Cognitive decline in multiple sclerosis (MS) is common, but unpredictable, and increases with disease duration. As such, early detection of cognitive decline may improve the effectiveness of interventions. To that end, the Symbol Digit Modalities Test (SDMT) is effective in detecting slow processing speed as it relates to cognitive impairment, and intraindividual variability (IIV) observed in trials assessing continuous reaction time (RT) may be a useful indicator of early cognitive changes. Here, we will assess cognitive IIV changes in adults with early MS.
MethodsAdults with relapsing-remitting MS (RRMS), <11 years since diagnosis, were recruited nationally. Baseline and two-year follow-up assessments included Brief International Cognitive Assessment in MS (BICAMS) and Cogstate computerized tests. Intraindividual variability in RT was calculated from psychomotor tasks and data were age-normalized.
ResultsA total of 44 of the 66 participants completed follow-up (mean age, 34.0 ± 5.5 years; 66 % female; mean disease duration, 4.1 ± 2.9 years; median Expanded Disability Status Scale (EDSS) score, 1.5 [0 to 6.0]). Participants were grouped by SDMT z-score median split. Groups did not differ in demographics or clinical features. The higher baseline SDMT group was faster (p = 0.05) in RT and less variable (lower IIV, p = 0.001). At the two-year follow-up, the higher SDMT group showed increased variability (p = 0.05) compared to the lower SDMT group, with no significant RT or BICAMS changes.
ConclusionsIn early MS, higher SDMT performance at baseline is associated with less cognitive variability but may indicate susceptibility to increased variability over time, highlighting the importance of monitoring IIV for early cognitive changes.
Cognitive decline is a common yet unpredictable aspect of multiple sclerosis (MS), with risk escalating throughout its duration (Sumowski et al., 2018). Detecting cognitive changes early is crucial for effective intervention to impede progression (Meide et al., 2018; Waskowiak et al., 2024). The Symbol Digit Modalities Test (SDMT) is a widely used measure of cognitive processing speed (Smith, 1982), requiring individuals to match numbers to corresponding symbols as quickly as possible, and scored according to the number of correct matches within a 90-second timeframe. The SDMT is considered the “gold standard” screening measure to identify cognitive impairment in MS (Benedict et al., 2017), detecting cognitive slowing as a robust predictor of overall cognitive functioning (Charvet et al., 2018; Parmenter et al., 2007). However, recent research suggests that increased variability in reaction times (RTs) across tasks, known as intraindividual variability (IIV), precedes the onset of cognitive slowing in many conditions including healthy aging (Jutten et al., 2024) and neurodegenerative conditions(Jones et al., 2020; Mumme et al., 2021) such as MS (Eilam-Stock et al., 2021; Wojtowicz et al., 2012, 2014).
Prior studies investigating cognitive decline in prodromal or early-stage progressive diseases have typically relied on single assessments of RT (Fengler et al., 2017; Grande et al., 2020; Mura et al., 2014). While some studies have failed to detect significant changes in RT during the early stages of neurological dysfunction, attention to IIV has emerged as a promising avenue. Reflecting the variation between measurements in the same individual taken at different time points, IIV in performance on cognitive tasks has been increasingly recognized as a sensitive marker of cognitive impairment. For example, it is a known marker of cognitive decline in the context of healthy aging and progressive neurological conditions (Christensen et al., 2005; Hultsch et al., 2000, 2002) including mild cognitive impairment (MCI), Alzheimer's disease and Parkinson's disease, among many others (Costa et al., 2019; Haynes et al., 2017; Tractenberg & Pietrzak, 2011). When compared to healthy controls, IIV is elevated at the group level in samples of people with MS (Bodling et al., 2012; Bruce et al., 2010; Mazerolle et al., 2013; Riegler et al., 2022; Wojtowicz et al., 2012, 2013; Wojtowicz et al., 2014), including in samples otherwise without any clinically measurable cognitive involvement (including with the SDMT) (Eilam-Stock et al., 2021). However, longitudinal characterization of IIV in relation to cognitive functioning in MS (as well as in other conditions) is needed to understand its predictive power.
