The goal of the present study is to describe the implementation of two Evidence-based treatments (EBT) for adolescent Cannabis Use Disorders (CUD) in the Spanish Public Health System, and its main clinical outcomes. Adolescent Community Reinforcement Approach (A-CRA) and Contingency Management (CM) were chosen as the most efficacious treatment programs for this population. A total of 26 adolescent cannabis users entered the study (91.7% male; age=16.50) at two outpatient clinical facilities in Spain. A quasi-experimental design was utilized, with one group receiving A-CRA only and the other A-CRA+CM. Implementation of both EBTs resulted feasible, with positive clinical outcomes. Results indicated that A-CRA has positive retention (81.3%) and abstinence rates (68.8%). Results for the group receiving A-CRA+CM were not significantly better than A-CRA in retention (100%) or abstinence (75.5%), although sample is too small to establish firm conclusions. Cannabis-related problems and depressive symptomatology also decreased during treatment. Several limitations prevent us from determining the clinical efficacy of A-CRA in this study. The process of translating EBT's to clinical contexts presented with many difficulties that need to be overcome. Recommendations are made for further attempts to implement EBTs in these contexts.
El objetivo de este estudio era describir la implementación en el Sistema Público de Salud de dos programas basados en la evidencia (PBE) para adolescentes con trastornos por consumo de cannabis, y sus principales resultados. La Aproximación de Reforzamiento Comunitario para Adolescentes (A-CRA) y el Control de Contingencias (MC) fueron elegidos como los programas de intervención más eficaces para esta población. Un total de 26 adolescentes participaron en el estudio (91.7% chicos; edad media=16.50 años) en dos centros de carácter ambulatorio en España. Se utilizó un diseño cuasi-experimental, donde un grupo recibió A-CRA y el otro A-CRA+MC. La implementación de ambos programas resultó factible, con resultados clínicos positivos. El A-CRA ofreció buenas tasas de retención (81.3%) y abstinencia (68.6%). Los resultados del grupo A-CRA+MC no fueron significativamente mejores que los del A-CRA en retención (100%) o abstinencia (75.5%), aunque el limitado tamaño muestral no permite establecer conclusiones firmes. Los problemas asociados al cannabis y la sintomatología depresiva se redujeron durante el tratamiento. Varias limitaciones nos impiden determinar la eficacia clínica del A-CRA en este estudio. El proceso de traslación de los PBE al contexto clínico presentó múltiples dificultades que deben ser abordadas. Se discuten recomendaciones para futuros intentos de implementación de PBE en estos contextos.
In Spain, 92% of adolescents in treatment under 15 years of age and 79% of those aged 15-19 report cannabis as their primary drug of abuse (European Monitoring Centre for Drugs and Drug Addiction, 2012). However, a review of the literature shows that no evidence-based treatment (EBT) aimed at this population has been implemented in our country. The need for such treatment programs for adolescent Cannabis Use Disorders (CUD) in Spain is very urgent. In the past years, several controlled studies have focused on treatment for CUD for adolescents (Dennis et al., 2004; Hendriks, van der Schee, & Blanken 2011; Martin & Copeland, 2005; Rigter et al., 2013; Walker et al., 2011). Among these, the Cannabis Youth Treatment study (CYT) is the largest published clinical trial (Dennis et al., 2004). Results indicated that Adolescent Community Reinforcement Approach (A-CRA) was the most cost-effective intervention, and it showed a non-significant trend for higher rates of recovery one year after treatment, when compared to MET/CBT5 (Motivational Enhancement Therapy/Cognitive Behavioral Therapy) and MDFT (Multidimensional Family Therapy). Despite the general effectiveness, however, the most powerful interventions tested so far with adolescent cannabis users achieved only modest abstinence rates and substance use reductions (Stanger & Budney, 2010). In this context, the integration of abstinence-based contingency management (CM) is a promising approach (Nordstrom & Levin, 2007; Stanger & Budney, 2010) that has proved to be an efficacious model for adolescent marijuana abuse (Kamon, Budney, & Stanger 2005; Stanger, Budney, Kamon, & Thostensen, 2009).
The goal of the present study was to describe a pilot implementation of two EBTs for adolescent CUDs in the Spanish Public Health System. A-CRA was chosen given its positive implementation rates and effectiveness (Godley, Garner, Smith, Meyers, & Godley, 2011), as well as its flexibility to address clients’ individual needs (Godley, White, Diamond, Passetti, & Titus, 2001). A-CRA was then partially combined with an abstinence-based CM program using a quasi-experimental design, given its demonstrated efficacy with adolescents (Stanger & Budney, 2010).We aimed to assess the clinical outcomes, determine the feasibility and limitations of the therapeutic approaches and their integration, and to discuss the barriers encountered in this specific context.
