Both the primary motor cortex (M1) and dorsolateral prefrontal cortex (DLPFC) rTMS have the potential to reduce certain chronic pain conditions. However, the analgesic mechanisms remain unclear, in which M1- and DLPFC-rTMS may have different impact on the release of dopamine receptor D2 neurotransmissions (DRD2). Using a double-blind, randomised, sham- and placebo-controlled design, this study investigated the influence of DRD2 antagonist on rTMS-induced analgesia and corticospinal excitability across the M1 and DLPFC. Healthy participants in each group (M1, DLPFC, or Sham) received an oral dose of chlorpromazine or placebo before the delivery of rTMS in two separate sessions. Heat pain and cortical excitability were assessed before drug administration and after rTMS intervention. DRD2 antagonist selectively abolished the increased heat pain threshold induced by DLPFC stimulation and increased pain unpleasantness. The absence of analgesic effects in DLPFC stimulation was not accompanied by plastic changes in the corticospinal pathway. In contrast, DRD2 antagonist increased corticospinal excitability and rebalanced excitation-inhibition relationship following motor cortex stimulation, although there were no clear changes in pain experiences. These novel findings together highlight the influence of dopaminergic neurotransmission on rTMS-induced analgesia and corticospinal excitability dependent on stimulation targets.
Transcranial magnetic stimulation (TMS) is a safe and non-invasive form of brain stimulation. Repetitive TMS (rTMS) delivered at a high frequency (≥ 5 Hz) has direct implications for pain management, which has been repeatedly demonstrated to improve chronic pain (Attal et al., 2021; Hosomi et al., 2019; Lefaucheur et al., 2006). Of note, rTMS over the primary motor cortex (M1) contralateral to painful side has level A evidence on neuropathic pain (Lefaucheur et al., 2020, 2014). Although relatively less studied, the dorsolateral prefrontal cortex (DLPFC) is also believed to improve certain chronic pain conditions, such as migraine (Granato et al., 2019; Leung et al., 2018; Sahu et al., 2019) and fibromyalgia (Altas et al., 2019; Fitzgibbon et al., 2018; Short et al., 2011). However, it is worth noting that rTMS only has a small to medium analgesic effect, which largely limits the clinical applications in pain conditions (Attal et al., 2021; Lefaucheur & Nguyen, 2019; Wang et al., 2023).
To date, the mechanisms underlying rTMS analgesia are still unclear, which may help improve rTMS efficacy. A line of evidence has indicated the involvement of dopamine system (DA), particularly dopamine receptor D2 neurotransmissions (DRD2), in pain sensations and potentially the modulation of pain perception by rTMS (DosSantos et al., 2016, 2018). It is well established that dopaminergic neurotransmissions are closely involved in reward (Arias-Carrión et al., 2010; Dreher et al., 2009) and motor control (Damier et al., 1999; Michely et al., 2015). In addition, a large number of studies have demonstrated dopamine engagement in both the transmission and modulation of pain experience (Hagelberg et al., 2002; Martikainen et al., 2005, 2015).
In the case of motor cortex, a pioneering study has demonstrated that human motor cortex rTMS induced dopamine release in ipsilateral putamen (Strafella et al., 2003), corresponding to the corticostriatal efferent originating in monkey motor cortex (Künzle, 1975). However, a more recent study indicated that motor cortex rTMS was not able to change either pain sensitivity or dopamine D2 receptor availability in any striatal regions (including putamen) (Lamusuo et al., 2017). Other studies indicated that these contradictory findings could be associated with the variances in dopamine D2 gene polymorphisms, such as 957TT homozygote and COMT Val158Met (Jääskeläinen et al., 2014; Lindholm et al., 2015; Ojala et al., 2022). Overall, more evidence is needed to establish the role of DRD2 neurotransmissions in the analgesic effect of motor cortex rTMS.
In contrast to the motor cortex, there is no evidence on how DRD2 neurotransmissions could impact rTMS analgesia over the DLPFC. A few studies indicated that both excitatory and inhibitory forms of DLPFC-rTMS could modulate dopamine release in the caudate nucleus (Ko et al., 2008; Strafella et al., 2001). Moreover, DLPFC-rTMS was demonstrated to induce dopamine release in cortical regions which are the main target areas of the mesocortical dopamine system, such as the anterior cingulate cortex (ACC) (Cho & Strafella, 2009). It is noted that the ACC plays a vital role in nociceptive transmission and may form a corticocortical pathway to reduce pain following DLPFC stimulation (Lorenz et al., 2003; Tracey & Mantyh, 2007; Ye et al., 2022). Overall, it is likely that dopaminergic neurotransmissions may play a different role in rTMS analgesia while targeting the DLPFC compared to the motor cortex.
This study was designed to investigate the different impacts of DRD2 neurotransmissions on rTMS analgesia between the motor cortex and DLPFC. Using oral intake of chlorpromazine, a primary DRD2 antagonist (Boyd-Kimball et al., 2018), this study evaluated rTMS analgesia across the motor cortex, DLPFC, and sham stimulation in a double-blind, randomised, sham- and placebo-controlled design. It is hypothesized that chlorpromazine would have a different impact on M1- and DLPFC-induced analgesia, based on their different effects on DRD2 neurotransmissions discussed above. As secondary outcome measures, corticospinal excitation (motor-evoked potentials, MEP) and inhibition (cortical silence period, CSP) were also systematically evaluated, which in one way could indicate the inhibitory effects of chlorpromazine and in another way could monitor the effects of rTMS on cortical excitability.
MethodsParticipantsSample size calculation was initially performed to determine the minimum sample size needed to power a mixed-design ANOVA [36]. Specifically, the significant level (alpha) and power were set to 0.05 and 0.8 respectively. In order to achieve the pre-defined effect size (ηp2=0.3) (de Andrade et al., 2011, 2014; Taylor et al., 2012), a total sample of 30 was needed.