In this study, we investigated cognitive IIV changes over a two-year period in a longitudinal cohort of recently diagnosed adults with relapsing remitting MS (RRMS). Our aim was to evaluate whether increases in IIV occurring in the early phase of MS correspond to changes on clinical cognitive measure. Identifying increases in IIV would provide valuable insight into the early trajectory of cognitive involvement in MS and inform timely interventions.
Material and methodsParticipantsIn this report, we analyze the cognitive measures from an adult MS cohort who were recruited to serve for comparison in a separate longitudinal study conducted by the US Network of Pediatric MS Centers (NPMSC) concerning the neurodevelopmental outcomes of pediatric MS (Krupp et al., 2023). Participants were recruited across the participating Network Centers and enrolled between March 2017 and March 2019. Each participant completed cognitive testing at both the baseline visit and the two-year follow-up visit occurring approximately two years from date of enrollment.
The adult MS cohort was required to have MS onset at ≥21 years of age and be 25–45 years (inclusive) at the time of enrollment, ensuring they were outside the expected window of neurodevelopment. Participants were included if they had a confirmed, diagnosis of RRMS based on the 2010 McDonald criteria (Polman et al., 2011) and a disease duration <11 years. Additional enrollment eligibility criteria required participants to be English speakers for >3 years and to have learned English before the age of 12 years.
All participants were screened at the enrollment for having any neurological disorder (other than MS) that could potentially and significantly impact cognition (e.g., head injury), a previous report of an IQ < 70, or any chronic or unstable medical condition (e.g., epilepsy, sickle cell disease, type 1 diabetes). Participants were required to earn a Wide-Range Achievement Test–Fourth Edition (Gary S. Wilkinson & Gary J. Robertson, 2006) (WRAT-4) reading recognition subtest standard score of ≥ 85. The reading recognition subtest served as a proxy for IQ and was used to screen participants for premorbid intellectual impairment or language-based learning disabilities that could affect cognitive performance. Additionally, at baseline and two-year following up cognitive testing, participants were required to have been free from relapse and/or steroid use in the preceding month.
Approval for the study was obtained from the Institutional Review Board of each participating institutions: NYU Grossman in New York, NY; Washington University in St. Louis, MO (S.M.); University of Alabama in Birmingham, AL; University of California, San Francisco, CA (E.W.); Children's Hospital, Broomfield, CO; SUNY University of Buffalo, Buffalo, NY. The Data Coordinating and Analysis Center (DCAC), located at the University of Utah, provided central administration and database management for the primary study. Before any research procedure, all adult participants were required to provide signed informed consent.
Cognitive assessmentsStudy participants completed a single session of cognitive testing and self-report questionnaires at baseline and a two-year follow-up, which included the administration of the Brief International Cognitive Assessment in MS (BICAMS) (Benedict et al., 2012) and computerized testing using the Cogstate platform (Cogstate, 2018). All cognitive testing was conducted according to the testing manual instructions by trained clinical coordinators, and the full assessment lasted approximately 60–90 min.
Brief international cognitive assessment for multiple sclerosisThe BICAMS was specifically developed to detect and measure cognitive impairment in MS (Benedict et al., 2012). The BICAMS includes the oral condition of the SDMT, as a measure of information processing speed (Smith, 1982; L. Strober et al., 2019). The learning trials included the Rey Auditory Verbal Learning Test (Schmidt, 1996) (RAVLT) and the Brief Visuospatial Memory Test–Revised (Benedict et al., 1996) (BVMT-R). The RAVLT is a verbal/auditory learning task requiring immediate recall of a list of 15 unrelated words over five exposures, with the total raw score summed over the five learning trials. The RAVLT was chosen as an alternative to the California Verbal Learning Test (Stegen et al., 2010) due to its availability in a single form for all ages and multiple alternative forms for repeat testing. The BVMT-R is a visual learning task involving three trials that require the participant to draw reproductions of their memory following short, 10-second exposures to stimuli during learning trials. The total raw score is then summed over the three learning trials. The raw scores for each measure were calculated and converted to age- (Benedict, 2007; Schmidt, 1996) (BVMT-R; RAVLT) or age-, gender-, and education- (SDMT) normative z-scores (Strober et al., 2020). These z-scores were averaged across the three measures to obtain a representative BICAMS z-score for each participant.