MethodParticipantsParticipants were recruited from those requesting treatment in clinical settings and through advertisements in pamphlets, on radio and in local newspapers. Any demand of treatment from an adolescent or their families related to drug use problems was considered for inclusion in the study. Inclusion criteria for individuals to participate were: (1) Being aged 12-18, (2) Individual or family report of cannabis use in the previous 30 days or delivering a positive urinalysis at intake, and (3) Living with a responsible adult who agreed to participate. Exclusion criteria included (1) Presenting a mental or physical disorder requiring more specific treatment, (2) Having a substance-use disorder requiring more intense or inpatient treatment, (3) Not living within 30minutes of the treatment facility, and (4) Not being fluent in Spanish. All participants and their families provided informed consent.
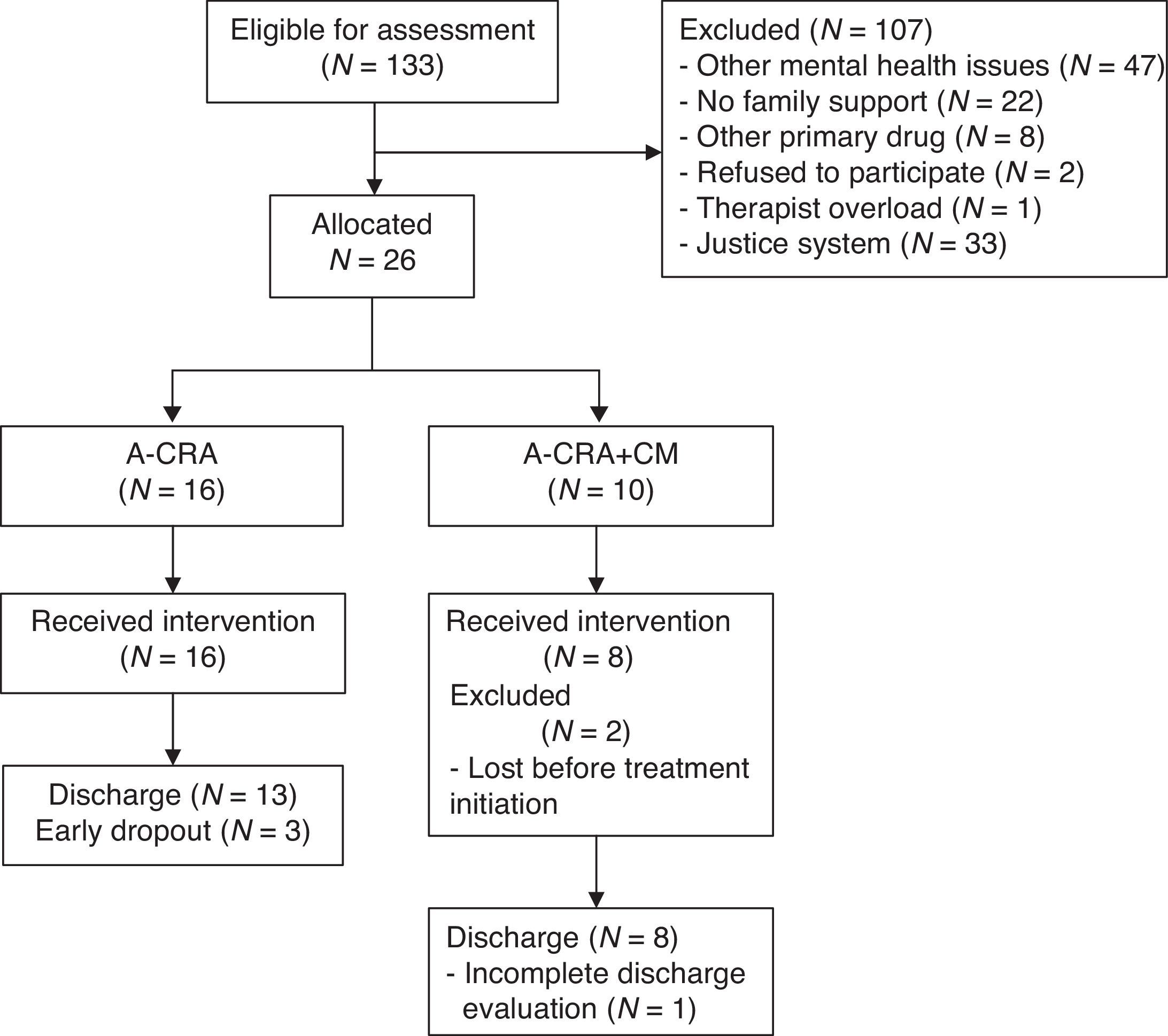
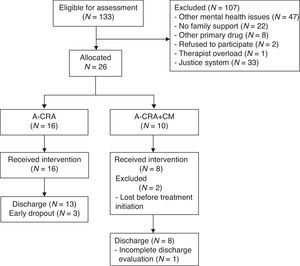
In the Principality of Asturias, 70 participants requested treatment and 19 (27.1%) met the inclusion criteria. In Madrid, 63 requested treatment and 7 (11.1%) met the inclusion criteria. All adolescents and their families meeting inclusion criteria agreed to participate. A total of 26 participants (19.55%) were allocated to one of the two treatment conditions. Two adolescents abandoned the study after allocation and before completing intake assessment (see Figure 1).