A group of 45 healthy, right-handed, TMS eligible (Rossi et al., 2011) adults were recruited in this study (age range: 21–64 years, mean ± SD: 25.98 ± 9.02, 27 females). Exclusion criteria included history or current diagnosis of psychiatric disorder, the experience of any form of chronic or constant pain during participation, or the use of psychoactive medication, as assessed by the Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998). No participant withdrew from this study, data from 45 participants were therefore analysed. All participants provided written informed consent before study commencement. This study was approved by the Ethics Committee in the Affiliated Hospital of Hangzhou Normal University (2022-E2-HS-012) and was conducted in accordance with the Declaration of Helsinki.
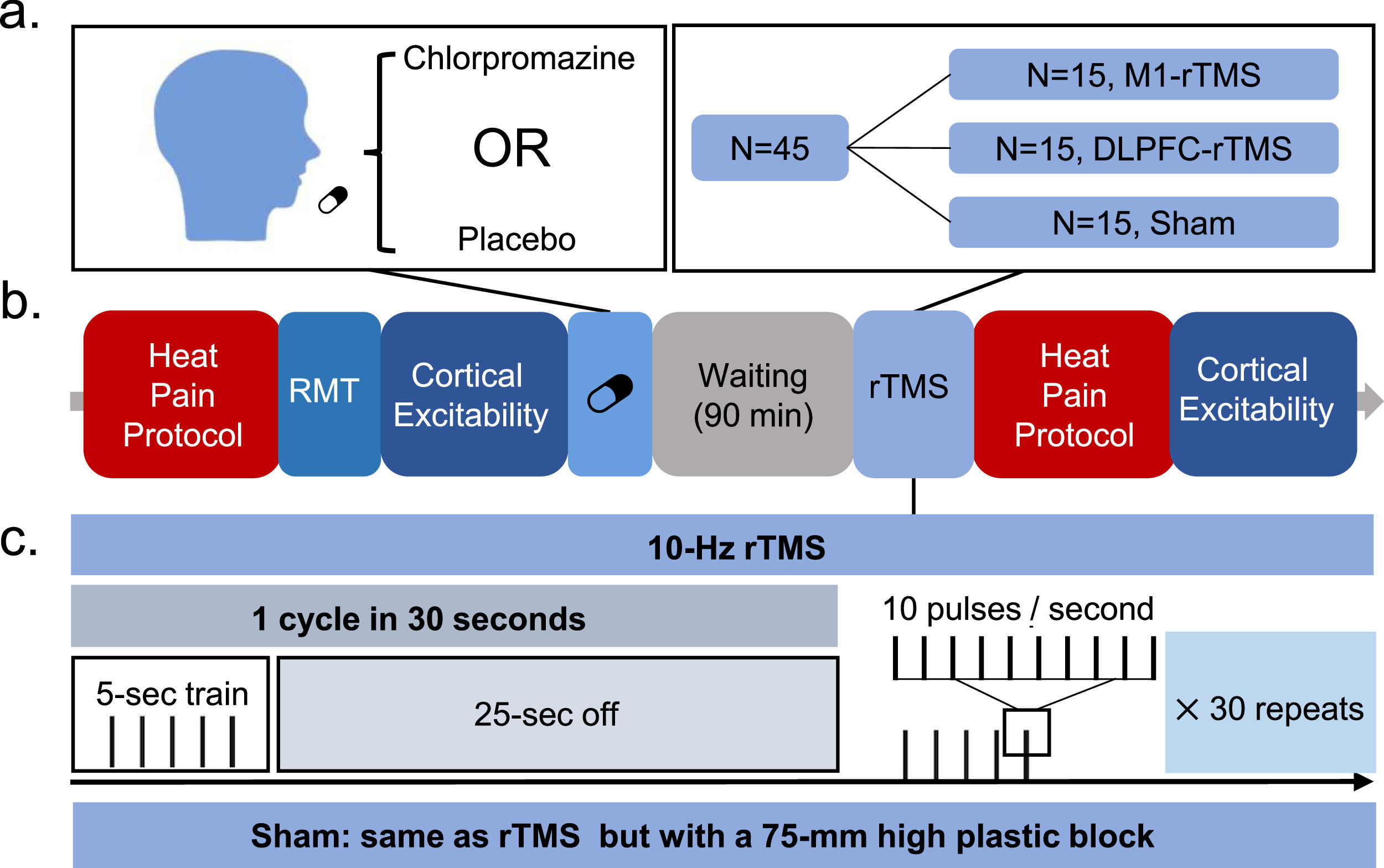
Study designThis was a double-blind, randomised, sham- and placebo-controlled study (Fig. 1). Participants randomised to each of the three groups (M1, DLPFC, Sham) visited the lab twice, with an interval of 7 days or longer. In each session, participants received an oral dose of chlorpromazine or placebo and rested for 90 min, followed by a single session of rTMS. Participants also underwent heat pain and cortical excitability assessment both before drug administration and after rTMS intervention.
Experiment procedure. A group of 45 participants were equally randomised to the three groups (M1, DLPFC, Sham) and visited the lab twice with an interval of 7 days or longer. In each session, participants received an oral dose of chlorpromazine (25 mg) or placebo and rested for 90 min, followed by a single session of rTMS. Participants also underwent heat pain and cortical excitability assessment both before drug administration and after rTMS intervention.
XC performed randomization with RESEARCH RANDOMIZER (https://www.randomizer.org/). Unique numbers from 1 to 45 were used to generate three sets of numbers each containing 15 numbers. Seven out of 15 participants in the Sham group received sham stimulation over the M1, with the rest targeting the DLPFC (Cheng et al., 2023; de Andrade et al., 2011). YW performed TMS treatment and BT collected the outcome measures. Both the participants and outcome assessor (BT) were blinded to the group allocation.
Pain protocolHeat pain threshold, intensity, and unpleasantness were used to evaluate pain experiences. A custom-built electrothermal device was able to heat from 20 to 60 °C with 0.5 °C steps. A 2 * 2-cm contact thermode was attached to the right volar forearm 5 cm from the wrist. The thermode started from body temperature of 37 °C with an increase rate of 0.5 °C. Each block lasted for 22 s followed by a 1-min break (Martin et al., 2013; Taylor et al., 2012). Pain threshold was defined as the temperature at which the stimulus became painful. For pain ratings, the thermode was continued to be heated until the participants reported a rating of 7/10 on a 0–10 scale. It is noted that a potential target temperature was adjusted until it reliably generated a 7/10 rating in 3/5 times (Martin et al., 2013). This temperate was then used to evaluate pain intensity and unpleasantness on a 0–10 visual analogue scale (VAS, 0-none, 10-extreme).