Cogstate, 2018 Brief BatteryThe computer-based (Cogstate, 2018) platform was used to administer the Cogstate (2018) Brief Battery (CBB), a computer-based assessment focusing on information processing speed, including simple and choice RT tests Detection (DET) and Identification (IDN), as well as the One-Back (ONB) test.
The computerized tasks involve a deck of cards on a green background screen, with participants indicating responses by pressing the keyboard keys "D" or "K" for "yes" or "no," respectively, across repeated trials. Each task includes instructions and a practice period prior to the test, lasting approximately 3–4 min each (for a total of ∼9 to 12 min). The CBB was comprised of the DET task (DET/simple RT), IDN task (IDN/choice RT), and ONB test (ONB/working memory). The ONB test was not included in this analysis. Scores were converted to age-normative z-scores based on a comprehensive global normative database (Cogstate, 2018).
Intraindividual variability derived from CogstateData cleaning was performed using built-in functions of the Research (Cogstate, 2018) Software (Version 2, 2020) (Cho et al., 2023). Data points were excluded if they met specific conditions: (1) DET speed > IDN speed, (2) DET accuracy ≤80 %, and (3) IDN accuracy ≤70 %. Trial-to-trial variability in RT was calculated across continuous simple and choice psychomotor tasks. The Cogstate (2018) RT and IIV values were automatically calculated for each participant for each task. RT was computed as the mean of log10-transformed RTs across tasks, while IIV was calculated as intraindividual standardized deviations (ISDs) of log10-transformed RTs across tasks (Cho et al., 2023), measured in milliseconds. Reaction time and IIV scores on each of the two tasks were converted to age-normative z-scores (Cogstate, 2018), with these z-scores averaged across the two tests to obtain an RT and IIV representative composite z-score for each participant (Bartlett et al., 2019; Charvet et al., 2018). For ease of comparison, all scores were transformed consistently so that positive z-scores indicate healthier performance, characterized by quicker speed and lower variability.
Statistical analysisParticipants were grouped according to Low and High SDMT based on a median split of their SDMT z-scores for further analyses. Baseline characteristics were compared between Low and High SDMT groups using either the t- (e.g., age, disease duration), Wilcoxon rank-sum median (e.g., Expanded Disability Status Scale Score (EDSS)) or chi-square (e.g., sex distribution, race and ethnicity distribution) tests. The z-score value of each cognitive variable was compared using paired sample t-test for within-group (Baseline vs. Follow-up) differences and independent t-test for between-group (Low SDMT vs. High SDMT) differences for baseline values and in mean change z-score values. Data were analyzed using SPSS version 28.0 (IBM Corp, Armonk, NY). Descriptive statistics (mean ± standard deviation) were calculated to determine participants’ demographic and clinical characteristics, and the Kolmogorov-Smirnov test was used to assess data normality. The Type I error (α) control was set at 0.05 for each comparison.
ResultsA total of 44 out of 66 participants completed the two-year follow-up assessment. Their mean age was 34.0 ± 5.5 years, and 66 % (n = 29) were female. In terms of race and ethnicity distribution, 69 % were White, 16 % were Black African or American, and 7 % were Hispanic or Latino. The mean disease duration was 4.1 ± 2.9 years, and the median EDSS score was 1.5 (range, 0–6.0).