ProcedureA quasi-experimental design with two treatment conditions was implemented. Participants were randomized between treatment conditions (A-CRA and A-CRA plus CM) unless otherwise considered by clinical judgment. Treatment goal regarding substance use was abstinence from cannabis and other illegal drugs in both groups. Participants who requested treatment for their CUD at the Asturias and Madrid outpatient facilities entered the study if meeting the criteria described above. Both clinical settings delivered A-CRA and A-CRA+CM. Before treatment entry, participants and responsible adults provided informed consent to enter the study. The Ethics Committee of the University of Oviedo approved this research study. All participants were evaluated at intake and post-treatment (3 months later). Urinalyses were carried out weekly during the first two weeks devoted to intake assessment and before treatment entry, and twice weekly during treatment.
This study was carried out simultaneously in two settings within the Spanish Public Health System. In Asturias, they were implemented in an outpatient setting delivering treatment for adolescents (ProgramaReciella, CESPA-Proyecto Hombre Asturias). In Madrid, at the Center for Drug Addiction belonging to MadridSalud.
Adolescent Community Reinforcement Approach (A-CRA). Translation of the A-CRA manual was carried out by experts in English and Spanish, who translated it into Spanish and then back-translated from Spanish to English. The A-CRA is an adaptation of the Community Reinforcement Approach (CRA) that was initially developed and tested with adults (Godley, Meyers, et al., 2001). The program is composed of 10 individual sessions with the adolescent and four family sessions, which include 2 sessions for caregivers alone and 2 sessions for the adolescent and caregivers together. A-CRA is aimed at increasing adolescents’ access to social reinforcers in the community through skills-training and engagement procedures. A-CRA clinicians use a positive non-confrontational approach to promote abstinence, establish positive peer relationships and improve family relationships. The nineteen specific A-CRA procedures can be used repeatedly in sessions in a flexible manner, based on clinical needs. Sessions last an average of 1hour. The therapeutic work in A-CRA extended over a period of 12 to 14 weeks.
Contingency Management. Only participants in the A-CRA+CM condition received vouchers, following a schedule created based on previous studies (Stanger et al., 2009). Since many regular cannabis users need at least two weeks to test negative after starting abstinence at cut-off level of 50ng/ml (Goodwin et al., 2008), the first two weeks of pre-treatment were considered a washout period. To facilitate compliance in providing specimens, during the first two weeks of pre-treatment participants received vouchers worth 4€ for each weekly urine specimen irrespective of its results. During 12 consecutive weeks during treatment, participants earned vouchers contingent on negative results. The CM schedule assigned an increasing voucher value for each consecutive negative specimen to reinforce continuous abstinence. The schedule began with a 3€ voucher, 0.50€ being added to each consecutive negative result, up to the maximum value of 14.5€. A bonus voucher worth 6€ was earned by participants for each continuous week of abstinence. A positive result or failure to provide a valid sample implied a positive urinalysis and the schedule being reset to the start (3€). Participants could move up again through the schedule, but catching up with their previously-achieved maximum value after providing two consecutive negative urine specimens. Maximum value of vouchers they could earn was 290€, which could be exchanged for leisure and sports activities.
Staff Training. The staff was made up of 5 therapists. All were Licensed Clinical Psychologists with expertise in the field of adolescent drug-use treatment. All of them received 15hours of training in the basics of A-CRA/CM program, its procedures and the assessment instruments by a Licensed Clinical Psychologist and Researcher with expertise in Community Reinforcement Approach. Their participation was integrated with the delivery of regular services to other young and adult patients ineligible for this study. Therapists were randomized between treatment conditions.
InstrumentsUrinalysisAdolescents provided two weekly urine specimens during a pre-treatment period devoted to intake assessment. During the treatment period participants provided one sample on the day of the therapeutic session and a second one in an additional visit to the center. Results were available for the adolescent and his/her responsible adult within 10minutes. Urinalyses (UA) were carried out using Instant Urine Drug Testing Kits by Perfelena® to detect the presence of several cannabis metabolites, with cutoff levels of 50ng/ml.