Resting motor threshold and cortical excitabilityResting motor threshold (RMT) was defined as the minimum intensity to induce motor-evoked potentials (MEPs) > 0.05 mV of the first dorsal interosseous (FDI) muscle in 5/10 trials. Single pulses to the hand region of the left M1 (45° to the midline, handle pointing backward) at 5 s ± 10% jitter intervals were sent by a figure-eight coil connected to a Magstim Rapid2 system (Magstim Company Ltd, UK). Coil position was measured relative to the nasion and inion to facilitate consistent re-positioning of the coil between two sessions (Che et al., 2019).
Corticospinal excitability was measured with MEP and CSP at rest and during a sustained voluntary FDI muscle contraction respectively (Hupfeld et al., 2020). The maximal voluntary contraction (MVC) was calculated and 20% of MVC was used for tonic contraction in CSP (Fling & Seidler, 2012; Liu et al., 2021). A total of 40 single pulses (20 for MEP and 20 for CSP) were consecutively delivered to the hand region of the left M1 at 120% RMT (45° to the midline, handle pointing backward). CSP was evaluated following MEP as the muscle contraction during CSP may have an impact on MEP (Conforto et al., 2004).
Drug administrationIn each session, participants orally took a single tablet of 25 mg of chlorpromazine or vitamin C, with the two drugs being identical in appearance. These two drugs were stored in two identical glass bottles with label A or B for blinding purposes. The sequence of drug administration was randomised across participants.
Repetitive transcranial magnetic stimulationrTMS protocol included 30 trains of 5-second stimulation given at 10 Hz, with the inter-train interval being set to 25 s (1500 pulses) (Bovy et al., 2019; Yılmaz et al., 2014). rTMS was delivered to the left M1 or DLPFC with an intensity of 90% and 110% RMT respectively (Lefaucheur & Nguyen, 2019). M1 was located by the ‘hotspot’, and the DLPFC target was determined by the Beam F3 methodology (Beam et al., 2009). The coil was set to be parallel (handle pointing backward) and perpendicular (handle pointing to left side) to the midline in the M1 and DLPFC site respectively (Lefaucheur & Nguyen, 2019). Sham stimulation was performed using the same protocol as M1 or DLPFC according to group allocation, but with a 75-mm high plastic block to avoid the penetration of magnetic field (Ojala et al., 2022).
Data analysisMEP was calculated by peak to peak. The calculation of CSP duration was based on the Mean Consecutive Difference (MCD) (Garvey et al., 2001), which was recommended in a recent review (Hupfeld et al., 2020). This method is briefly described here: (1) All silent period trials were rectified using the absolute value and then were averaged; (2) The MCD of 100 ms of pre-stimulus EMG was calculated, in which the MCD is the mean successive difference between individual data points; (3) Thresholds were set at: ± MCD x 2.66 (i.e. 3 standard deviations), which covers 99.76% of possible pre-stimulus EMG data points; (4) Silent period onset was defined as the time point at which the post-stimulus EMG falls below the variation threshold for three consecutive data points, while the silent period offset was determined as the time point at which the post-stimulus EMG returns above the variation threshold for three consecutive data points. Both MEP and CSP were expressed as the ratio between post- and pre-stimulation, which has been commonly used to measure TMS-induced plasticity(de Freitas Zanona et al., 2022; Liu et al., 2021).
Statistical analysisUsing SPSS (version 22; IBM Corp, Armonk, NY), statistical analyses were performed using two-way ANOVAs to examine the main and interaction effects of Drug (2 levels: chlorpromazine, placebo) and Time (2 levels: pre, post) for each group. Post-hoc pairwise comparisons were conducted to further explore the significant main and interaction effects, with the α-level set to 0.05 and Bonferroni corrected.
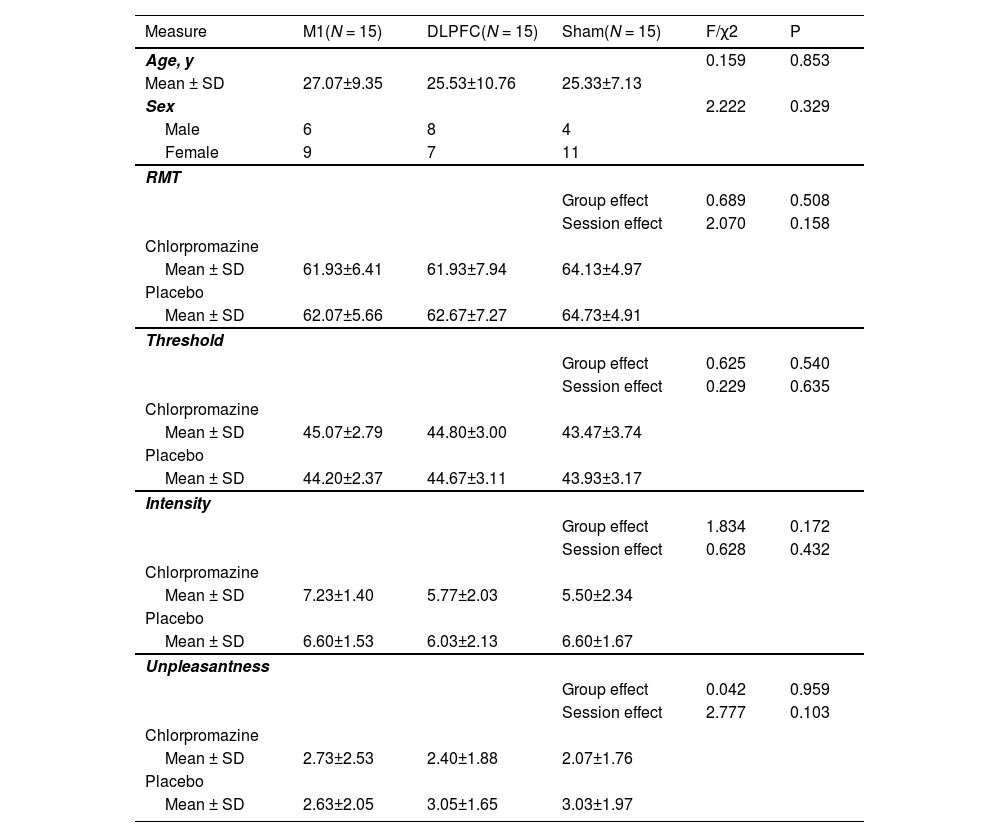
ResultsDemographic informationThere was no group effect on gender or age (all PBonferroni > 0.05). Two-way ANOVAs on baseline data also revealed no group effect (three groups) or testing session effect (two sessions) on either RMT, pain threshold, pain intensity or unpleasantness (all PBonferroni > 0.05) (Table 1).