Participants were categorized into higher (n = 22; SDMT z-score: 1.6 ± 1.0) and lower (n = 22; SDMT z-score: 0.8 ± 0.9) baseline SDMT performance groups using a median split z-score of −0.6. The groups did not differ significantly in demographic or clinical features, as reported in Table 1.
Demographic and clinical characteristics of the n = 44 participants with MS divided into Low (n = 22) and High (n = 22) SDMT groups, respectively.
EDSS: Expanded Disability Status Scale.
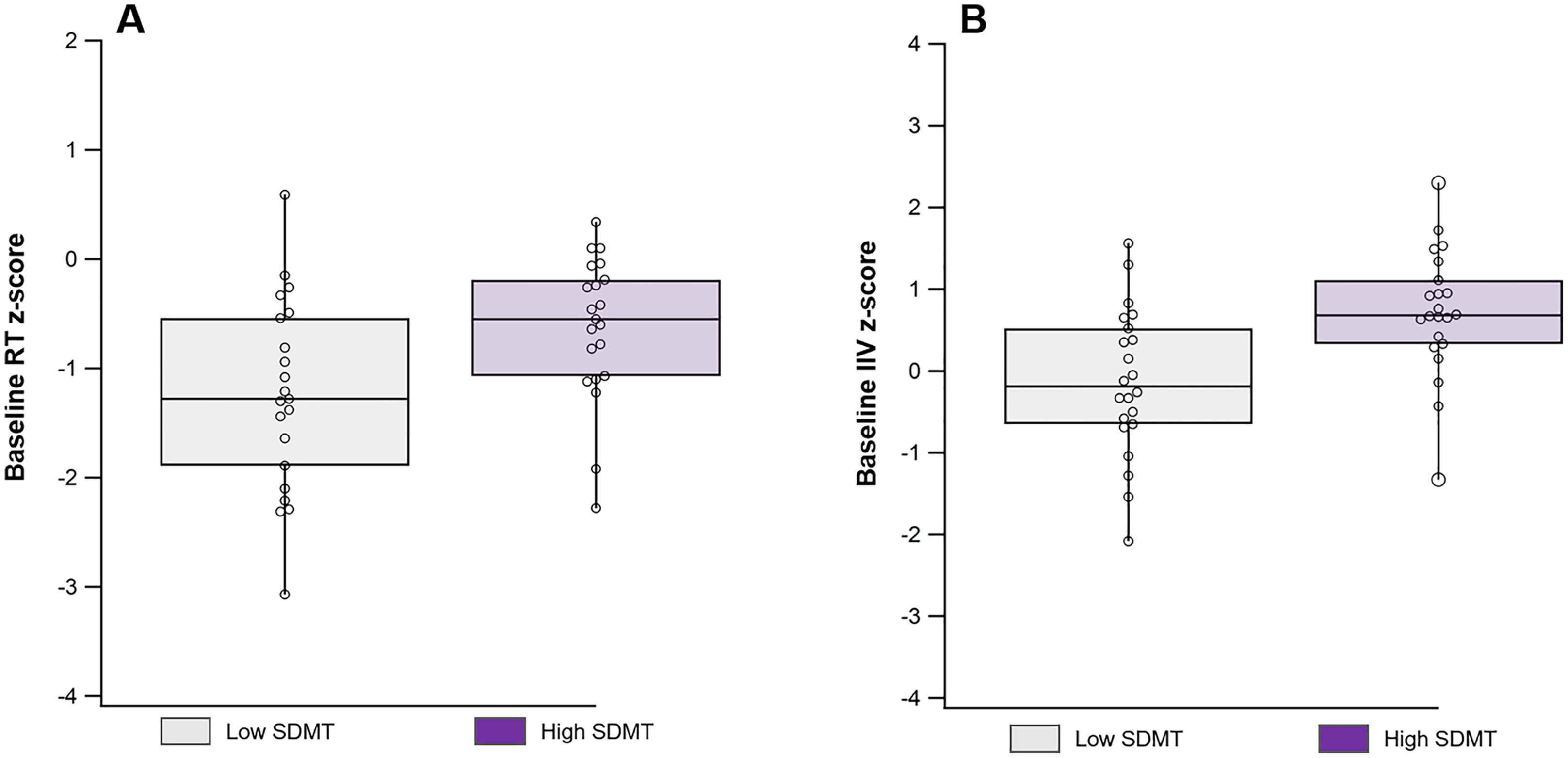
At baseline, the High SDMT group demonstrated significantly faster performance (mean Cogstate, 2018 RT z-scores: −0.82 ± 1.10 vs. −1.41 ± 1.19, p = 0.05) and lower variability (mean Cogstate, 2018 IIV z-scores: 0.71 ± 0.77 vs. −0.14 ± 0.90, p = 0.001) compared to the Low SDMT group (Fig. 1).
