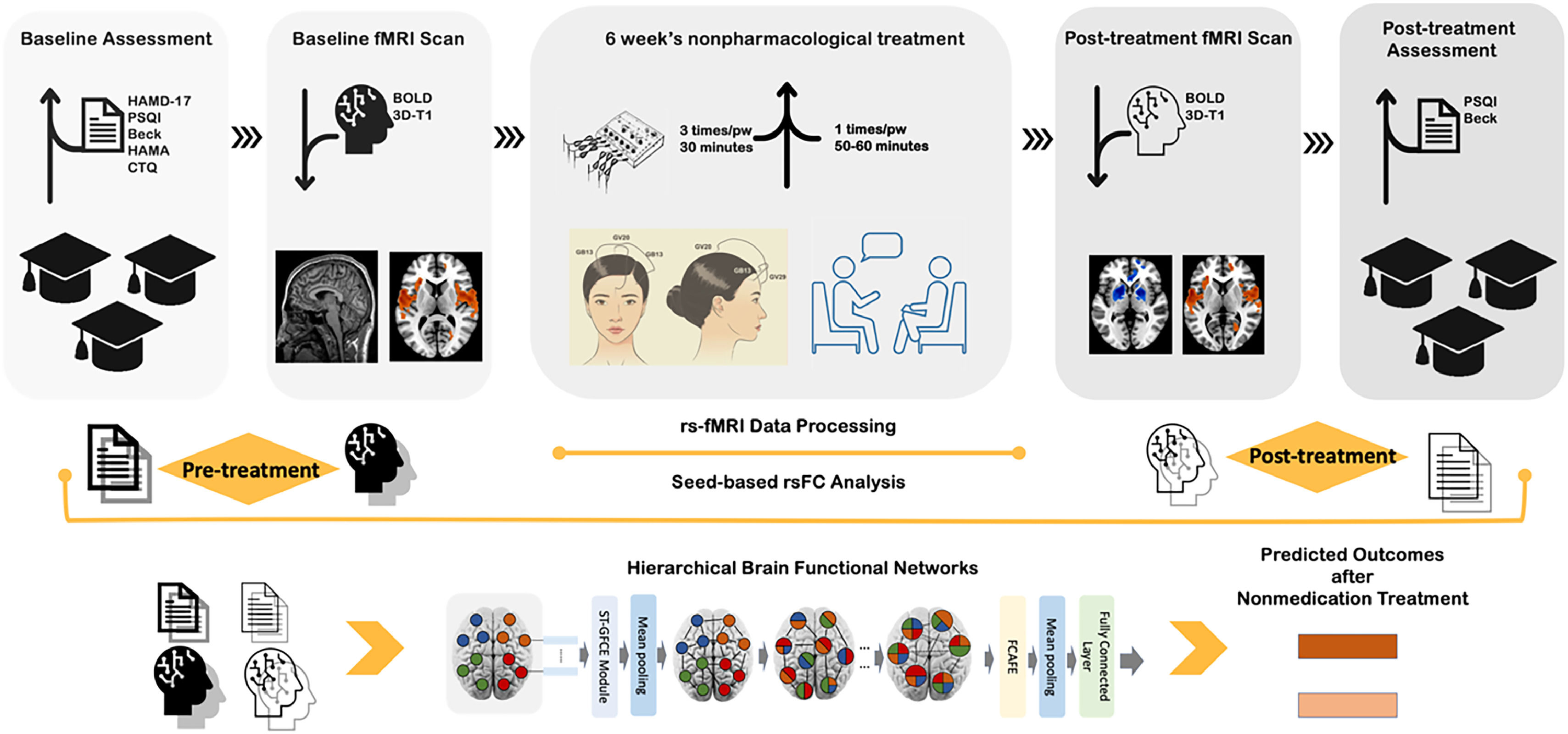
College students with subclinical depression often experience sleep disturbances and are at high risk of developing major depressive disorder without early intervention. Clinical guidelines recommend non-pharmacotherapy as the primary option for subclinical depression with comorbid sleep disorders (sDSDs). However, the neuroimaging mechanisms and therapeutic responses associated with these treatments are poorly understood. Additionally, the lack of an early diagnosis and therapeutic effectiveness prediction model hampers the clinical promotion and acceptance of non-pharmacological interventions for subclinical depression.
MethodsThis study involved pre- and post-treatment resting-state functional Magnetic Resonance Imaging (rs-fMRI) and clinical data from a multicenter, single-blind, randomized clinical trial. The trial included 114 first-episode, drug-naïve university students with subclinical depression and comorbid sleep disorders (sDSDs; Mean age=22.8±2.3 years; 73.7% female) and 93 healthy controls (HCs; Mean age=22.2±1.7 years; 63.4% female). We examined altered functional connectivity (FC) and brain network connective mode related to subregions of Default Mode Network (sub-DMN) using seed-to-voxel analysis before and after six weeks of non-pharmacological antidepressant treatment. Additionally, we developed an individualized diagnosing and therapeutic effect predicting model to realize early recognition of subclinical depression and provide objective suggestions to select non-pharmacological therapy by using the newly proposed Hierarchical Functional Brain Network (HFBN) with advanced deep learning algorithms within the transformer framework.
ResultsNeuroimaging responses to non-pharmacologic treatments are characterized by alterations in functional connectivity (FC) and shifts in brain network connectivity patterns, particularly within the sub-DMN. At baseline, significantly increased FC was observed between the sub-DMN and both Executive Control Network (ECN) and Dorsal Attention Network (DAN). Following six weeks of non-pharmacologic intervention, connectivity patterns primarily shifted within the sub-DMN and ECN, with a predominant decrease in FCs. The HFBN model demonstrated superior performance over traditional deep learning models, accurately predicting therapeutic outcomes and diagnosing subclinical depression, achieving cumulative scores of 80.47% for sleep quality prediction and 84.67% for depression prediction, along with an overall diagnostic accuracy of 82.34%.
ConclusionsTwo-scale neuroimaging signatures related to the sub-DMN underlying the antidepressant mechanisms of non-pharmacological treatments for subclinical depression. The HFBN model exhibited supreme capability in early diagnosing and predicting non-pharmacological treatment outcomes for subclinical depression, thereby promoting objective clinical psychological treatment decision-making.
The World Health Organization estimated that approximately 280 million people worldwide suffer from depression (WHO, 2024), with nearly 1/3 of cases occurring in China (Lu et al., 2021). Recently, subclinical depression among university students has garnered significant clinical attention, with global studies reporting a prevalence rate of 30–40% among college students (An et al., 2022; Bertha & Balázs, 2013; Cukrowicz et al., 2011; Ge et al., 2024), with 90% also suffering from sleep disturbances (Geoffroy et al., 2018; Tsuno et al., 2005). This rising clinical concern could be attributed to several factors, including challenges in adjusting to the dramatic transition from adolescence, sleep disturbances, intense academic pressure, social media impacts, financial stress, and inadequate mental health education. University students are particularly vulnerable to mental health challenges due to pressures related to academics, career preparation, and social relationships. Moreover, subthreshold depression—an early indicator of Major Depressive Disorder (MDD) —is notably prevalent in this population, raising concerns about an increased risk of future depressive diagnoses. In China, over 50 million students belong to subthreshold depression, with 15% having suicidal thoughts (Gao et al., 2020; Qu et al., 2023). The issue could also be attributed to the increased fear and anxiety that resulted from the COVID-19 pandemic. During the pandemic, Chinese college students exhibited a prevalence of depressive symptoms and sleep disturbances of as high as 54.95% and 48.18%, respectively (Zhang et al., 2023b).