Demographic and baseline information across groups and drugs.
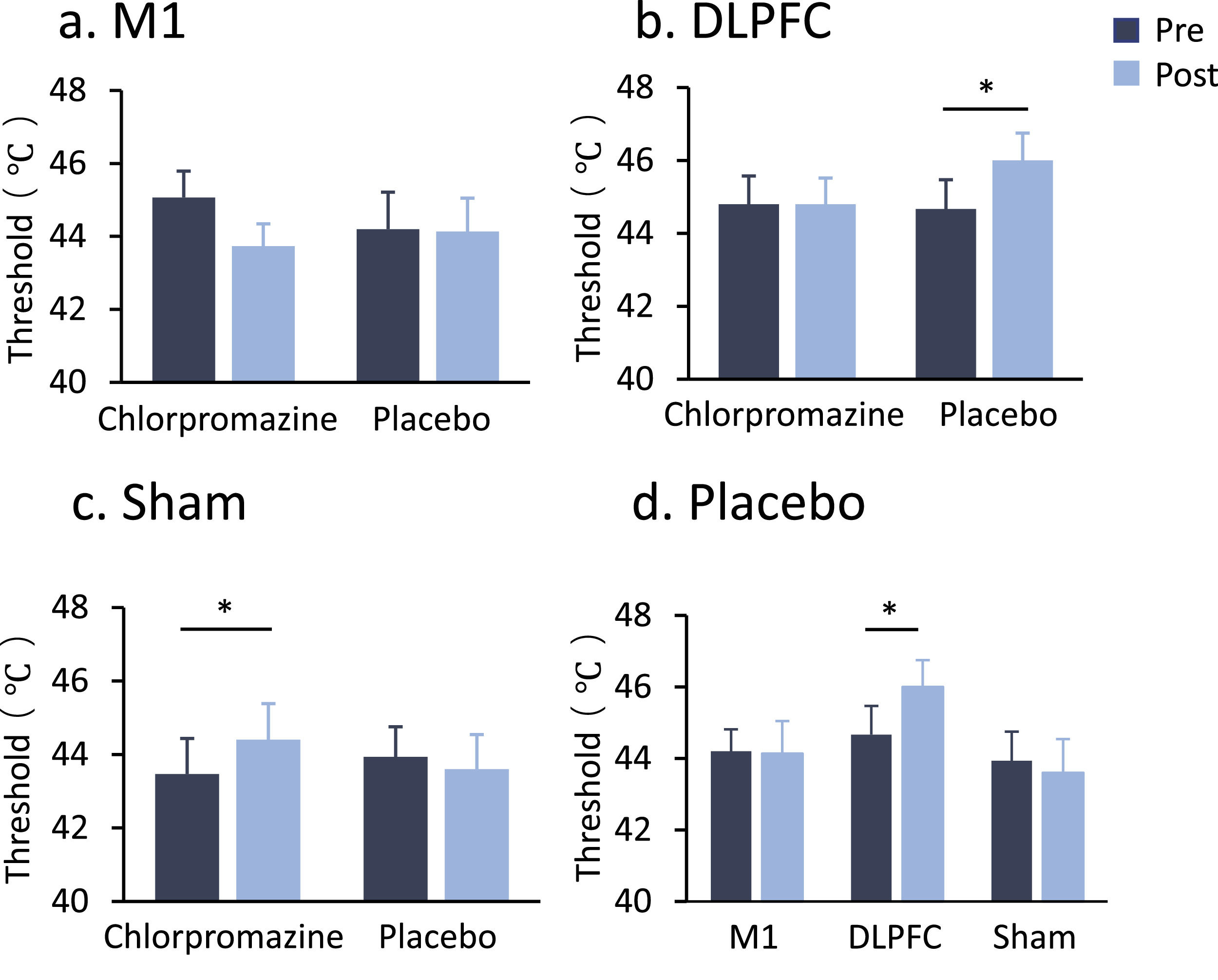
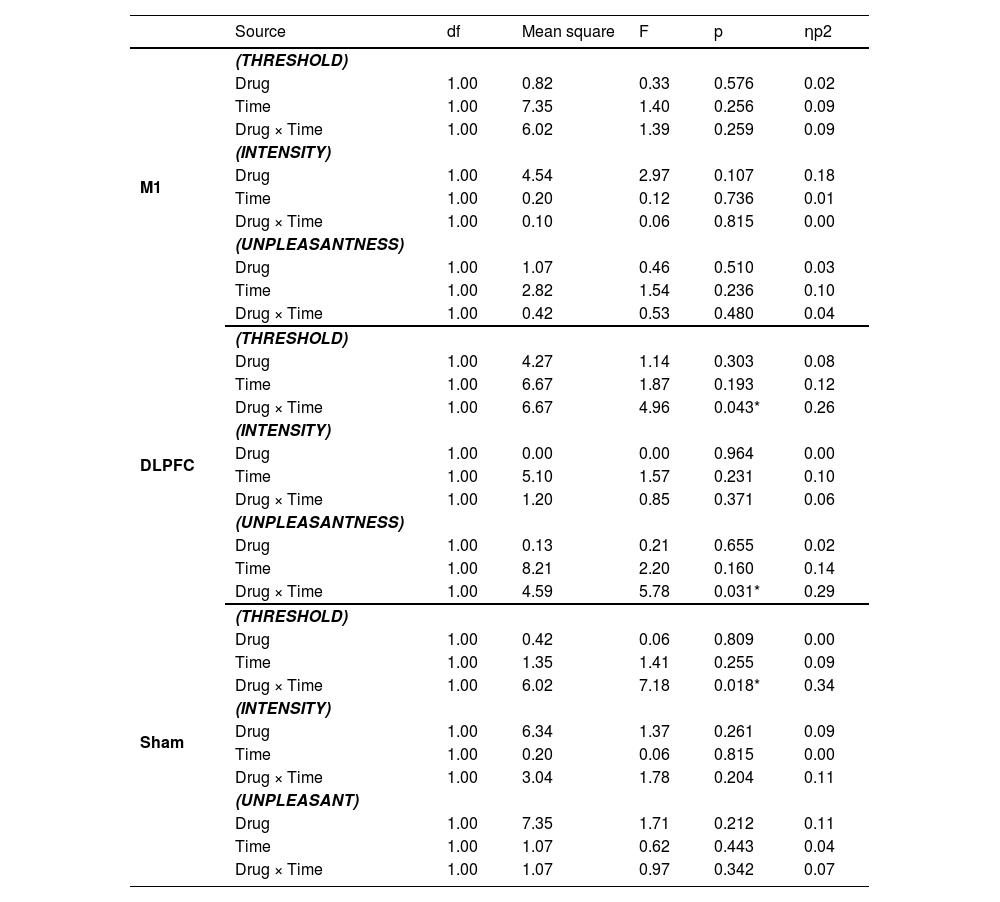
In the stimulation of the DLPFC, a two-way ANOVA indicated a significant drug * time interaction (F1,14 = 4.96, P = 0.043, ηp2=0.26) (see Table 2 and Fig. 2b). Post-hoc comparisons indicated that DLPFC-rTMS increased pain threshold from pre- to post-stimulation in the placebo condition (Meanpre = 44.67, Meanpost = 46.00, PBonferroni = 0.008). Meanwhile, this effect was abolished in the chlorpromazine condition (PBonferroni = 1.000). In the Sham stimulation, there was also a drug * time interaction (F1,14 = 7.18, P = 0.018, ηp2=0.34) (Fig. 2c), with post-hoc comparisons indicating increased pain threshold from pre- to post-stimulation in the chlorpromazine condition (Meanpre = 43.47, Meanpost = 44.40, PBonferroni = 0.042), but not in the placebo condition (PBonferroni = 0.668). In the motor cortex group, no significant main or interaction effect was observed (all PBonferroni > 0.05) (Fig. 2a).
Main results.
