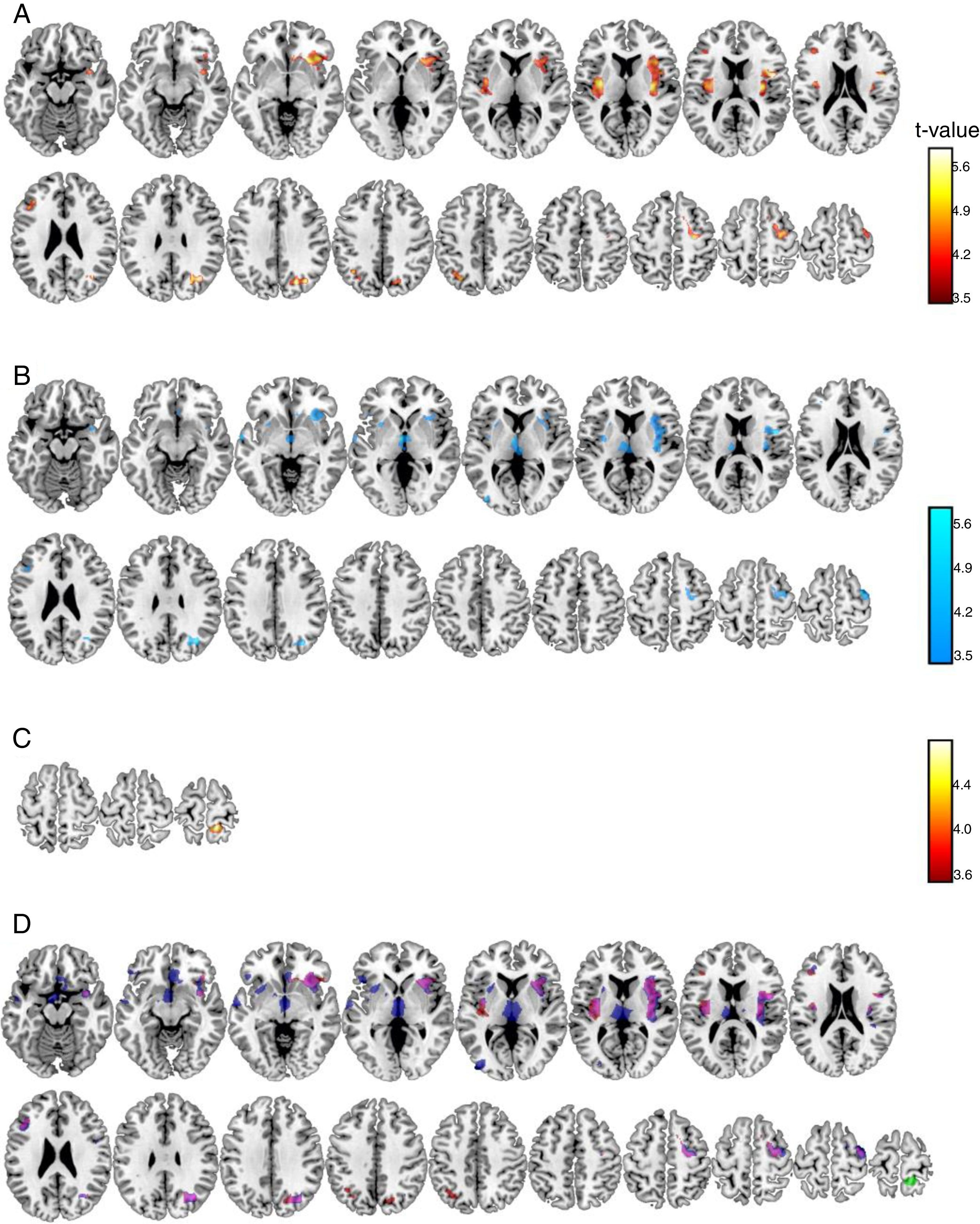
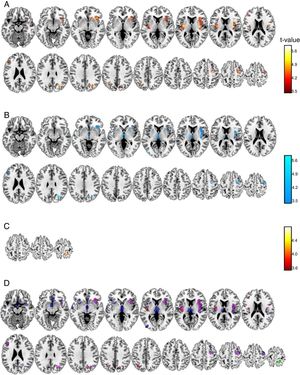
Voxel-by-voxel analysis. At the voxel level a set of grey matter areas were significantly related to pain scores (Table 1, Figure 1) in anesthesia and pain stages, but only a cluster in the precuneus was significant at the analgesia stage. Relationships were positive for the anesthesia and analgesia stages, but were negative for the pain stage. Anesthesia and pain stages partially overlap in a number of areas (Figure 1D) including left and right insula, frontal superior or precentral gyrus areas. There were, however, unique areas of association, as Thalamus, rostral anterior cingulate or middle temporal gyrus at the pain stage.