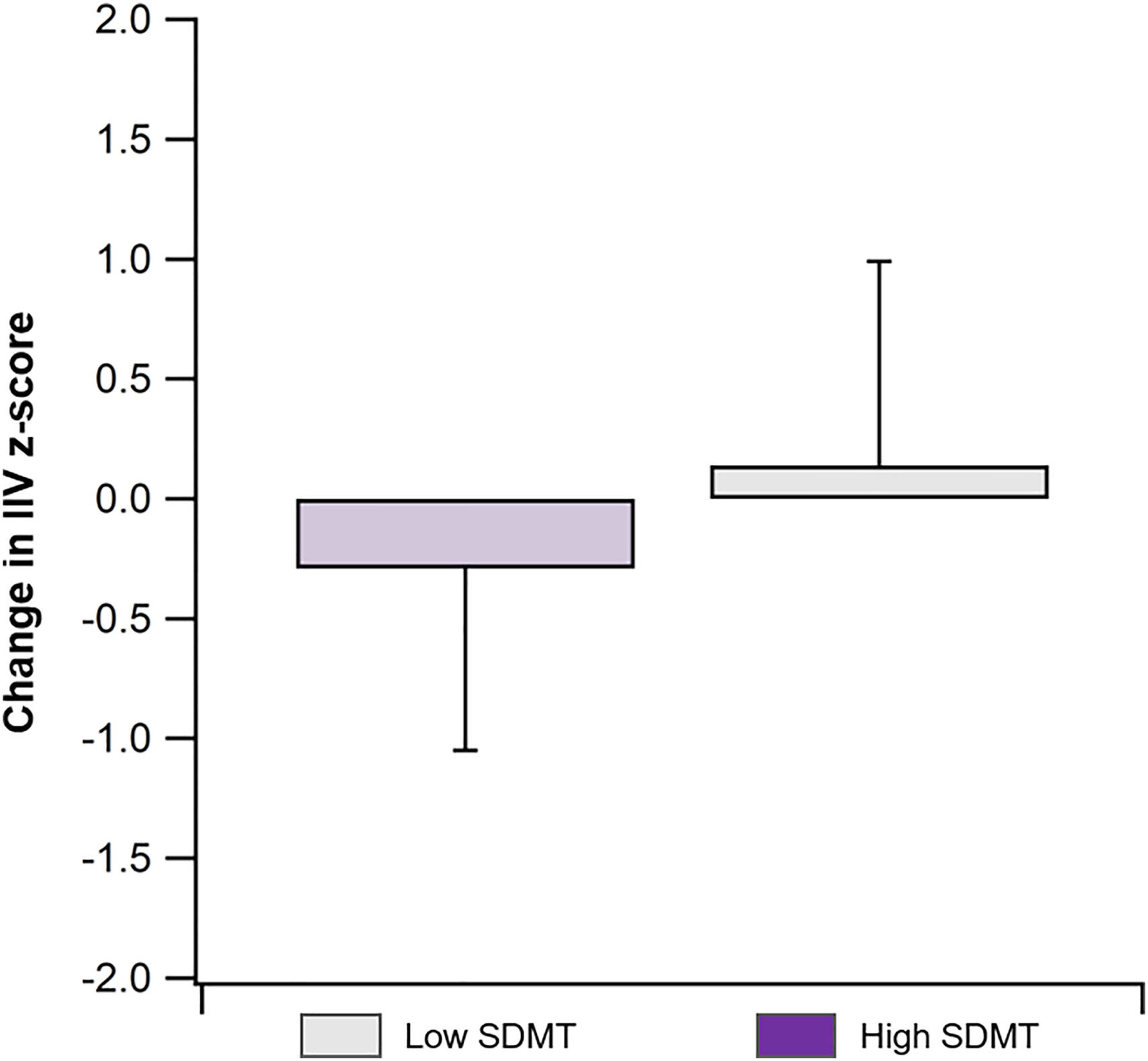
Over the two-year follow-up period (Table 2), the High SDMT group exhibited a significant increase in IIV (Baseline vs. Follow-up IIV z-score: 0.71 ± 0.78 vs. 0.42 ± 0.77, t = 1.76, Cohen's d = 0.37, p = 0.05), with a greater increase compared to the Low SDMT group (IIV z-score change: −0.29 ± 0.76 vs. 0.14 ± 0.85, t = 1.75, Cohen's d = 0.53 p = 0.05; Fig. 2). However, there were no significant changes in RT (Baseline vs. Follow-up: −0.82 ± 1.11 vs. −0.89 ± 0.93, t = 0.28, Cohen's d = 0.06, p = 0.39; Table 3) or BICAMS performance (Baseline vs. Follow-up: 0.40 ± 0.70 vs. 0.44 ± 0.78, t = −0.24, Cohen's d = 0.05, p = 0.41; Table 3) within the High SDMT group during this period.
Comparison of baseline standard cognitive assessment z-scores and CBB composite z-scores (mean ± SD) between Low and High SDMT groups.
SDMT: Symbol Digit Modalities Test; RAVLT: Rey Auditory Verbal Learning Test; BVMT-R: Brief Visuospatial Memory Test-Revised; BICAMS: Brief International Cognitive Assessment in MS; RT: Reaction Time; IIV: Intraindividual Variability.
Within group comparison of baseline vs. two-year follow-up standard cognitive assessment z-scores and CBB composite z-scores (mean ± SD). * indicates a p-value <0.05.
SDMT: Symbol Digit Modalities Test; RAVLT: Rey Auditory Verbal Learning Test; BVMT-R: Brief Visuospatial Memory Test-Revised; BICAMS: Brief International Cognitive Assessment in MS; RT: Reaction Time; IIV: Intraindividual Variability.
There was no significant correlation between change in IIV z-score and change in RT z-score in the High SDMT group (r = 0.02, p = 0.93), while a moderate correlation was observed in the Low SDMT group (r = 0.44, p = 0.04).
DiscussionIn this longitudinal and observational study examining cognitive functioning in a cohort of younger adults with early MS, we observed that IIV is an important measure of cognitive function during the initial stages of the disease. Consistent with previous findings (Eilam-Stock et al., 2021; Wojtowicz et al., 2013, 2014), our baseline assessment revealed higher IIV in individuals with lower SDMT scores compared to those with higher SDMT scores who can be presumed to have greater cognitive preservation. However, over the two-year follow-up period, we observed an increase in variability among participants with higher baseline SDMT scores, while their SDMT performance remained stable. In contrast, individuals with lower SDMT scores did not exhibit any significant change in variability over the same timeframe.