Heat pain threshold by stimulation target and drug pre-treatment. (a) There was no significant main or interaction effect in the M1 stimulation. (b) DLPFC stimulation increased pain threshold in the placebo condition (PBonferroni = 0.008), and this effect was eliminated by the pre-treatment of chlorpromazine. (c) Chlorpromazine increased pain threshold following Sham stimulation (PBonferroni = 0.042). * denotes PBonferroni < 0.05, ** denotes PBonferroni < 0.01. (d) DLPFC-rTMS increased heat pain threshold in the placebo condition (PBonferroni = 0.008).
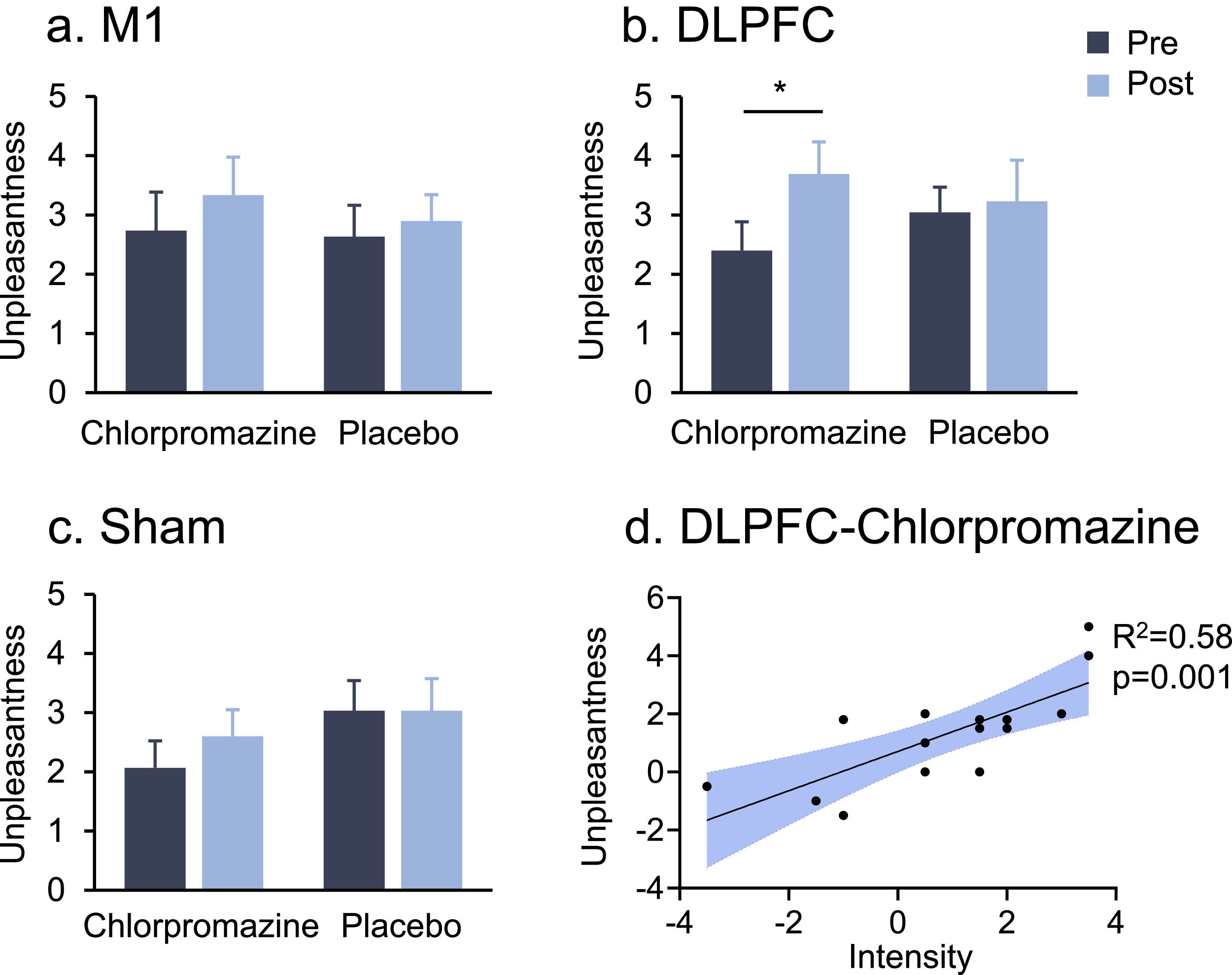
In the stimulation of the DLPFC, a two-way ANOVA indicated a significant drug * time interaction on pain unpleasantness (F1,14 = 5.78, P = 0.031, ηp2=0.29) (Fig. 3b). Post-hoc comparisons indicated that chlorpromazine increased pain unpleasantness from pre- to post-stimulation (Meanpre = 2.40, Meanpost = 3.69, PBonferroni = 0.024). No significant main or interaction effect on pain unpleasantness was observed in the M1 or Sham stimulation (all PBonferroni > 0.05) (Fig. 3a,c).