Voxel level significant associations between subjective pain scores and gray matter volume at the post-surgical stages.
| Pain Stage | Brain Areas | H | k | Peak T | X | Y | Z |
|---|---|---|---|---|---|---|---|
| Anesthesia | Occipital Sup / Mid /Cuneus | R | 615 | 5.88 | 37 | -66 | 28 |
| Insula/Putamen/Frontal Inf Orb | R | 2966 | 5.82 | 46 | 2 | 15 | |
| Frontal Sup 2/Precentral | R | 550 | 4.71 | 27 | -15 | 57 | |
| Insula/Rolandic Oper | L | 1155 | 4.68 | -35 | -7 | 9 | |
| Frontal Inf Tri | L | 242 | 4.67 | -38 | 32 | 24 | |
| Parietal Inf/Angular | L | 258 | 4.65 | -29 | -66 | 43 | |
| Pain | Occipital Mid /Sup/Angular | R | 595 | 6.83 | 36 | -67 | 30 |
| Insula/Putamen | R | 3908 | 6.49 | 45 | 2 | 15 | |
| Thalamus | L/R | 2752 | 5.72 | -6 | -6 | 1 | |
| Frontal Sup 2/Precentral | R | 901 | 5.22 | 34 | -6 | 63 | |
| Temporal Sup/Mid | L | 342 | 5.2 | -65 | -4 | -5 | |
| Occipital Mid | L | 262 | 5.05 | -41 | -84 | 4 | |
| Putamen/Insula | L | 388 | 4.77 | -30 | 11 | 0 | |
| Frontal Inf Tri | L | 298 | 4.66 | -44 | 24 | 27 | |
| Insula | L | 715 | 4.6 | -33 | -6 | 9 | |
| Frontal Med Orb/Cingulate Ant | R | 693 | 4.44 | 8 | 24 | -5 | |
| Frontal Inf Orb 2/Tri | L | 276 | 4.25 | -47 | 26 | 2 | |
| Analgesia | Postcentral/Precuneus | R | 403 | 4.77 | 14 | -45 | 72 |
Note. L: left, R: right; Peak T, the max t-test value in the cluster;, Y, and Z are in MNI space.
Brain grey matter volumes significantly associated to pain scores at Anesthesia (A), Pain (B), and Analgesia (C) stages. Scales indicated the t-values. Overlap (D) of these associations (red: positive, anesthesia stage; blue: negative, pain stage; green: positive, analgesia stage; magenta indicate anesthesia-pain overlapping).
Volumes. No significant association was observed between pain scores and subcortical volumes (all p>.2).
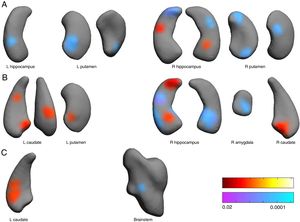
Shape. Significant associations (Figure 2) were observed between shapes of left and right hippocampus and putamen nuclei with pain scores at the anesthesia stage. However, the reverse pattern of relationships is observed for pain stage scores. At pain stage, distances around right basomedial amygdala were negatively associated to pain scores, but surface distances at right caudate head, left caudate head and tail, and left posterior putamen were positively associated to pain scores. At analgesia stage, distances around medial caudate head were positively associated to pain scores, but those in right lateral brainstem around the chief sensory nucleus of the trigeminal were negatively related to pain scores.
Associations between subcortical nuclei shape and pain scores at the anesthesia (A), pain (B) and analgesia (C) stages. Red color indicates surface positively related to pain scores. Blue colors indicated surface negatively associated to pain scores. L: left hemisphere, R: right. Color bars indicated p-values (red-yellow for positive and blue-violet for negative relationships).
Our results contribute to disentangle in part the nature of pain-brain structure associations. The sign of these relationships and the brain areas that are related to pain scores depended on the patients’ pain stage. When patients are under analgesic effect, only volumes of a small right postcentral/precuneus cluster are correlated with pain scores. However, when patients are under the anesthesia effects, regions in the thalamus, insula, prefrontal, parietal and occipital are positively associated to pain scores. Moreover, this pattern of difference holds for the subcortical shape analysis, in which hippocampus and putamen shapes are related to pain scores in the anesthesia stage, but caudate and brainstem are related to analgesia pain scores. Importantly, there are apparent outward and inward changes of these structures associated to pain scores. Furthermore, the associations at the pain stage are similar to those for the anesthesia one in cortical volumes, specially insula and frontal, but they are in stark contrast regarding subcortical shapes, as hippocampus, amygdala, caudate and putamen are related to pain scores in pain stage, but only hippocampus and putamen in the anesthesia stage. Similar areas of the right hippocampus are associated to pain scores, but in opposite direction, so that distances of the nuclei surface are positively related to pain scores at anesthesia, but negatively to those in pain stage.