Substance use SeverityThe presence of Cannabis Abuse in the previous 12 months was assessed by the therapist following DSM-IV-TR criteria. The Teen-Addiction Severity Index (T-ASI) (Kaminer, Burkstein, & Tarter, 1991) was utilized to collect information on patterns of drug use at intake: age of onset of cannabis use, months using cannabis, days of alcohol and cannabis use (in the last 30), report of legal issues and illicit drug use. This instrument has shown high inter-rater agreement, with an average correlation across scales of .78. The Spanish version (Fernandez-Artamendi et al., 2012) of the Cannabis Problems Questionnaire for Adolescents (CPQ-A) was used to assess the severity of cannabis-related problems. The CPQ-A consists of 27 items with a dichotomous response format, and has shown high reliability with Spanish adolescents (Cronbach's alpha=.86).
PsychopathologyThe Child Behavior Checklist (CBCL) (Achenbach, 1991) is a self-report instrument for detecting emotional and behavioral problems in the past six months for children and adolescents aged 6 to 18. It consists of 113 questions, scored on a three-point Likert scale, and provides scores on three global scales: internalizing and externalizing symptoms, and global symptomatology. Internal consistency of subscales ranges between .78 and .97, and inter-rater reliability is between .93 and .96. Beck Depression Inventory-II (Beck, Steer, Ball, & Ranieri, 1996). A comprehensive screening of depressive symptoms with 21 items rated from 0 to 3, with high reliability (Cronbach's alpha=.91)
Feedback from therapistsTo assess the opinion of the therapists on the experience of implementing A-CRA and CM, two short scales were created based on previous literature (Nelson, Steele, & Mize, 2006). One scale assessed the opinion of therapists exclusively on A-CRA and the other on CM only. Each scale included nine questions, with Likert-type response options ranging from 1 (very low/bad) to 5 (very high/good). Qualitative data was collected on the therapist's impressions by means of a telephone interview in which the following topics were discussed: ‘Treatment adequacy for the population attended’, ‘Integration of treatment programs in regular service’, ‘Positive and negative aspects of the implementation’ and ‘Limitations of the intervention’.
Data analysesThe flow of participants from first request to discharge is described by means of a flow chart to facilitate reading (Hartley, 2012), detailing the prevalence of each inclusion/exclusion criterion (see Figure 1). Descriptive statistics were utilized to report baseline characteristics of participants. Statistical differences between groups were sought using Fisher Exact Test to compare frequencies, and in order to prevent Family-wise errors, ANOVA was used to conduct comparisons between means. Details on implementation are reported with descriptive statistics.
Dependent t-test statistics and Fisher Exact Tests were performed to assess changes in psychopathology between intake and end of treatment. Participants who did not provide intake and discharge assessments were excluded from these analyses.
To compare substance use outcomes between groups, ANOVA and Fisher Exact Test were utilized, selecting participants based on an intent-to-treat model (Austin, Macgowan, & Wagner, 2005). Abstinence was analyzed using results from UAs, calculating: longest duration of continuous cannabis abstinence (two consecutive weekly samples=1 week), point-prevalence abstinence at months 1, 2 and 3 (discharge), percentage of negative specimens delivered (compared to all due samples) and percentage of adolescents “in-recovery” based on UAs (abstinence in the prior 30 days at the end of treatment). Missing samples were considered positive in order to report a conservative estimation of abstinence, except for continuous abstinence, where they were interpolated (considered negative only if they were preceded and followed by a negative UA; otherwise positive). Only 9% of due samples were missing and required interpolation. Confidence interval used for all analyses was 95%. Effect sizes were calculated using Cohen's d.
Descriptive statistics were applied on the items of ad hoc scales about A-CRA and CM. The most representative data from the qualitative reports was selected by the research team and was used to support the discussion of the results provided by quantitative data.
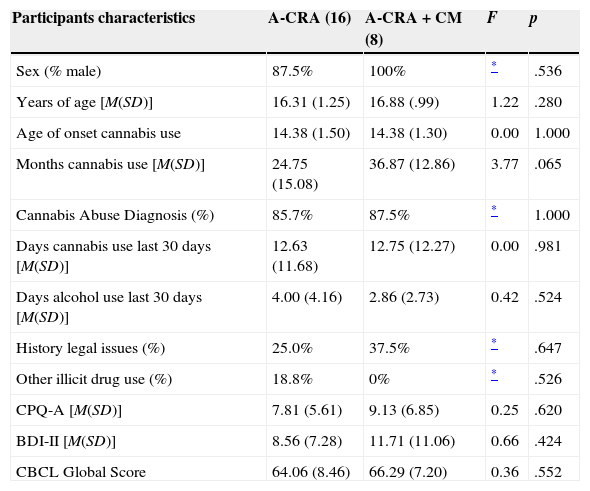
ResultsParticipantsBaseline characteristics of participants included in the study are shown in Table 1. Most participants were male (91.7%), and mean age was 16.50 (SD=1.18). Mean age at first cannabis use was 14.38 (SD=1.41), with an average of 28.79 months (SD=15.26) using this drug. Of the total sample, 86.4% met the criteria for cannabis abuse as defined in the DSM-IV-TR, with an average of 2.18 symptoms (SD=1.22). Participants reported an average of 12.67 days (SD=11.61) of cannabis use in the last 30 days, and 3.60 days (SD=3.69) of alcohol use. Other experimental illegal drug use was reported by 12.5% of the sample. Nearly one-third (29.2%) was or had been involved in legal issues. Participants presented a mean score of 8.25 (SD=5.94) on CPQ-A and 9.52 (SD=8.47) on BDI-II. Average global CBCL score was 64.74 (SD=8).