Heat pain unpleasantness by stimulation target and drug pre-treatment. (a) and (c) There were no significant main or interaction effect in the M1 or Sham stimulation. (b) DLPFC stimulation increased pain unpleasantness in the pre-treatment of chlorpromazine (PBonferroni = 0.024). (d) There was a strong positive correlation between increased pain unpleasantness and intensity induced by DLPFC stimulation in the pre-treatment of chlorpromazine (R2 = 0.58, p = 0.001). * denotes PBonferroni < 0.05.
In terms of pain intensity, no significant main or interaction effect was observed in either of these three targets (all PBonferroni > 0.05, see Supplementary Materials). There was a strong positive correlation between increased pain unpleasantness and intensity induced by DLPFC stimulation following chlorpromazine pre-treatment (R2= 0.58, p = 0.001) (Fig. 3d).
Group comparisonsA two-way ANOVA indicated a significant group * time interaction on pain threshold in the placebo condition (F1,14 = 3.90, P = 0.028, ηp2=0.16) (Fig. 2d). Post-hoc comparisons indicated that only DLPFC-rTMS increased heat pain threshold from pre- to post-stimulation, further confirming the analgesic effect of DLPFC stimulation. There were no significant results in either pain intensity or unpleasantness in the placebo condition (results not shown).
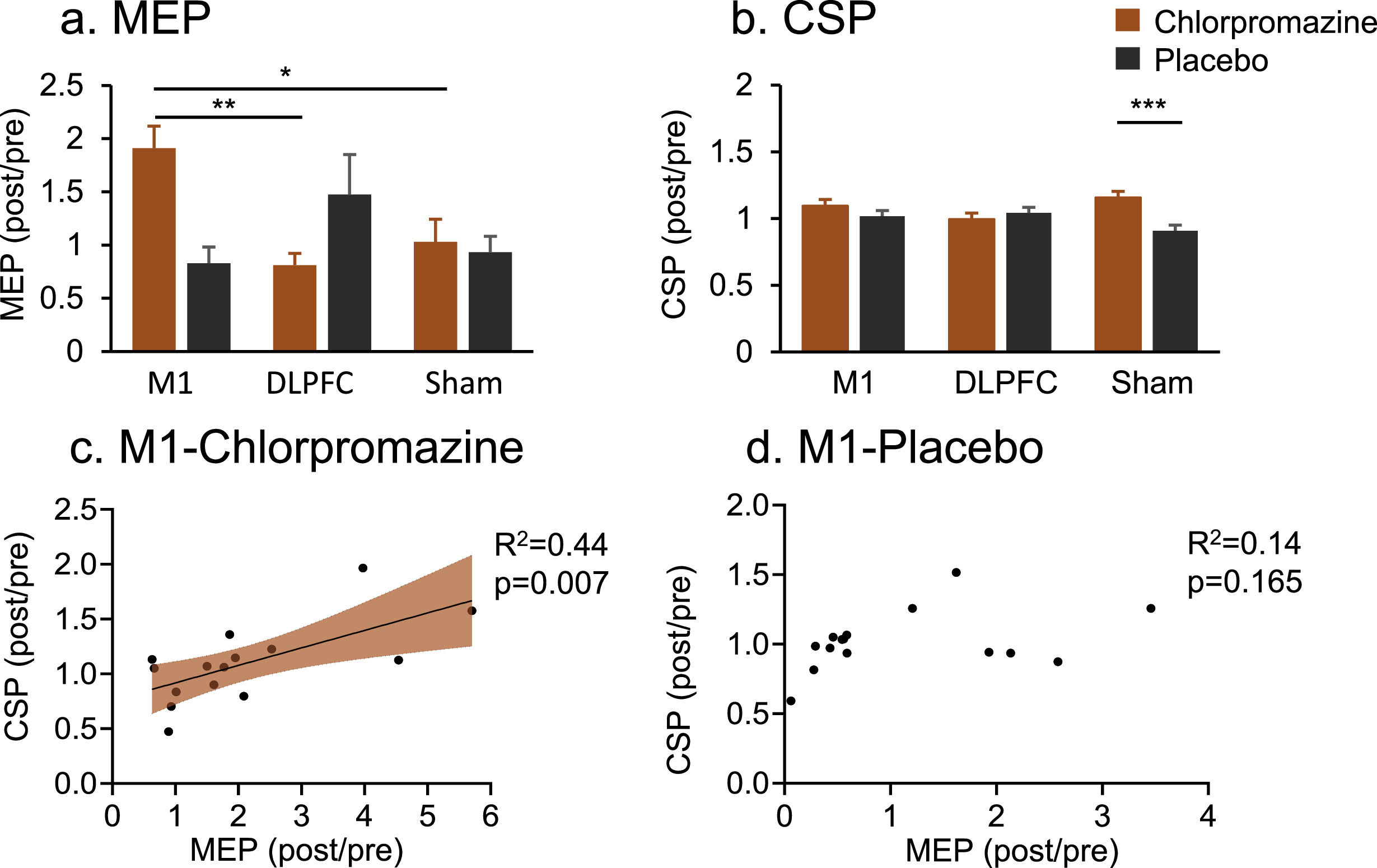
Corticospinal excitabilityMEP data indicated a significant interaction effect (F1,44 = 4.21, P = 0.022, ηp2=0.17) (Fig. 4a). Post-hoc comparisons indicated that chlorpromazine increased MEP amplitude following M1 stimulation (Mean = 2.11) compared to the target of DLPFC (Mean = 0.86, PBonferroni = 0.003) and of Sham (Mean = 0.46, PBonferroni = 0.018) stimulation.