Brain anatomy related with pain has become subject of research in the last years under the assumption that volume and shape are related to function in pain processing. Our results, abstracting from the direction of these associations, agree with literature, including those studies that have used orofacial and head regions (DaSilva et al., 2008; Gerstner, Ichesco, Quintero, & Schmidt-Wilcke, 2011; Schmidt-Wilcke, Ganssbauer, Neuner, Bogdahn, & May, 2008). Moreover, subcortical nuclei, especially hippocampus (Berger et al., 2018), caudate (Li et al., 2017) and putamen (Tsai et al., 2018) appear to have an important role in subjective pain. However, it seems that is not a matter of nuclei volume, but of nuclei shape. Grey matter analysis indicated that areas in the pain matrix, some of them involved in the default mode network and in cognitive control and emotion regulation (Garcia-Larrea & Peyron, 2013), plays an important role in pain sensitivity and pain control (Baliki, Geha, Apkarian, & Chialvo, 2008; Grant, Courtemanche, & Rainwille, 2011; Loggia et al., 2013).
Last decades of research on brain imaging of pain experience demonstrated that a set of brain structures are involved in the processing of noxious stimuli, embracing the brain areas we have observed are associated to subjective pain scores, as prefrontal cortex, hippocampus, thalamus, amygdala, or insula (Baliki & Apkarian, 2015; Salomons, Iannetti, Liang, & Wood, 2016; Tanasescu, Cottam, Condon, Tench, & Auer, 2016). These areas process all dimensions of pain by changing its activation in the presence of noxious events (Atlas, Lindquist, Bolger, & Wager, 2014) and even, its structure in response to chronic (Kairys et al., 2015; Krause et al., 2014) or repetitive pain (Erpelding et al., 2012). Some of these areas appear to code the emotional dimension of pain, such as cingulate, prefrontal and insular cortices, while some others appear to code the intensity and quality of pain, such as somatosensory cortex, posterior insula (Baliki & Apkarian, 2015; Coppieters et al., 2016; Segerdahl, Mezue, Okell, Farrar, & Tracey, 2015) or thalamus.
Reported anatomical alterations in chronic pain had lead to the idea that pain can cause changes in brain structure. However, our and recent reports cast some doubts over the long-term effects of pain on brain, as volumes appear to increase after patients recovered from the chronic pain condition (Erpelding et al., 2012; Rodriguez-Raecke et al., 2013), and over the cause-effect direction (Tracey & Bushnell, 2009).
Our results put a puzzle in which volume (and shape) apparently can be either increased or decreased in relation with pain scores. That is, when the direction of the associations and the stages of pain after surgery are taken into account, a more remarkable complexity of the pain-brain structure relationship emerged, as positive and negative relationships are observed within the same structure (e.g., right hippocampus) or area (e.g., insula). The puzzle cannot be completely solved with the present data, but they do provide us a clue to its solution. Firstly, it seems that these associations varies as a function of the pain intensity, so that the higher the level (pain stage>anesthesia>analgesia) the larger the amount of pain-brain structure significant relationships and the larger the amount of cortical and subcortical structures related to pain. Secondly, in general, the directions of the observed pain-brain structure relationships (positive/negative) are also a function of the average pain scores. For cortical grey matter, positive associations are found for lower pain average stages, but for subcortical nuclei shape positive associations (surface structure protrusions) are found for the higher pain average condition, especially in left hemisphere nuclei.
Although investigating with acute actual oral surgery-provoked pain precludes the use of large samples, it can be thought that more powerful effects will be observed if a larger sample were used. Also, although it is very common in the literature, the subjective pain score is a complex measure of a complex subjective phenomenon and much would be gained if emotional and non-emotional components of pain could be disentangled. In this vein, the design of objective pain measures (Diaz-Piedra et al., 2014) that allow the separation of the two factors regarding brain structure would make an important contribution to solve the pain-brain structure puzzle.
Our results suggest that whether pain (acute or chronic) is associated to changes in brain structure and, especially, if there is a reduction in pain-related brain areas caused by pain can be a mislead question. Here we have shown that apparent reduction and increments of grey matter volume and subcortical nuclei deformations can be observed when working with surgery-related pain, depending of the post-surgery stage (i.e., the intensity of the actual pain): anesthesia, pain and analgesia, that is, the pain intensity. These results suggest that finding reduced grey/white matter volume associated to chronic pain conditions will depends of the actual pain level rather than the pain chronicity.
This investigation was partially supported by Research Groups #CTS-138, #CTS-176 and #CTS-1028. (Junta de Andalucía, Spain). LTM also was supported by the Talentia Scholarship Program of the Regional Ministry for Innovation, Science and Enterprise (Junta de Andalucía) and this research was conducted as part of her PDh project. The authors would like to thank to Unidad de Excelencia Salud, Comportamiento y Cerebro.