Baseline characteristics of participants.
| Participants characteristics | A-CRA (16) | A-CRA+CM (8) | F | p |
|---|---|---|---|---|
| Sex (% male) | 87.5% | 100% | * | .536 |
| Years of age [M(SD)] | 16.31 (1.25) | 16.88 (.99) | 1.22 | .280 |
| Age of onset cannabis use | 14.38 (1.50) | 14.38 (1.30) | 0.00 | 1.000 |
| Months cannabis use [M(SD)] | 24.75 (15.08) | 36.87 (12.86) | 3.77 | .065 |
| Cannabis Abuse Diagnosis (%) | 85.7% | 87.5% | * | 1.000 |
| Days cannabis use last 30 days [M(SD)] | 12.63 (11.68) | 12.75 (12.27) | 0.00 | .981 |
| Days alcohol use last 30 days [M(SD)] | 4.00 (4.16) | 2.86 (2.73) | 0.42 | .524 |
| History legal issues (%) | 25.0% | 37.5% | * | .647 |
| Other illicit drug use (%) | 18.8% | 0% | * | .526 |
| CPQ-A [M(SD)] | 7.81 (5.61) | 9.13 (6.85) | 0.25 | .620 |
| BDI-II [M(SD)] | 8.56 (7.28) | 11.71 (11.06) | 0.66 | .424 |
| CBCL Global Score | 64.06 (8.46) | 66.29 (7.20) | 0.36 | .552 |
No statistically significant differences (p>.05) were found between groups in baseline characteristics (Table 1). Regarding gender, the only two girls in the study entered the A-CRA group.
The average number of A-CRA sessions per patient was 10.92 (SD=2.59), with a range of 4 to 16. Adolescents in the A-CRA+CM group received an average of 155.81€ (SD=88.29) in vouchers during the treatment program, ranging from 14€ to 248.5€. The overall budget of the CM program was 1,246.5€. Although external donations were not actively sought, 20.98% of vouchers were funded by companies contacted that wanted to donate.
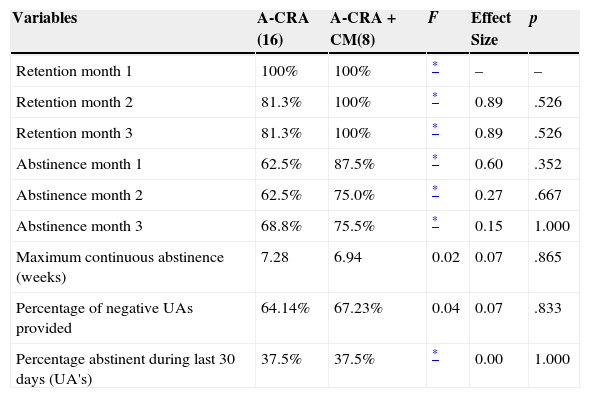
RetentionAfter 1 month, 100% of participants continued attending treatment in both groups. Whereas retention decreased to 81.3% in A-CRA at months 2 and 3, it remained 100% in the A-CRA+CM group. No significant differences were found between groups (p> .05) (see Table 2).
Retention and abstinence outcomes by treatment group.
| Variables | A-CRA (16) | A-CRA+CM(8) | F | Effect Size | p |
|---|---|---|---|---|---|
| Retention month 1 | 100% | 100% | * | – | – |
| Retention month 2 | 81.3% | 100% | * | 0.89 | .526 |
| Retention month 3 | 81.3% | 100% | * | 0.89 | .526 |
| Abstinence month 1 | 62.5% | 87.5% | * | 0.60 | .352 |
| Abstinence month 2 | 62.5% | 75.0% | * | 0.27 | .667 |
| Abstinence month 3 | 68.8% | 75.5% | * | 0.15 | 1.000 |
| Maximum continuous abstinence (weeks) | 7.28 | 6.94 | 0.02 | 0.07 | .865 |
| Percentage of negative UAs provided | 64.14% | 67.23% | 0.04 | 0.07 | .833 |
| Percentage abstinent during last 30 days (UA's) | 37.5% | 37.5% | * | 0.00 | 1.000 |
Treatment outcomes.