MEP and CSP results. (a) Chlorpromazine increased MEP amplitude following M1 stimulation compared to DLPFC (PBonferroni = 0.003) and Sham (PBonferroni = 0.018) stimulation. (b) Chlorpromazine increased CSP latency compared to placebo in the Sham stimulation (PBonferroni = 0.000). (c) Increased MEP was strongly associated with CSP induced by M1 stimulation following chlorpromazine pre-treatment (R2= 0.44, p = 0.007). (d) This pattern of relationship was not significant following placebo pre-treatment (p = 165). * denotes PBonferroni < 0.05, ** denotes PBonferroni < 0.01, ***denotes PBonferroni < 0.001.
In terms of CSP, there was also a significant interaction effect (F1,44 = 3.78, P = 0.031, ηp2=0.15) (Fig. 4b). Post-hoc comparisons indicated that chlorpromazine resulted in a larger CSP compared to placebo in the Sham group (Meanchlorpromazine = 1.13, Meanplacebo = 0.91, PBonferroni = 0.000).
Correlation analysis indicated that increased MEP was strongly associated with CSP induced by M1 stimulation following chlorpromazine pre-treatment (R2 = 0.44, p = 0.007) (Fig. 3c). Further analysis found that this pattern of relationship was not significant following placebo pre-treatment (p = 0.165) (Fig. 3d).
Safety assessmentThere was no serious adverse effect by monitoring patients’ vitality, and physical and mental health. There was a chance to experience mild headache (3, 8, 0 in the M1, DLPFC, Sham group) and/or mild scalp discomfort (6, 8, 1 in the M1, DLPFC and Sham group), but the sensations dissolved within minutes or hours. Two subjects reported dizziness after taking chlorpromazine, but symptoms disappeared after having a rest. Overall, the protocol was safe and well-tolerated.
DiscussionUsing a double-blind, randomised, sham- and placebo-controlled design, this study investigated the influence of dopamine on rTMS-induced analgesia across the motor cortex and the DLPFC. DRD2 antagonist was found to selectively abolish the increased heat pain threshold induced by DLPFC stimulation as well as to increased pain unpleasantness. Further analysis indicated that the absence of analgesic effects in the DLPFC stimulation was not associated with plastic changes in the corticospinal pathway. Meanwhile, DRD2 antagonist did not interact with motor cortex rTMS in the modulation of pain experiences, whereby it increased corticospinal excitability and rebalanced corticospinal excitation and inhibition following motor cortex stimulation.
Our data demonstrated for the first time that DRD2 antagonist selectively reversed the increased heat pain threshold induced by DLPFC stimulation (Fig. 2b). In fact, pain threshold in experimental settings was the most consistent outcome measure of DLPFC-induced analgesia as demonstrated by our previous meta-analysis (Che et al., 2021a). We also evaluated heat pain induced pain intensity and unpleasantness, in which DRD2 antagonist increased pain unpleasantness in the DLPFC stimulation (Fig. 3b). Moreover, increased pain intensity was strongly correlated with unpleasantness in the chlorpromazine-DLPFC interaction, although increased pain intensity did not reach statistical significance (Fig. 3d). These data together provide novel findings that the analgesic effect of DLPFC-rTMS relies on DRD2 neurotransmissions.
The absence of DLPFC-induced analgesia in the pre-treatment of DRD2 antagonist could be associated with the antinociceptive effects of DRD2 neurotransmissions. Previous studies have already demonstrated that DLPFC-rTMS is able to induce endogenous dopamine D2 release in the striatal regions and other regions along the mesocortical dopamine system (Cho & Strafella, 2009; Ko et al., 2008; Strafella et al., 2001). Moreover, a large number of studies have confirmed the antinociceptive effects of DRD2 neurotransmissions as well as a disruption of dopaminergic functioning across different chronic pain conditions (Hagelberg et al., 2003a,b; Ledermann et al., 2016; Scott et al., 2006; Wood et al., 2007). For instance, dopamine D2 binding potential in the striatal regions was associated with pain threshold (Hagelberg et al., 2002; Martikainen et al., 2005). Further study revealed decreased dopamine D2 binding potential in chronic back pain patients compared to healthy controls (Martikainen et al., 2015). Therefore, the absence of pain threshold changes in the DLPFC condition could result from the abolishment of DRD2 neurotransmissions which has an antinociceptive effect.
In line with the role in antinociception, DRD2 antagonist is also able to reduce sensations of reward and/or pleasure that could work against the analgesic effects of DLPFC-rTMS. Dopamine system is well-known for the involvement in reward and pleasure sensations (Wise, 1980, 2004). Similarly, DLPFC-rTMS has been cleared by the Food and Drug Administration (FDA) for treating major depression disorders, due to its capability in modulating brain regions such as the ACC and amygdala that are critical for the generation and modulation of emotions (Cash et al., 2021; Eshel et al., 2020; Fox et al., 2012). In chronic pain conditions, DLPFC-rTMS was also found to reduce the emotional aspects of pain, such as fearful, sickening, and tiring emotions (Che et al., 2021a). Our data of increased pain sensitivity and unpleasantness are therefore likely result from the suppression of reward and pleasure sensations induced by DRD2 antagonist.