While IIV has been robustly demonstrated to be a marker of early cognitive impairment (Costa et al., 2019, 2019; Eilam-Stock et al., 2021; Wojtowicz et al., 2014), there remains little data to evaluate its change over time in relation to cognitive functioning. The finding of elevated IIV at baseline among those participants with SDMT scores in the lower end of the intact range (i.e., remaining unimpaired) is consistent with the model of variability as the earliest indicator of risk for cognitive decline, reflecting a hypothetical noise of neuronal networks attributed to initial neurodegenerative processes (Hultsch et al., 2000; Palop et al., 2006).
Given the model of IIV as the earliest indicator of cognitive decline, the two-year follow-up findings suggest that once there is cognitive impairment, measured here by slowing on the SDMT, IIV becomes less clinically relevant (Bielak et al., 2010; Costa et al., 2019). That is, once cognitive decline becomes detectable with clinical measurement, we would not expect a continued corresponding increase in variability. In support of this hypotheses, our findings show that at the two-years follow-up participants with the higher SDMT scores at baseline had an increase in variability over time at the group level. This increase may indicate that some participants in that group develop an increased risk for clinical cognitive decline over time.
These results contribute to a growing body of literature supporting the utility of cognitive IIV as a sensitive measure for the earliest detection of cognitive involvement in MS (Hultsch et al., 2000; MacDonald et al., 2006; Palop et al., 2006). While we have the strength of a nationally recruited and observational longitudinal cohort, broader generalization is limited by the smaller sample size. Further, while participants were not recruited on the basis of cognitive impairment, there was not full capture of clinical events and interventions during the two-year interval between baseline and follow-up testing. Therefore, in that period, participants may have developed cognitive impairment or even received treatment or rehabilitation that could have influenced functioning at follow-up testing. This study involved a “real-world” cohort where participants might have started new medications. While most disease modifying therapies (DMTs) have not been proven to affect cognitive outcomes (Niccolai et al., 2017), newer and more efficacious medications may have a cognitive-preserving effect (as measured by SDMT) (Landmeyer et al., 2020). Additionally, common symptomatic medications used between testing time points, such as stimulants (often prescribed for fatigue) and gabapentin (commonly used for pain and sensory dysfunctions) (Niccolai et al., 2017; Salinsky et al., 2005), may have influenced cognitive outcomes.
Nonetheless, these findings clearly warrant continued study using IIV as a longitudinal measurement of cognitive functioning in MS as well as in other chronic neurological conditions. Carefully designed studies specifically addressing IIV and its change over time will be critical in establishing its predictive power at the group level. While our study provides valuable insights into the relationship between IIV and cognitive function over time, further research is needed to elucidate the underlying mechanisms driving changes in IIV and its predictive utility at the individual level (Cho et al., 2023), as well as influence of DMT and other mediations on cognitive function.
Ultimately, by identifying individuals at highest risk for cognitive decline based on their IIV profiles, we can tailor interventions aimed at preserving cognitive functioning rather than attempting to recover it once it has been lost. This personalized approach has the potential to significantly enhance patient outcomes and quality of life by intervening at an earlier stage of cognitive involvement, and potentially slowing its progression. Future studies should therefore also focus on refining the normative-based interpretation of IIV measurement and its reliable change to optimize prediction for future risk at the level of the individual level. This work can lead to the identification and evaluation of intervention strategies targeted towards those identified as high risk based on their IIV profile at the earliest possible point in the course of disease.
ConclusionThe findings from this observational cohort suggest that IIV may serve as the earliest detectable measure of cognitive change at a two-year follow-up in individuals with early MS. Further research is warranted to elucidate the underlying mechanisms driving changes in IIV and its potential as a prognostic marker in MS and other progressive conditions, leading to the development of potentially scalable and home-based strategies for detecting early markers of cognitive decline.
This work was supported by the National MS Society grants # RFA-2104–37483, RG-1507-05285, and SI-1808-32326 and the Parekh Center for Interdisciplinary Neurology.