The depression-sleep disturbance relationship is not only intricate but also comorbid, creating a bidirectional link that has been extensively explored in numerous studies since the 1970s (Pearlman & Greenberg, 1970). For instance, patients with insomnia and subthreshold depression were previously reported to show a significant deterioration of insomnia when they stopped taking sleep-promoting medications (Wichniak et al., 2011). Furthermore, college students with an evening chronotype and poorer sleep quality were found to be at a higher risk of more severe depressive symptoms (Zhang et al., 2023b). It is also noteworthy that subclinical depression, a significant precursor to and a risk factor for MDD (Zhang et al., 2023c), can develop into a MDD if not properly intervened. In subclinical depression randomized controlled trials (RCTs), anti-depressants have not often shown no better therapeutic effects than placebos (Baumeister, 2012). Furthermore, research evidence suggests that anti-depressants might be less useful in treating patients with more severe depression, as well as functional impairments or suicidal ideations (Kroenke, 2017). Given the limited research evidence supporting the effectiveness of pharmacotherapeutic treatments in managing subclinical depression, current clinical guidelines recommend non-pharmacological interventions as the first-line treatment for early depression.
Psychotherapy, the most commonly employed anti-depressant intervention for subclinical depression, has been shown in numerous studies to reduce MDD incidences (Cuijpers et al., 2014). For instance, a meta-analysis involving 32 RCTs revealed that psychotherapy might be the most effective non-pharmacological treatment for subclinical depression in adults (He et al., 2022). Moreover, findings from the annual Healthy Minds Study that involved 96,000 US students across 133 campuses who completed web surveys during the 2021–22 academic year revealed a significant increase in the number of students participating in counseling or alternative therapy. Specifically, the number of students participating in ≥ 1 therapy or counseling session within a year rose from 30 to 37% (>1/3 of the surveyed students), marking a new high. According to a systematic review and network meta-analysis of combined treatments for a new episode of depression, included 676 RCTs, 105,477 participants and 63 treatment classes, reported that combined treatments showed better effectiveness for a new episodes of depression at the intervention level (Mavranezouli et al., 2024).
Electroacupuncture (EA), another promising Y anti-depressant treatment, entails applying electrical stimulation to acupuncture needles, resulting in improved therapeutic effects relative to traditional acupuncture (Yin et al., 2022). It has been widely studied in recent years for its safety, effectiveness, ease of operation, and affordability. The Mayo Clinic adopted acupuncture as a regular therapy in April 2024 to help with pain management, anxiety, and sleep problems, et al. showing its effectiveness and safety. According to clinical research, EA exerts therapeutic effects comparable to classic tricyclic anti-depressants in alleviating MDD symptoms, with no side effects (Luo et al., 1998; Zhao et al., 2019). Moreover, clinical research evidence from two reliable RCTs (Yeung et al., 2011; Yin et al., 2022) revealed that compared to placebo group, EA on Baihui (GV20) and Yintang (GV29) [covering the Prefrontal Cortex (PFC)] significantly improved the depression and subjective sleep scores after treatment. Nonetheless, additional high-quality RCTs are required to further elucidate the effectiveness of different non-pharmacological interventions, including EA and Psychotherapy. It is also noteworthy that the underlying neuroimaging mechanisms, as well as neural markers associated with combined non-pharmacological treatment responses in young patients with subclinical depression, remain unclear, necessitating further research.
The Default Mode Network (DMN), the most studied functional brain network in recent rs-fMRI research, is highly relevant in depression diagnosis and treatment response. The DMN is responsible for self-referential thinking, memory retrieval, emotional regulation, introspection, and internal focus, faculties that are closely linked to depression (Barreiros et al., 2024; Deng et al., 2016; Liang et al., 2020; Xu et al., 2023). Therefore, it is plausible that research targeting the synchronous activation of distinct brain regions is flourishing based on the notion that anti-depression neuro-responses are related to DMN and its downstream modulation of connected brain regions (Bestmann et al., 2004; Beynel et al., 2020; Zweerings et al., 2019). Based on Independent Component Analysis (ICA), the DMN can be divided into two sub-regions: the anterior DMN (aDMN) and posterior DMN (pDMN) (Damoiseaux et al., 2008; Lei et al., 2013, 2014). The Anterior Cingulate Cortex (ACC) and Precuneus (PCU), integral components located in the aDMN and pDMN, respectively, formed the central hub within the DMN and have been established to correlate with depression complicated by comorbid sleep disorders (Godlewska et al., 2018; Rubart et al., 2022; Yu et al., 2020). In a previous study, compared to Healthy Controls (HCs), mental disorder patients showed a stronger ACC connectivity in the aDMN and a weaker connectivity between the PCU and the Posterior Cingulate Cortex (PCC) within the pDMN (Liemburg et al., 2012; Sendi et al., 2021). Furthermore, a leave-one-out analysis implied that, following six weeks of treatment, activity in the ACC could predict anti-depressant response status at individual participant levels (Godlewska et al., 2018). The sub-DMN is also crucially involved in the neuromodulation and communication between other brain networks in depression patients, especially during rest. For instance, a rs-fMRI study involving 114 MDD patients linked the PCU to subjective sleep quality in depression patients (Ma & Zhang, 2022; Rubart et al., 2022). Furthermore, a study on DMN-related changes among adolescents with depression predicted future depression risk based on the ACC and anterior dorsomedial PFC, with the increase in depressive symptoms at previous time points significantly predicting changes in FCs between the PCC and PCU (Afzali et al., 2022). Despite such extensive research insights, the correlation between the sub-DMN and neuroimaging signatures of non-pharmacological treatment responses in college students with subthreshold depression is yet to be fully elucidated.
Notably, the lack of individualized therapeutic effect prediction models limits the global applicability and popularity of non-pharmacological interventions for subclinical depression treatment. Consequently, robust, and intelligent predictive models are urgently required to enhance clinical therapeutic selection and pre-evaluation of therapeutic outcomes for subthreshold depression. Deep learning in Artificial Intelligence (AI) has demonstrated considerable potential in understanding and modeling the brain's complex structures and functions. Although the Functional Brain Network (FBN) has been widely used in constructing FBN models, conventional FBN construction often relies on a predefined brain region atlas to identify nodes (Tzourio-Mazoyer et al., 2002), limiting node interaction modeling to a single scale. However, extensive research has revealed that the brain functions hierarchically in physiology and anatomy (Mastrandrea et al., 2017; Raut et al., 2020; Vidaurre et al., 2017). Owing to the brain's hierarchical architecture in both structure and function, the single-scale FBN has been associated with several limitations, including inherently flat methods that do not learn hierarchical representations of the brain connectome (Ying et al., 2018). To overcome the hierarchical prediction details overlooked in conventional approaches, we propose the adaptive use of the Hierarchical Functional Brain Network (HFBN) within the transformer framework. Recently, the HFBN model has been successfully applied in diagnosing early Alzheimer's Disease (Zhang et al., 2024).
Herein, we hypothesized that: (1) compared to HCs, sDSDs would show abnormal rsFCs related to sub-DMN in some brain regions, as well as abnormal rsFCs between sub-DMN to other brain networks; (2) after six weeks of non-pharmacological treatment, sDSDs might have altered rsFCs and changed brain network connective mode related to sub-DMN (aDMN or pDMN); and (3) HFBN model could perform better in diagnosing and predicting non-pharmacological treatment outcomes than traditional deep learning models. To the best of our knowledge, this is the first rs-fMRI study that focuses on neuroimaging signatures of non-pharmacological treatment response in subclinical depression among college students and constructing a deep learning diagnosis-prediction model based on the transformer framework. The study's flow chart and design are shown in Fig. 1.
MethodsThis rs-fMRI study was approved by the Ethics Committee of The First Affiliated Hospital of Guangzhou University of Chinese Medicine (NO. ZYYECK [2019] 068). It is part of a four-site, single-blind RCT clinical trial on the effectiveness of non-pharmacological treatments on subclinical depression, registered in the Chinese Clinical Trial Registry (ChiCTR1900028530) https://www.chictr.org.cn. The metabolic genome findings of this clinical trial have already been published (Jiang et al., 2024), and the study protocol was as in Wang et al. (2020).
ParticipantsSample size calculation uses G* power (Faul et al., 2007), based on previous RCTs of EA on depression (Yeung et al., 2011; Yin et al., 2022; Zhao et al., 2019). After 6 to 8 weeks of intervention, the average depression scale scores in the post-EA group were 9.8 ± 3.1 and 3.9 ± 3.2 in the control group. With the alpha value of 0.05, the statistical power (1-β) = 0.95, and a 10% dropout rate, the minimized sample size is 72.