Psychopathology
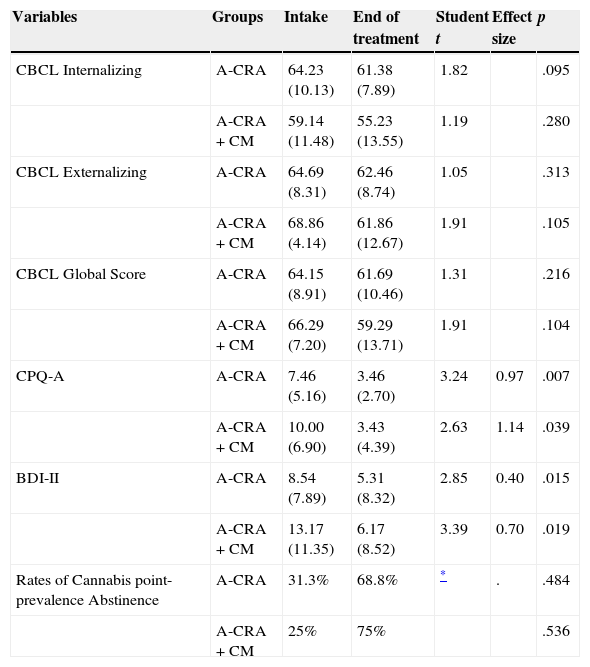
Clinical changes in each group were assessed for all participants who completed assessments at intake and end of treatment (N=20; n for A-CRA=13; n for A-CRA+CM=7). Point-prevalence abstinence rates were calculated for all participants in the study (N=24), considering missing samples as positive (conservative approach). Although all clinical scores decreased and abstinence rates increased during treatment in both groups, the only significant changes were on cannabis-related problems (p=.007 for A-CRA; p=.039 for A-CRA+CM) and depressive symptoms (p=.015 for A-CRA; p=.019 for A-CRA+CM)(see Table 3).
Rates of point-prevalence abstinence as assessed by UAs at months 1, 2 and 3 were higher in the A-CRA+CM group, with a final rate of 75.5% at the end of treatment, but differences were non-significant. In A-CRA, abstinence at the end of treatment was 68.8%. Maximum continuous abstinence was 7.28 weeks in A-CRA and 6.94 in A-CRA+CM, with no significant differences between groups. Regarding rates of adolescents in-recovery, in both groups 37.5% of adolescents were abstinent and in the community in the last 30 days of treatment. See Table 2.
Feedback from therapistsFour therapists completed questionnaires on A-CRA and A-CRA+CM. Therapists’ opinions on both approaches were very positive (4.5 in both cases). Clinicians considered CM to work better (4) with adolescents than A-CRA (3.25), and were more likely to continue using CM techniques (5) than A-CRA treatment (3.75). The experience of implementing both programs was positive (A-CRA: 4.5; CM: 3.5). Regarding detected barriers, clinicians considered their significance was low: Lack of institutional support (A-CRA: 1.5; CM: 1), lack of training (1.25; 1), lack of interest from patients (2; 1.5) and lack of clinical utility (1.5; 1). The lack of time/high caseload was highlighted as the more significant barrier (3.25; 2.5). All therapists considered very important (5) to continue extending implementation of EBTs in regular practice at the Spanish Public Health System.
DiscussionThis is the first study to implement an EBT for adolescent CUD in Spain, and more specifically in the Public Health System. Implementing A-CRA and CM in this context was feasible and had positive results. However, the clinical effectiveness of A-CRA and CM when compared to other interventions utilized in these contexts still needs further study. The addition of CM to A-CRA as a way of improving outcomes by reinforcing abstinence needs more research too. We found significant barriers that alerted us to certain obstacles in the implementation of evidence-based programs in the Public Health System, giving us useful information on how to proceed with future implementation efforts.
Implementation processSeveral factors hindered the implementation of the treatment program and limited the eventual sample size of the study to 26 participants. During the 24 month course of this project only 133 eligible adolescents sought treatment in the participating clinical settings. The second factor that limited the sample size was the establishment of inclusion and exclusion criteria. Eventually, 107 adolescents were ineligible for the study based on exclusion criteria, mostly the presence of other mental health issues referred to more specific interventions and lack of family support. Given the influence of family involvement (Cerezo, Méndez, & Alto, 2013) and comorbid disorders (López-Villalobos, Andrés-de Llano, Sánchez-Azón, Sanguino-Andres, & Alberola-López, 2012) on disruptive behaviors such as substance abuse, these exclusions might have contributed to our positive results. These adolescents were referred to more specialized services. Future research studies could include these and other clinical profiles of adolescent cannabis users since A-CRA has shown to be effective among young adults (Smith, Godley, Godley, & Dennis, 2011), adolescent using other drugs (Slesnick, Prestopnik, Meyers, & Glassman, 2007) and presenting internalizing and/or externalizing disorders (Godley et al., 2014). Qualitative reports from therapists underscore this fact, since they considered A-CRA and A-CRA+CM were suitable for a broader population of adolescents.