Our findings on corticospinal excitability also provide insights on the mechanisms of DLPFC-induced analgesia. Specifically, DLPFC stimulation did not induce significant changes in either corticospinal excitation or inhibition indexed by MEP and CSP respectively. Moreover, DRD2 antagonist did not interact with DLPFC in the modulation of corticospinal excitability. Previous studies have demonstrated inconsistent findings on whether DLPFC-rTMS is able to induce corticospinal excitability. For instance, one study demonstrated decreased pain experience and increased MEP and intracortical inhibition following DLPFC-rTMS (Fierro et al., 2010). Meanwhile, DLPFC-rTMS did not change MEP or intracortical inhibition in another study, although there was a clear increase in heat pain threshold same as our data (De Martino et al., 2019). Moreover, one study from our group indicated that DLPFC-rTMS is more likely to induce cortical plasticity in the frontal and insular cortices that are associated with pain reduction (Ye et al., 2022). Overall, our data suggest that DLPFC-induced analgesia is not likely to result from excitability changes in the motor pathway.
It is noted that chlorpromazine was found to increase heat pain threshold in the sham stimulation in our data (Fig. 2c). In fact, this effect could be dated back to 1950s when the drug was initially introduced (Hougs & Skouby, 1957). Chlorpromazine was reported to induce ‘an indifference to pain’ (Lane & Ross, 1985). Our data of increased heat pain threshold therefore replicate this effect. Moreover, we have provided interesting findings that chlorpromazine increased CSP for the first time in the sham condition (Fig. 3b). CSP is thought to indicate GABAB-mediated intracortical inhibition (Werhahn et al., 1999), which has a negative association with both chronic pain (Parker et al., 2016) and induced pain (Liu et al., 2021). Increased pain threshold here aligns nicely to the enhancement of intracortical inhibition.
We also provided novel findings that DRD2 antagonist selectively increased MEP amplitude following M1 stimulation compared to other targets. This increased pattern of MEP was strongly associated with increased CSP latency, although increase in CSP did not reach statistical significance following M1 stimulation. Of note, the MEP-CSP dyad was not significant in placebo conditions, indicating that DRD2 antagonist is able to rebalance corticospinal excitation and inhibition in M1 stimulation. It has been debated how dopamine, glutamate, and GABA neurons could interact with each other in neurotransmissions (Carlsson & Carlsson, 1990; Sun et al., 2020; Taber & Fibiger, 1997). Our data indicated that dopaminergic transmissions could modulate corticospinal excitability and furthermore reshape excitation-inhibition relationship potentially mediated by glutamate and GABA neuronal activity.
It is quite surprising that rTMS over the M1 region did not induce significant changes either in heat pain sensitivity or heat pain ratings. It is well-established that M1-rTMS is effective in managing neuropathic pain and experimentally induced pain (Attal et al., 2021; Che et al., 2021b). A closer examination of the literature on induced pain reveals that rTMS has a consistent analgesia on cold pain across a large number of studies (Che et al., 2021a; de Andrade et al., 2011, 2014; Moisset et al., 2015; Summers et al., 2004). Meanwhile, heat pain data are relatively scarce, and the results are inconsistent (Johnson et al., 2006; Lamusuo et al., 2017). These data together highlight the importance of nociceptive fibres (e.g. A δ and C fibres) that can be selectively targeted by different pain protocols (Mylius et al., 2007). These findings also help to explain the null findings in heat pain experience in the M1-chlorpromazine interaction, although chlorpromazine was found to rebalance corticospinal excitation and inhibition in this condition.
There were some limitations in this study. Here we presented evidence on pain experiences and corticospinal excitability, without neural circuits evidence from functional magnetic resonance imaging (fMRI) or positron emission tomography (PET). These imaging technologies would reveal neural pathways whereby dopamine modulates TMS-induced analgesia. Blood samples were not collected which was done before to monitor the pharmacokinetics of chlorpromazine following oral administration (Whitfield et al., 1978). Oral administration of CPZ begins to appear in systemic circulation after a mean lag time of ∼ 20 min and is subsequently absorbed for an average of 2.9 h. Our design included a lag of 90 min following oral administration and a subsequent rTMS session for ∼ 20 min which together would fall into the period of the peak concentration of chlorpromazine (Whitfield et al., 1978). A heat pain protocol was used in this study, results of which need to be validated in other pain protocols such as cold pain or capsaicin induced hyperalgesia. In addition, it remains to be determined how dopamine could affect rTMS effects in chronic pain conditions, such as migraine and fibromyalgia that can benefit from DLPFC stimulation (Altas et al., 2019; Fitzgibbon et al., 2018; Granato et al., 2019; Leung et al., 2018; Sahu et al., 2019; Short et al., 2011). Although baseline pain levels were not considered during recruitment, our data presented baseline pain levels at 6–7 (Table 1). This is consistent with recent TMS trials reporting a medium level of pain at baseline (∼ 7/10) (Attal et al., 2021; Wang et al., 2023; Che et al., 2021b).
In conclusion, dopamine antagonist selectively abolished rTMS effects on pain sensitivity while targeting the DLPFC. In addition, dopamine antagonist is able to rebalance corticospinal excitation and inhibition in motor cortex rTMS. These findings highlight the influence of dopaminergic neurotransmission on rTMS-induced pain experiences and corticospinal excitability dependent on stimulation targets.
Data availabilityAll the data and codes generating findings of this work are available upon the request from the corresponding author.
DisclosuresssXC is supported by the National Natural Science Foundation of China (32000781), Zhejiang Provincial Natural Science Foundation of China (Y23C090003), the Key Research and Development Program of Zhejiang Province (2022C03038), and the Hangzhou Municipal Health Commission (2021WJCY130). YW is supported by the Postgraduate Research Innovation Project of Hangzhou Normal University (2022HSDYJSKY298).
CRediT authorship contribution statementYing Wang: Conceptualization, Formal analysis, Investigation, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. Bolin Tan: Conceptualization, Formal analysis, Investigation, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. Shuyan Shi: Conceptualization, Formal analysis, Investigation, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. Yang Ye: Conceptualization, Formal analysis, Investigation, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing. Xianwei Che: Conceptualization, Formal analysis, Investigation, Project administration, Software, Supervision, Writing – original draft, Writing – review & editing.