From June 2020 to March 2021, 207 college students recruited from two campuses of Guangzhou University of Chinese Medicine, all over 18 years old, signed an informed consent form, allowing for data sharing and publication for scientific purposes. The inclusion and exclusion criteria were as described in Wang et al. (2020). Students with subclinical depression and comorbid sleep disorders received six weeks of non-pharmacological treatments; EA was administered three times per week for 30 min per session, whereas counseling was provided once a week for 50–60 min per session.
Clinical measurementsThe primary measurement was Beck Depression Inventory-Second Edition (BDI-II) scores (Beck, 1996). BDI-II is a brief, self-rated measure that is easily scored and administered (Scogin & McElreath, 1994). Furthermore, a 50% change in the BDI-II score demonstrated high sensitivity and specificity for predicting depression remission (Reeves et al., 2012; Riedel et al., 2010). The secondary outcome was the Pittsburgh Sleep Quality Index (PSQI) scores (Buysse et al., 1989). PSQI measure comprises 19 self-rated questions covering seven sleep quality components (each weighted equally on a 0–3 scale) with a global score ranging from 0 to 21, with scores ≥ 5 indicating poor sleep quality (Buysse et al., 1989). Additionally, a ≥ 3-point reduction in the PSQI score indicated a clinically significant improvement in sleep quality, defined as Minimally Clinically Important Difference (MCID) by (Costandi et al., 2023).
fMRI data acquisition and pre-processingAll students' MRI images were obtained using a 3.0 Tesla Siemens Prisma scanner with a 64-channel head coil. Students were instructed to lie still, wear sponge earphones to reduce noise, and avoid systemic thinking during the procedures. Foam pads were put in the gap between head and coil to minimize head motion and improve image quality. Clinical diagnosis sequences were scanned first to exclude brain-structural abnormality. Blood Oxygen Level Dependent (BOLD) fMRI data were acquired using Gradient Echo Planar Imaging (GRE-EPI) sequences with the following parameters: TR=500 ms, TE=30 ms, slice thickness=3 mm, slice spacing=1 mm, Field of View (FOV)=220 × 220 mm, matrix=64 × 64, flip angle=90°, and time points=960, collected in 8 min. The DICOM was converted to the NIFTI format using MRIcroGL's dcm2niix converter (https://www.nitrc.org/projects/mricrogl/), and quality assessed. The Data Processing and Analysis for Brain Imaging (DPABI) toolbox version 6.8 (http://rfmri.org/DPABI) and SPM 12 (https://www.fil.ion.ucl.ac.uk/spm/software/spm12/) were employed for MRI data processing (Yan et al., 2016) using MATLAB (version R2022b).
The data pre-processing steps were as follows: (1) Discarding the first ten volumes to eliminate magnetic field instability; (2) Slice timing and realignment, excluding participants with head motion or rotation >3mm or 3°, respectively; (3) Spatial normalization to the MNI space using the standard EPI template with a resampling voxel size of 3 × 3 × 3 mm³; (4) Smooth normalizing images with a 6 mm Full-Width at Half Maximum (FWHM) to reduce the spatial noise; (5) Regressing out covariates, including head motion parameters, cerebrospinal fluid signals, and white matter signal; and (6) Bandpass filtering (0.01∼0.08 Hz) and detrending to remove cardiac and high-frequency physiological noise, and low-frequency drift.
Data quality controlThe baseline fMRI data of sDSDs-071 and HC-066 subjects were excluded due to insufficient rs-fMRI data time points. Furthermore, six students (sDSDs-groups-019, 032, 039, 042, 052, 064) dropped out of the study during the six-week non-pharmacological treatment course, resulting in some missing post-treatment fMRI data. Additionally, post-treatment fMRI data of the sDSDs-group-081 student was excluded due to excessive head motion (>3 mm or 3°). During the six weeks of treatment course, patient compliance remained high, 11 students dropped out, with a dropout rate of 9.6%, lower than the 12% dropout rate reported in a systematic review of acupuncture RCTs (Jeon et al., 2021). Finally, 96 sDSDs (with 96 paired pre/post-scan images) and 92 HCs (with 46 paired pre/post-scan images) were included in the final rsFC analysis.
Seed-based FC analysisThe SPM 12 version of Automated Anatomical Labelling (AAL) software (Tzourio-Mazoyer et al., 2002) was used to select core sub-DMN regions as ROIs or seeds, including ROI 1=AAL 31, ROI 2=AAL 32 (for represent aDMN); and ROI 3=AAL 67, ROI 4=AAL 68 (for represent pDMN) (DeMaster et al., 2022; Lei et al., 2013). Their Montreal Neurological Institute (MNI) coordinates (x, y, z) were anterior cingulate and paracingulate gyri L/R (−6, 52, −2 / 2, 36, 22) and precuneus L/R (0, -56, 30 / 2, −56, 26). The RESTplus toolbox was used to calculate altered FCs related to sub-DMN before and after six weeks of non-pharmacological treatment (Jia et al., 2019). Pearson's correlation coefficients were also calculated between the seeds’ time series and the remaining voxels in the entire brain. Finally, FC matrixes were obtained via conversion of the correlation values to Z values through Fisher Z transformation. Two-sample t-tests were performed to evaluate the abnormal FCs related to sub-DMN between sDSD and HCs and the different FCs between post-treatment SDSDs and post-scanned HCs. The altered FCs related to sub-DMN after non-pharmacological treatment within sDSDs were estimated by paired t-tests (gender, age, and education as covariates). The significant level was voxel p<0.001, cluster p<0.05, with a family-wise error (FWE) correction threshold of p<0.05 at the cluster level.
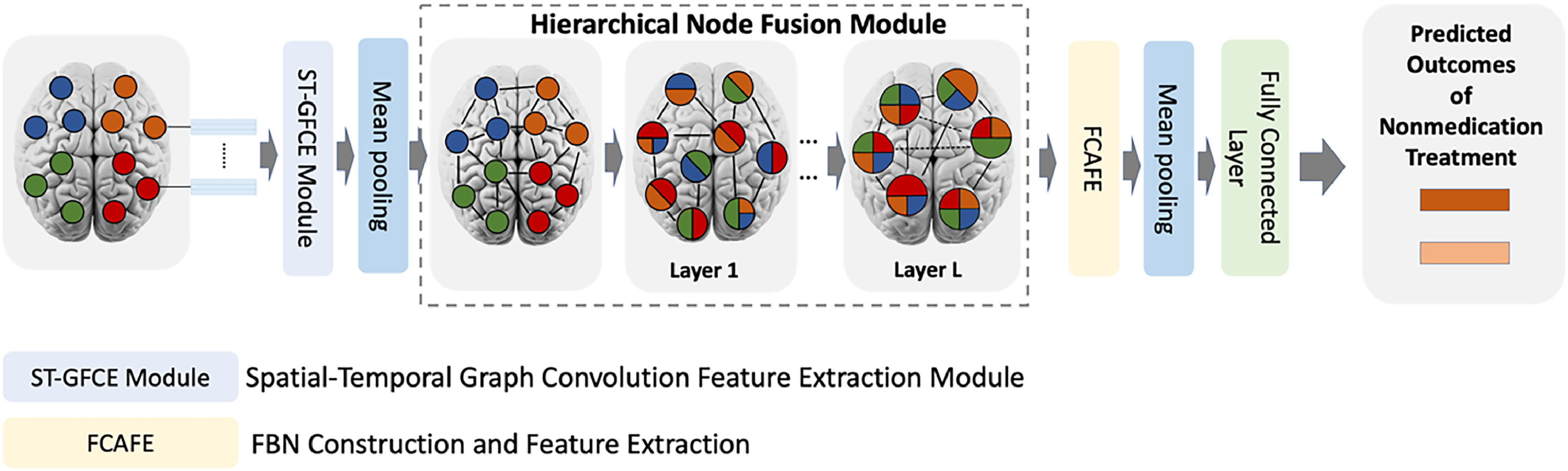
Construction of the HFBN model for diagnosing and predicting therapeutic outcomesThe HFBN is a sparse attention mechanism-based diagnostic model for constructing and analyzing multi-scale Functional Brain Networks (FBN). Herein, the HFBN was employed to construct and analyze multi-scale FBNs using rs-fMRI data to predict the therapeutic outcomes of non-pharmacological treatments. Its architecture comprised two key modules: The Spatial-Temporal Graph Convolutional Feature Extraction (ST-GCFE) module and the Hierarchical Node Fusion (HNFM) module. Fig. 2 shows the architecture of HFBN model. The former extracts spatiotemporal features from rs-fMRI time series signals via spatiotemporal graph convolution operations, effectively capturing low-level spatial-temporal features from rs-fMRI signals for subsequent construction of multi-scale FBNs. On the other hand, HNFM provides a node fusion technique for hierarchically merging the fine-grained brain nodes into coarse-grained nodes to construct and analyze multi-scale FBNs. This node fusion process allows HNFM to gradually refine and integrate the ST-GCFE-extracted multi-scale features and learn the hierarchical representation of FBNs for understanding and analyzing the brain's structural and functional organization. Our previous methodological research (Zhang et al., 2024) details these two modules and their application in the proposed deep network.