Since therapists’ participation in the study was voluntary and based on time availability, qualitative reports showed that they could not have integrated more study cases in their regular caseloads; although only .01% of cases were eventually excluded due to this reason. Previous studies have shown that organizational issues can be a significant barrier to implement EBTs (Lundgren, Chassler, Amodeo, D’Ippolito, & Sullivan, 2012). Lack of time also prevented therapists from utilizing some outreach techniques inherent to the A-CRA approach, such as treatment delivery outside the clinical setting (Godley, Meyers, et al., 2001). Given the greater complexity of A-CRA compared to CM, this might have contributed to lower scores from therapists on their evaluation. In future experiences, utilizing other formats for A-CRA could help delivering treatment to broader samples in these public contexts. Previous experiences using group format with A-CRA in the US (Godley, Smith, Meyers, & Godley, 2009; Slesnick et al., 2007), and CRA in Spain (Garcia-Fernandez et al., 2011) have obtained positive results. Other approaches such as telehealth procedures have shown to be effective for psychological treatments (Peñate, 2012).
Regarding CM, previous studies have shown practitioners as scarcely motivated for its implementation by comparison with other new evidence-based behavioral techniques (McGovern, Fox, Xie, & Drake, 2004). Actually, CM is an unfamiliar approach for many treatment providers (McGovern et al., 2004), who find it too expensive (Kirby, Benishek, Dugosh, & Kerwin, 2006). Whereas previous feasibility studies have found many significant obstacles to the implementation of CM (Killeen, McRae-Clark, Waldrop, Upadhyaya, & Brady, 2012),in our case, there were no significant barriers. According to therapists, “it helps to motivate adolescents by offering new positive reinforcers in line with therapeutic goals”. The main reported limitation was “the difficulties to include such programs within the budget of the institution”. In this regard, this study did not actively seek altruist donations, but 20.98% of vouchers were funded by companies that proposed to donate. Some protocols exist that contribute to building a voucher program with up to 38% of companies providing services free of charge or with some discount (García-Rodriguez, Secades-Villa, Higgins, Fernández-Hermida, & Carballo, 2008).
Clinical outcomesNo evidence-based treatments or systematic UAs were being regularly delivered by the collaborating clinical settings. Lack of experimental control on extant treatment programs implemented at these institutions obliged us to dismiss creating a control group, which thus prevented us from comparing the effectiveness of the two EBT conditions to standard treatment programs. The limited sample size might also have prevented us from obtaining significant differences in effectiveness between both approaches. These limitations notwithstanding, this experience has shed some light on the clinical outcomes of selected EBTs when implemented in this public context.
When evaluating clinical outcomes, only cannabis-related problems and depressive symptoms showed significant decreases after treatment, although a non-significant reduction can be observed in other clinical variables. The lack of a control group and the limited sample size do not allow us to determine whether these were direct effects of A-CRA or CM. Retention rates showed that A-CRA and A-CRA+CM generate engagement, with no significant improvements associated to the utilization of CM. Nevertheless, and in line with previous studies with adults (Budney & Higgins, 1998; Secades-Villa, García-Rodríguez, Higgins, Fernández-Hermida, & Carballo, 2008), adding CM to CRA proved feasible, and both techniques were readily integrated, with no additional difficulties. Further studies should analyze whether this rates are a significant improvement when compared to engagement in treatment as usual at these resources.
Most participants achieved high rates of abstinence, with an average 70.8% point-prevalence rate at the end of treatment and 37.5% of adolescents in-recovery, according to UA's. This latter rate is higher than the self-reported rate of 24% across conditions and follow-ups in the CYT study (Dennis et al., 2004). In our study, utilization of UA's might be contributing to improved abstinence outcomes in both groups (Sánchez-Hervás et al., 2010). A-CRA resulted a useful approach to reduce cannabis use among adolescents in our study, with similar rates of adolescents in-recovery across conditions. The same is shown regarding continuous abstinence, where adolescents achieved an average of 7 weeks in both groups, without significant differences between them. This rate is similar to the 7.6 weeks achieved in the CM group in the study of Stanger et al. (2009). Due to the lack of a control group we cannot determine whether our results are fully attributable to the interventions implemented, or if adding CM to A-CRA had significant additive effects on this approach. Future studies with larger samples are needed to establish conclusions on differences in efficacy between conditions.