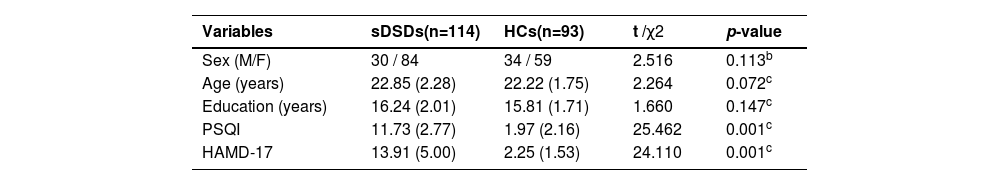
ResultsDemographic and clinical characteristicsTable 1 shows a summary of the demographic and disease-related clinical characteristics. Continuous variables were examined using an independent two-sample t-test between sDSd and HC. The categorical variable (sex) using percentages and analyzed with the chi-square test, with statistical significance at p<0.05.
Demographic and clinical characteristics of participantsa.
This study involved 207 college students in total, including 114 unmedicated first-episode subclinical depression students with comorbid sleep disorders (males=30; females=84; age range=18–25 years; mean age=22.85 (±2.28) years; HAMD-17 ≥7 points and <17 points; PSQI>5 points) and 93 HCs (males=34; females=59 females; age range=18–25 years; mean age=22.22 (±1.75) years; HAMD-17<7 points; PSQI<5 points).
The two groups showed no significant differences in sex, age, or education (p>0.05). However, they showed significant differences in PSQI and HAMD-17 scores, with average scores of 11.73 (±2.77), 13.91 (±5.00) in sDSDs group, and 1.97 (±2.16), 2.25 (±1.53) in HC groups, respectively (p<0.001).
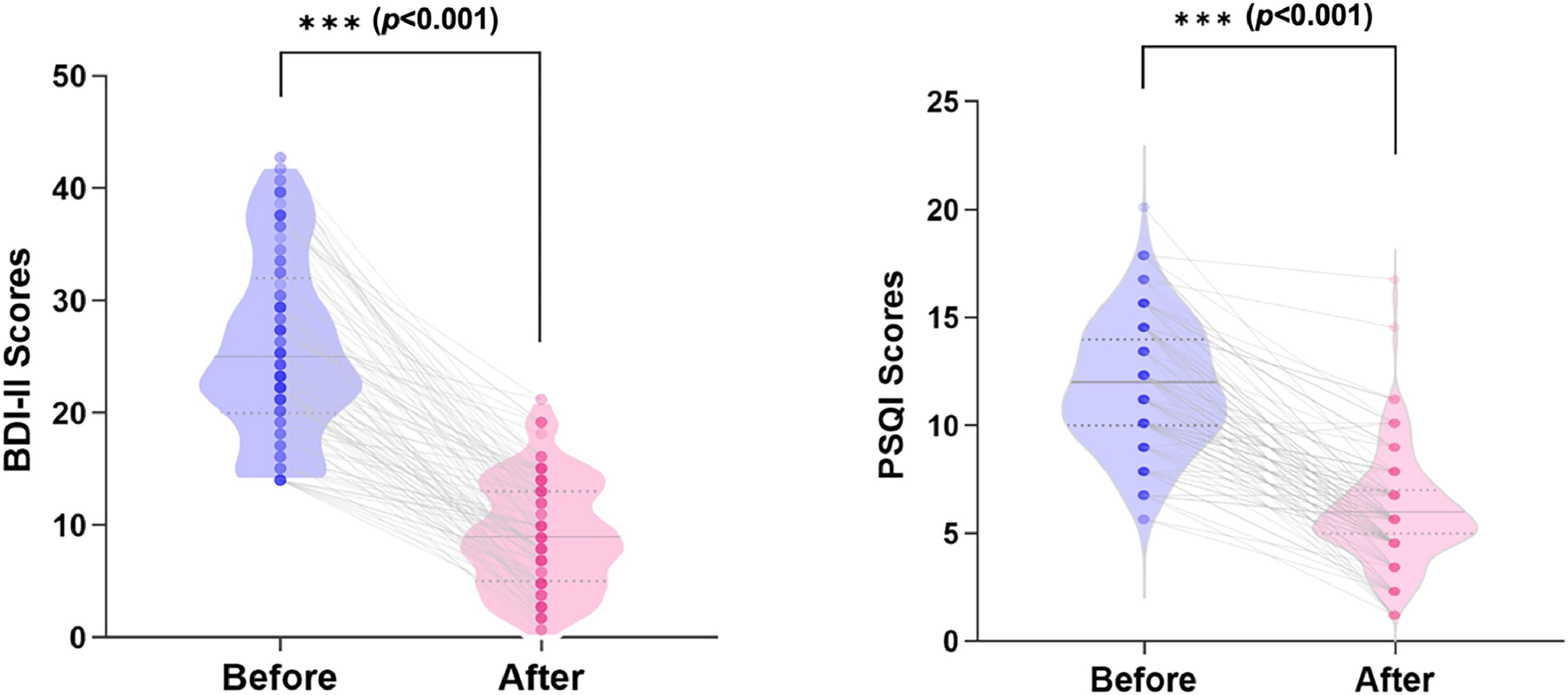
Clinical measurements after non-pharmacological treatment in the sDSDsIn baseline, the mean scores of the primary (BDI-II) and secondary (PSQI) measurements were 25.78 (±7.75) and 11.73 (±2.77) (p<0.001), respectively (Table 2). After 6 weeks of non-pharmacological treatment, 79 (74.5%) students were in clinical remission in depression; 91 (85.8%) students achieved MCID in sleep disorders, and the means of the core clinical measurements were markedly reduced to 8.99 (± 5.03) and 5.95 (± 2.39) for BDI-II and PSQI, respectively (p<0.001) (Fig. 3).
Clinical measurements before and after nonpharmacological treatment in sDSDs.
| Variables | pre-treatment(n=96) | post-treatment(n=96) | Clinical Remission Raten (%) | t | p-value |
|---|---|---|---|---|---|
| BDI-II | 25.78 (7.75) | 8.99 (5.03) | 79 (74.5%) | 22.320 | <0.001a |
| PSQI | 11.73 (2.77) | 5.95 (2.39) | 91 (85.8%) | 20.389 | <0.001a |
| CTQ | 36.91 (9.70) | - | - | - | - |
| HAMA | 19.41 (6.58) | 13.84 (4.15) | - | - | - |
Note: BDI-II = The Beck Depression Inventory of Second Edition; PSQI = The Pittsburgh Sleep Quality Index; CTQ = The Childhood Trauma Questionnaire; HAMA = Hamilton Anxiety Rating Scale.
Improve clinical outcomes following 6 weeks of non-pharmacological treatment for subclinical depression college students. The violin plots show the sample distribution with mean and median of the BDI-II Scores and PSQI Scores from the baseline to post-treatment; symbols refer to the clinical scores of each treated individual, lines illustrate clinical measurements reduction according to nonmedication treatments, followed by the paired t-tests (p<0.001).
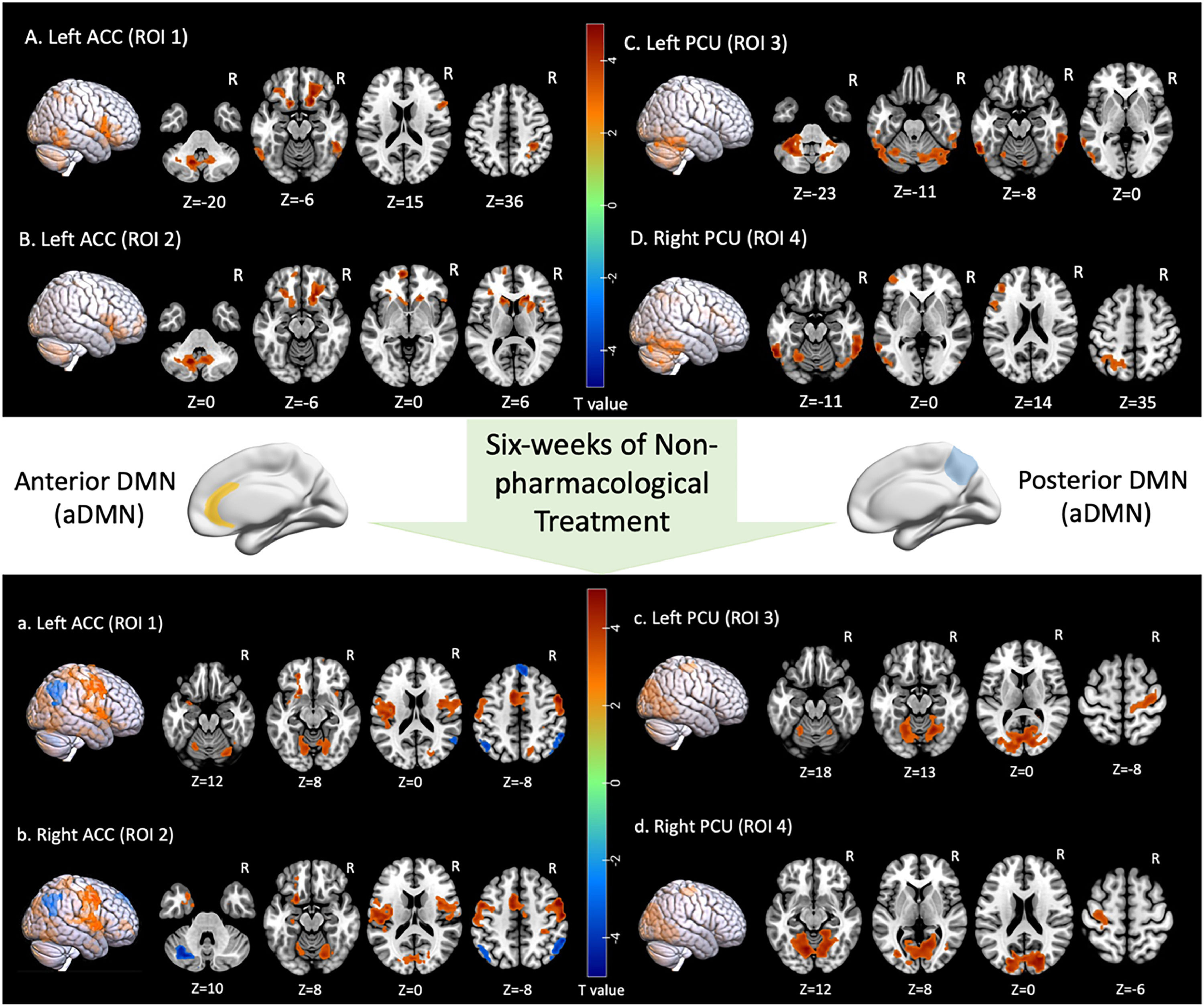
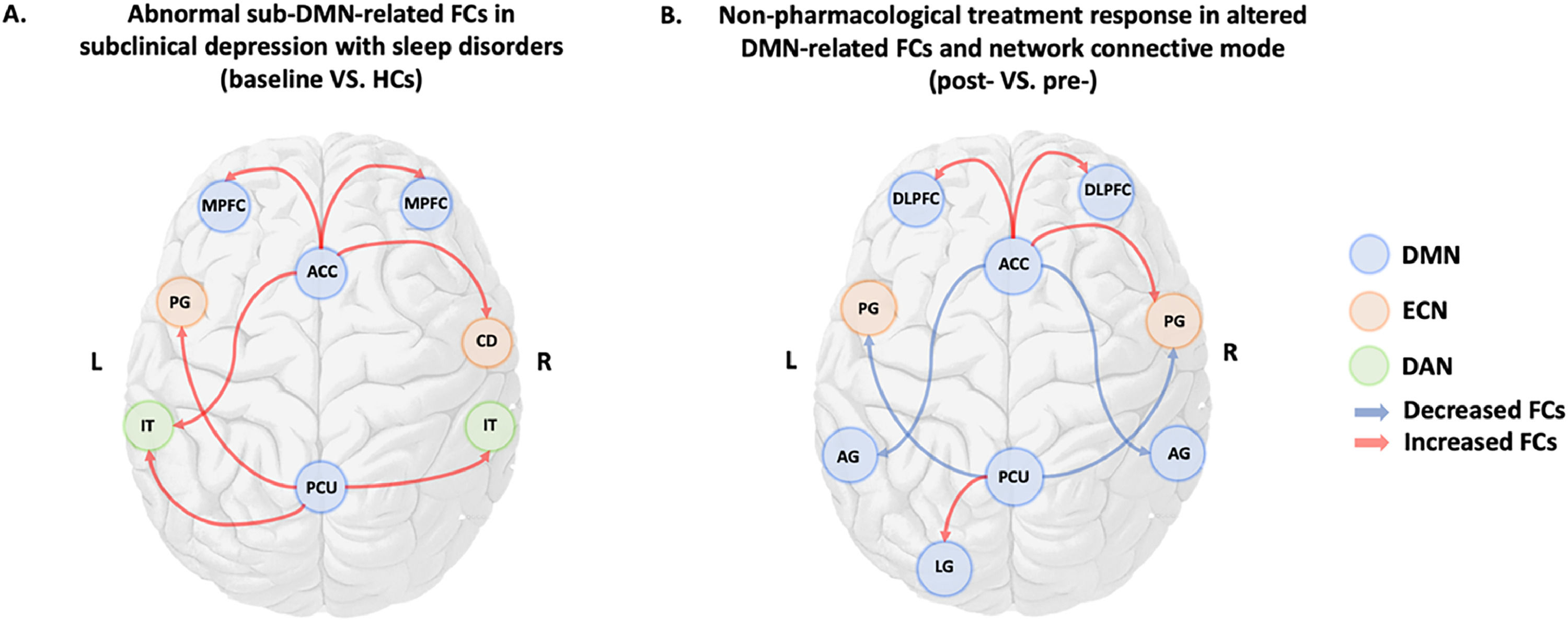
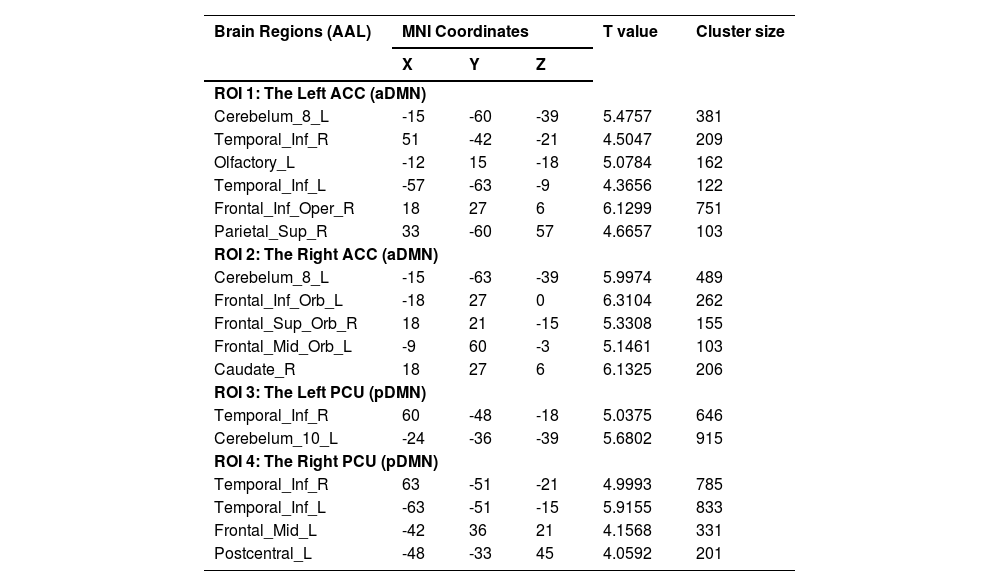
Two-sample t-tests were performed between two groups based on cluster-forming (p<0.001 at the voxel level) and cluster-extent (pFWE-corrected<0.05) thresholds. This study found statistically significant differences in whole-brain FCs between the sDSDs and the HCs in the four core sub-DMN regions. At baseline, the sDSDs had higher FCs related to sub-DMN than HCs (Table 3). These alterations were detected in the bilateral medial Prefrontal Cortex (mPFC), right caudate, and left inferior temporal gyrus, which correlated with the ACC (a part of the aDMN) (Fig. 4-AB). On the other hand, brain activity in the left precentral and inferior temporal regions correlated with brain activity in the PCU (a part of the pDMN) (Fig. 4-CD). Furthermore, higher FCs between sub-DMN with other brain networks show a connective mode mainly in the ECN, DMN, and DAN at baseline (Fig. 5A).