LimitationsOur results indicate that using A-CRA and A-CRA+CM in the Public System is feasible. However, several methodological issues limit the extent of our findings. First, the lack of a control group prevented us from comparing these EBTs with treatment as usual and determining whether significant decreases in clinical symptomatology are due to the interventions implemented. Low utilization of treatment services and strict inclusion/exclusion criteria restricted the sample size to 26 participants, limiting the power of our statistical analyses and excluding other potential beneficiaries. Therapists had to deliver EBTs while providing treatment as usual to other patients, which consumed much of their time and prevented them from deploying all the outreach techniques suggested in the A-CRA manual, which could have improved enrollment and retention. No monitoring of therapists could be conducted by means of videotaping or recordings, so fidelity to the model relied upon intensive training (15hours) and continuous clinical assistance. Utilizing UA's in the A-CRA group was necessary to monitor abstinence but it might have had an effect on these rates given the natural consequences provided by the family based on its results. This could have concealed between-group differences, minimizing the effects of the vouchers schedule in the A-CRA+CM group.Only one follow-up at the end of treatment was conducted, so further research should analyze the stability of treatment effects and the feasibility of multiple follow-ups.
ConclusionsThis is the first study to implement EBTs with adolescent cannabis users in Spain, and the first reported experience of such interventions in the Public System. Since there was no control group we could not determine the clinical efficacy of A-CRA and CM compared to other regular services. When compared to previous studies, A-CRA and CM resemble positive results achieved by previous studies regarding retention or abstinence, and their utilization in this context was feasible. Treatment outcomes indicated that CM did not have a significant effect on abstinence or retention rates of A-CRA. However, this needs to be further analyzed given our small sample size. Despite significant improvements in cannabis-related problems and depressive symptoms, we cannot attribute them exclusively to the interventions implemented due to the absence of a comparison group (Table 3).
Clinical outcomes by treatment group.
| Variables | Groups | Intake | End of treatment | Student t | Effect size | p |
|---|---|---|---|---|---|---|
| CBCL Internalizing | A-CRA | 64.23 (10.13) | 61.38 (7.89) | 1.82 | .095 | |
| A-CRA+CM | 59.14 (11.48) | 55.23 (13.55) | 1.19 | .280 | ||
| CBCL Externalizing | A-CRA | 64.69 (8.31) | 62.46 (8.74) | 1.05 | .313 | |
| A-CRA+CM | 68.86 (4.14) | 61.86 (12.67) | 1.91 | .105 | ||
| CBCL Global Score | A-CRA | 64.15 (8.91) | 61.69 (10.46) | 1.31 | .216 | |
| A-CRA+CM | 66.29 (7.20) | 59.29 (13.71) | 1.91 | .104 | ||
| CPQ-A | A-CRA | 7.46 (5.16) | 3.46 (2.70) | 3.24 | 0.97 | .007 |
| A-CRA+CM | 10.00 (6.90) | 3.43 (4.39) | 2.63 | 1.14 | .039 | |
| BDI-II | A-CRA | 8.54 (7.89) | 5.31 (8.32) | 2.85 | 0.40 | .015 |
| A-CRA+CM | 13.17 (11.35) | 6.17 (8.52) | 3.39 | 0.70 | .019 | |
| Rates of Cannabis point-prevalence Abstinence | A-CRA | 31.3% | 68.8% | * | . | .484 |
| A-CRA+CM | 25% | 75% | .536 |
No comparable data from other controlled studies or interventions applied in this context has been found that allow us to compare the efficacy of the programs utilized here. This research sheds light on the obstacles that need to be overcome to conduct such studies here on and it encourages further exploration of A-CRA and CM, as well as other alternative EBTs, within the Public Health System to determine their effectiveness.In this sense, some recommendations for improving the use and acceptability of these EBTs in the Public System are suggested: (1) Extending their utilization to broader samples of adolescent drug users; (2) utilizing a group format that could make A-CRA more feasible for overloaded resources; (3) improving recruitment strategies to promote adolescents’ utilization of outpatient services when experiencing drug-use problems; (4) utilizing existing protocols to help funding CM programs through donations (García-Rodriguez et al., 2008), facilitating its implementation in diverse clinical settings; and (5) extending the use of CM techniques to reinforce clinical evaluations and completion of assessment instruments, which would increase participants’ compliance with the evaluation process.
FundingThis study was funded by the Ministry of Science and Innovation of Spain (Ref. MICINN-08-00309-2008). The authors would like to express their great appreciation to “Proyecto Hombre Asturias–ProgramaReciella” and “MadridSalud” for collaborating in this study. Our grateful thanks are also extended to all therapists, patients and their families for their cooperation. Finally, we would like to thank all companies and associations who provided financial support to utilize their activities and services as vouchers.
Available online 21 June 2014