The abnormal rsFCs related to sub-DMN in baseline (sDSs>HCs).
| Brain Regions (AAL) | MNI Coordinates | T value | Cluster size | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| ROI 1: The Left ACC (aDMN) | |||||
| Cerebelum_8_L | -15 | -60 | -39 | 5.4757 | 381 |
| Temporal_Inf_R | 51 | -42 | -21 | 4.5047 | 209 |
| Olfactory_L | -12 | 15 | -18 | 5.0784 | 162 |
| Temporal_Inf_L | -57 | -63 | -9 | 4.3656 | 122 |
| Frontal_Inf_Oper_R | 18 | 27 | 6 | 6.1299 | 751 |
| Parietal_Sup_R | 33 | -60 | 57 | 4.6657 | 103 |
| ROI 2: The Right ACC (aDMN) | |||||
| Cerebelum_8_L | -15 | -63 | -39 | 5.9974 | 489 |
| Frontal_Inf_Orb_L | -18 | 27 | 0 | 6.3104 | 262 |
| Frontal_Sup_Orb_R | 18 | 21 | -15 | 5.3308 | 155 |
| Frontal_Mid_Orb_L | -9 | 60 | -3 | 5.1461 | 103 |
| Caudate_R | 18 | 27 | 6 | 6.1325 | 206 |
| ROI 3: The Left PCU (pDMN) | |||||
| Temporal_Inf_R | 60 | -48 | -18 | 5.0375 | 646 |
| Cerebelum_10_L | -24 | -36 | -39 | 5.6802 | 915 |
| ROI 4: The Right PCU (pDMN) | |||||
| Temporal_Inf_R | 63 | -51 | -21 | 4.9993 | 785 |
| Temporal_Inf_L | -63 | -51 | -15 | 5.9155 | 833 |
| Frontal_Mid_L | -42 | 36 | 21 | 4.1568 | 331 |
| Postcentral_L | -48 | -33 | 45 | 4.0592 | 201 |
Note: Two sample t-tests were performed for abnormal FCs between sDSDs and HCs groups; MNI Coordinates refer to the Montreal Neurological Institute stereotaxic space; Cluster-forming threshold (pvoxel-level<0.001), and cluster-extent threshold (pFWE-corrected<0.05). L=left, R=right, ACC=anterior cingulate cortex, PCU=precuneus, a/pDMN=anterior/posterior default mode network.
Alteration in sub-DMN related FCs pre- and post-non-pharmacological treatment for sDSDs. (A, B) brain regions showing abnormal FCs related to aDMN/ACC; (C, D) brain regions showing abnormal FCs related to pDMN/PCU; (a,b) brain regions showing treatment response in aDMN/ACC-related FCs; (c,d) brain regions showing treatment response in pDMN/PCU-related FCs.
Sub-DMN related brain network connective mode shift after non-pharmacological treatment in sDSDs.
(A). Baseline brain networks connective mode in sDSDs compared to HCs with increased FCs within DMN and other networks.
(B). Shifted connective mode after 6 weeks of nonpharmacological treatment with altered FCs within DMN and ECN.
Note: Blue circle: DMN=default mode network; Orange circle: ECN=executive control network; Green circle: DAN=dorsal attention network; ACC=anterior cingulate cortex; PCU=precuneus; DLPFC=dorsolateral prefrontal cortex; MPFC=medial prefrontal cortex; CD=caudate; IT=inferior temporal gyrus; TP=temporal cortex; PG=precentral gyrus; AG=angular gyrus; LG=lingual gyrus.
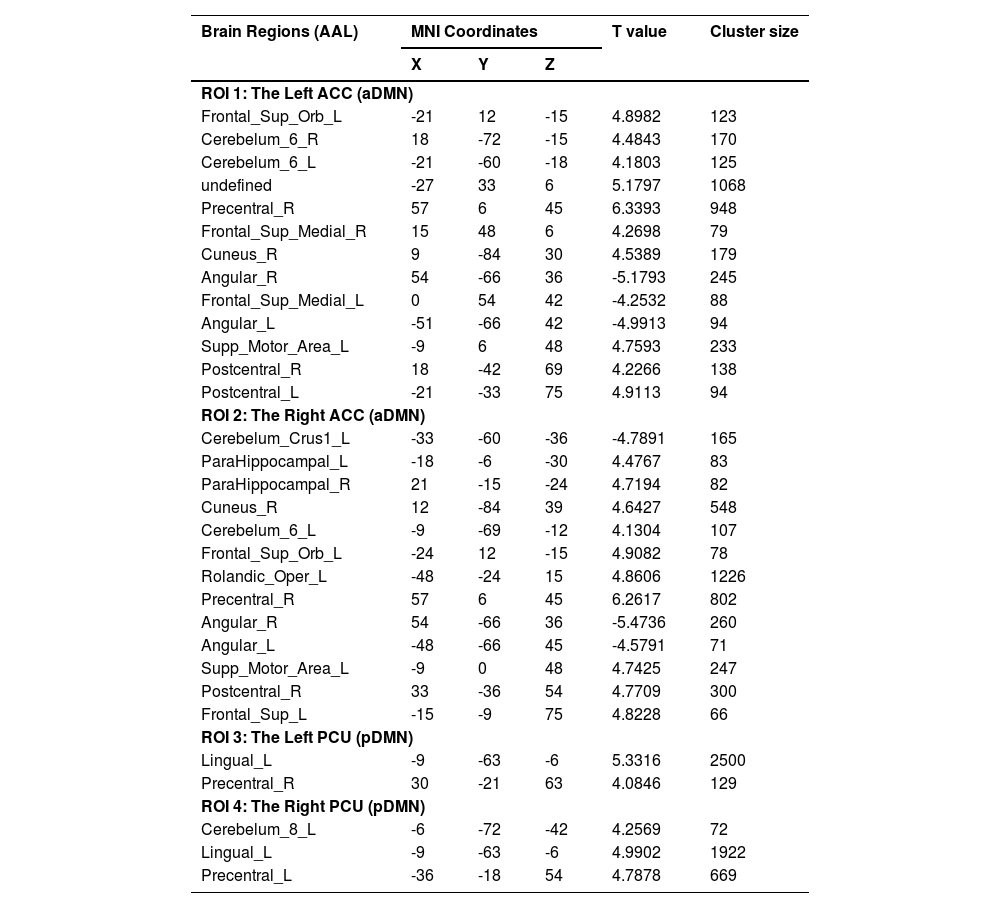
Paired t-tests were performed to estimate FC alterations before and after non-pharmacological treatment in sDSDs group (Table 4). After treatment, FCs significantly changed in the bilateral Dorsolateral Prefrontal Cortex (dlPFC), angular gyrus, and right precentral gyrus, which correlated with the ACC (a part of the aDMN) (Fig. 4-ab). Meanwhile, FCs significantly altered in the left lingual and bilateral precentral gyrus, which correlated with the PCU (a part of the pDMN) (Fig. 4-cd).
Altered rsFCs related to sub-DMN after 6 weeks of non-pharmacalogical treatments (post>pre).
| Brain Regions (AAL) | MNI Coordinates | T value | Cluster size | ||
|---|---|---|---|---|---|
| X | Y | Z | |||
| ROI 1: The Left ACC (aDMN) | |||||
| Frontal_Sup_Orb_L | -21 | 12 | -15 | 4.8982 | 123 |
| Cerebelum_6_R | 18 | -72 | -15 | 4.4843 | 170 |
| Cerebelum_6_L | -21 | -60 | -18 | 4.1803 | 125 |
| undefined | -27 | 33 | 6 | 5.1797 | 1068 |
| Precentral_R | 57 | 6 | 45 | 6.3393 | 948 |
| Frontal_Sup_Medial_R | 15 | 48 | 6 | 4.2698 | 79 |
| Cuneus_R | 9 | -84 | 30 | 4.5389 | 179 |
| Angular_R | 54 | -66 | 36 | -5.1793 | 245 |
| Frontal_Sup_Medial_L | 0 | 54 | 42 | -4.2532 | 88 |
| Angular_L | -51 | -66 | 42 | -4.9913 | 94 |
| Supp_Motor_Area_L | -9 | 6 | 48 | 4.7593 | 233 |
| Postcentral_R | 18 | -42 | 69 | 4.2266 | 138 |
| Postcentral_L | -21 | -33 | 75 | 4.9113 | 94 |
| ROI 2: The Right ACC (aDMN) | |||||
| Cerebelum_Crus1_L | -33 | -60 | -36 | -4.7891 | 165 |
| ParaHippocampal_L | -18 | -6 | -30 | 4.4767 | 83 |
| ParaHippocampal_R | 21 | -15 | -24 | 4.7194 | 82 |
| Cuneus_R | 12 | -84 | 39 | 4.6427 | 548 |
| Cerebelum_6_L | -9 | -69 | -12 | 4.1304 | 107 |
| Frontal_Sup_Orb_L | -24 | 12 | -15 | 4.9082 | 78 |
| Rolandic_Oper_L | -48 | -24 | 15 | 4.8606 | 1226 |
| Precentral_R | 57 | 6 | 45 | 6.2617 | 802 |
| Angular_R | 54 | -66 | 36 | -5.4736 | 260 |
| Angular_L | -48 | -66 | 45 | -4.5791 | 71 |
| Supp_Motor_Area_L | -9 | 0 | 48 | 4.7425 | 247 |
| Postcentral_R | 33 | -36 | 54 | 4.7709 | 300 |
| Frontal_Sup_L | -15 | -9 | 75 | 4.8228 | 66 |
| ROI 3: The Left PCU (pDMN) | |||||
| Lingual_L | -9 | -63 | -6 | 5.3316 | 2500 |
| Precentral_R | 30 | -21 | 63 | 4.0846 | 129 |
| ROI 4: The Right PCU (pDMN) | |||||
| Cerebelum_8_L | -6 | -72 | -42 | 4.2569 | 72 |
| Lingual_L | -9 | -63 | -6 | 4.9902 | 1922 |
| Precentral_L | -36 | -18 | 54 | 4.7878 | 669 |
Note: Paired t-tests were performed for altered FCs in sDSd group after 6 weeks of non-pharmacological treatments; MNI Coordinates refer to the Montreal Neurological Institute stereotaxic space; Cluster-forming threshold (pvoxel-level<0.001), and cluster-extent threshold (pFWE-corrected<0.05); L=left, R=right, ACC=anterior cingulate cortex, PCU=precuneus, a/pDMN=anterior/posterior default mode network.
Two-sample t-tests were performed between two groups based on cluster-forming (p<0.001 at the voxel level) and cluster-extent (pFWE-corrected<0.05) thresholds. The whole brain voxel analyses and seed-based functional connectivity analyses were all conducted within HC group and with post-treatment group (Supplementary Tables 1–2, Supplementary Figs. 1–2), while none of them hold up to correction for multiple comparisons (pFWE-corrected<0.05).
The sub-DMN-related brain network connective mode in sDSDs after treatmentThe DMN and ECN responded to such intervention (Fig. 5B), with higher sub-DMN-related FCs in baseline decreased after non-pharmacological treatment (Table 4, Fig. 5B). Moreover, therapeutic neural responses were characterized by alterations in the collaborative brain network mode, primarily in the DMN and ECN.
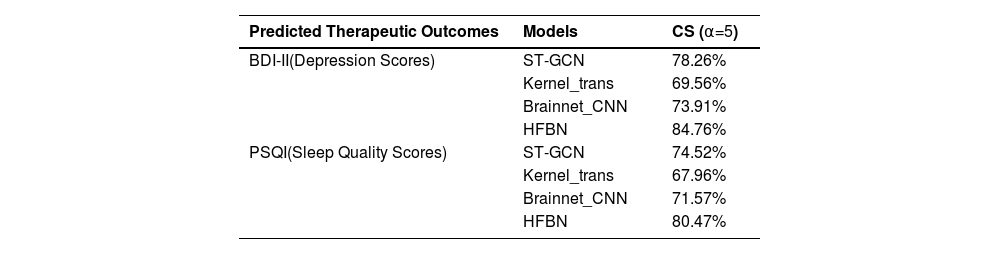
Performance of the HFBN model in early diagnosis and therapeutic outcomes predictionTable 5 shows the model prediction performance. A Cumulative Score (CS) was set to evaluate outcome prediction accuracies within a threshold of α=5. The accuracies were determined as follows: CS(α)=Ne≤αN×100%; where Ne≤α is the number of samples in which the absolute prediction error e is not > the α threshold. The model prediction was considered accurate if the error between the predicted and actual values was ≤ 5. To enhance the reliability of the results, all data were processed using five-fold cross-validation. The HFBN model achieved the highest CS scores of 80.47 and 84.67 compared to traditional deep learning models for predicting sleep quality and depression, respectively (Table 5).
Predictive performance of HFBN versus traditional deep learning models.
| Predicted Therapeutic Outcomes | Models | CS (α=5) |
|---|---|---|
| BDI-II(Depression Scores) | ST-GCN | 78.26% |
| Kernel_trans | 69.56% | |
| Brainnet_CNN | 73.91% | |
| HFBN | 84.76% | |
| PSQI(Sleep Quality Scores) | ST-GCN | 74.52% |
| Kernel_trans | 67.96% | |
| Brainnet_CNN | 71.57% | |
| HFBN | 80.47% |
Note: CS=Cumulative Scores; ST-GCN=Spatial Temporal Graph Convolutional Networks; Kernel_trans=Kernel Transformer Networks; CNN=Convolution Neural Networks; HFBN=Hierarchical Functional Brain Networks.
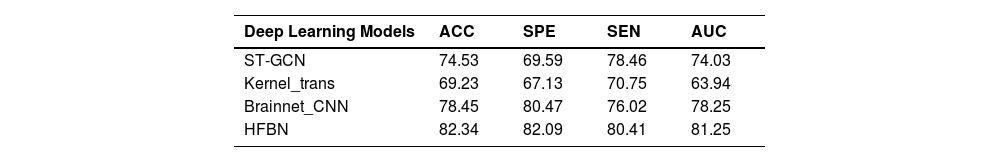
Table 6 shows the model diagnoses performance of early depression. Compared to other traditional deep learning models, the HFBN model exhibited superior classification results, achieving an accuracy of 82.34%, 3.89% higher than that of the second-best model, BrainNetCNN (Table 6).
Diagnostic performance of HFBN versus traditional deep learning models (in %).
| Deep Learning Models | ACC | SPE | SEN | AUC |
|---|---|---|---|---|
| ST-GCN | 74.53 | 69.59 | 78.46 | 74.03 |
| Kernel_trans | 69.23 | 67.13 | 70.75 | 63.94 |
| Brainnet_CNN | 78.45 | 80.47 | 76.02 | 78.25 |
| HFBN | 82.34 | 82.09 | 80.41 | 81.25 |
Note: ACC=accuracy (in %); SPE=specificity (in %); SEN=sensitivity (in %); AUC=area under ROC curve; ROC=Receiver Operating Characteristic
This study detected two scales of neuroimaging signatures: the altered sub-DMN-related FCs and a shifted brain network connective mode, after non-pharmacological treatment for subclinical depression with sleep disorders. Furthermore, the HFBN intelligence model performed outstandingly in diagnosing subclinical depression and pre-evaluating the therapeutic outcomes of non-pharmacological therapy.
The rsfMRI indicators in subclinical depression and comorbid sleep disordersUntreated, first-episode sDSDs show relatively "pure" rsfMRI abnormality in the baseline. In FC scale, the higher rsFCs related to the ACC were detected in the bilateral mPFC, which regulates decision-making and emotional responses (Zhang et al., 2023a), the right caudate, a part of the reward system associated with motivation and emotional processing (Kaliuzhna et al., 2023), and the left inferior temporal gyrus, associated with visual processing and memory, all showing altered depression activities (Wu et al., 2023). Additionally, lower rsFCs were detected in the left precentral and inferior temporal regions, which are associated with motor control and memory processing (Parr et al., 2023), and correlated with brain activity in the PCU (a part of the pDMN).
In the brain network scale, consistent with previous research, this study found that subclinical depression often manifests as alterations in brain regions related to emotional regulation and cognitive control. Additionally, the higher FCs between the aDMN and pDMN correlated with the ECN, which regulates cognitive control and decision-making, and the DAN, which governs attentional control (Huang et al., 2024; Korgaonkar et al., 2023). Specifically, our findings indicated that the bilateral mPFC, a part of the ECN, exhibited increased connectivity, attributable to the heightened emotional regulation efforts and decision-making processes in individuals with subclinical depression. According to research, subclinical depression patients often exhibit FC upregulation within the ECN (Dunlop et al., 2023; Zhu et al., 2023), indicating heightened activity in response to emotional and executive challenges. It is also noteworthy that owing to their robust and efficient neural connectivity patterns, young individuals with mild depression often exhibit higher connectivity between the DMN and ECN without necessarily having a cognitive impairment.
This study also discovered that higher FCs between sub-DMN and DAN in subclinical depression are detailed in the precentral gyrus and the right caudate, parts of the DAN, which are crucial for maintaining attention and cognitive control. Furthermore, increased connectivity between the DAN and DMN could indicate difficulties in disengaging from self-referential thought processes (Yang et al., 2023). According to research, the relationship between the DAN, DMN, and ECN may be disrupted in subclinical depression (Zhu et al., 2023), potentially resulting in attentional biases and sustained attention difficulties. Such functional impairments could manifest in subclinical depression as increased activity in the DAN during tasks requiring sustained attention or cognitive flexibility (Chang et al., 2023). Notably, this increased connectivity may be a compensatory mechanism for counteracting the attentional deficits commonly evident in subclinical depression.
Neuroimaging signatures of non-pharmacological treatment in subclinical depressionSuccessful anti-depression treatment normalizes connectivity within the DMN, Salience Network (SN), and ECN (Hidalgo-Lopez et al., 2023; Prompiengchai & Dunlop, 2024). Consistent with previous research, we identified a shifted connective mode between the ECN and DMN as the non-pharmacological treatment response in subthreshold depression. Evidence-based depression interventions, such as pharmacotherapy (Henssler et al., 2022; Zisook et al., 2011), Transcranial Magnetic Stimulation (TMS) (Cardenas et al., 2022; Hidalgo-Lopez et al., 2023; Liston et al., 2014), and transcranial Direct Current Stimulation (tDCS) (Chan et al., 2021; Filmer et al., 2019), have widely reported alterations in FCs within key brain networks and decreased hyperconnectivity in the DMN and ECN, potentially reflecting symptom remission.
This study showed that the treatment response correlated with the altered rsFCs related to ACC (part of aDMN), the increased rsFC observed in the bilateral dlPFC (part of DMN) and right precentral gyrus (part of ECN). Pre-treatment hyperactivity in the subgenual ACC, the core regions of emotional processing and effective decision-making, has been linked to better response to antidepressant medications (Godlewska et al., 2018; Yu et al., 2020). Conversely, hypoactivity in the dlPFC, which performs executive functions, correlates with poor outcomes (Cardenas et al., 2022; Corlier et al., 2023). Therefore, an ideal treatment should enhance FCs in ACC and dlPFC, as observed in our results. Our findings in pDMN-related treatment response show decreased connectivity between DMN and ECN in the bilateral precentral gyrus, which is involved in visual processing and motor control, and increased connectivity within DMN between left lingual and pDMN. The PCU (part of pDMN) is disrupted in depression and insomnia and provides a neural basis for the comorbidity of depression and insomnia. In such patients, fMRI studies have revealed significant disruptions in connectivity between the PCU and other DMN regions involved in arousal, attention, and emotional regulation (Ma & Zhang, 2022; Rubart et al., 2022). Effective treatment for comorbid depression and insomnia, such as combined cognitive behavioral therapy (CBT) (Fang et al., 2024; Haller et al., 2024) and pharmacotherapy (Dunlop et al., 2023; Gerlach et al., 2022), can normalize PCU activity and connectivity while also improving connectivity with mood-regulating regions affected by depression. This dual normalization can help improve mood and sleep quality.
Depression and sleep disorders overlap in neural circuits and treatment effectDepression and sleep disorders often share overlapping neural circuits exhibit a bi-directional relationship in brain regions and functional networks in terms of activity and connectivity (Alvaro et al., 2013; Fang et al., 2019; Sun et al., 2022). Core brain regions like the amygdala and ACC have been shown to contribute to hyperarousal insomnia in both conditions. The analyses revealed that youngsters with sDSDs had heightened activity in ACC-related FC, which facilitates arousal and attention across wakefulness and sleep. Moreover, altered connectivity often manifests as reduced functional interactions within the DMN during mind-wandering in wakefulness and specific sleep stages. This notion may explain the alterations in sub-DMN-related FC in those youngsters.
Depression and sleep disorders may be treated using the same drugs and interventions. Treatments like cognitive behavioural therapy for insomnia can reduce hyperarousal and improve connectivity in depression-relevant brain regions. Our post-treatment findings in decreased sub-DMN-related-rsFC between ECN and within-DMN correlated with this effective treatment outcome. Several clinical trials have reported that cognitive behavioural therapy (Takano et al., 2023), mindfulness meditation (Li et al., 2023), exercise (Huang et al., 2023), and light therapy (Chen et al., 2024; Verma et al., 2023) can improve sleep quality and reduce depressive symptoms. Similarly, fMRI studies have shown that depression treatments, such as antidepressant medications (Azari et al., 2024) and psychotherapy (Davis et al., 2023), can improve both mood and sleep quality. Although these interventions primarily target depressive symptoms, they can also normalize sleep architecture and reduce insomnia, which is consistent with our findings. In the future, researchers should explore the neural mechanisms underlying the intersection of subclinical depression and sleep disorders to provide valuable insights for developing potential therapeutic targets and personalized treatment approaches.
Deep-learning predictive model of nondrug treatment for subclinical depressionThis HFBN model allows for the early diagnosis and precise quantification of non-pharmacological treatment effectiveness, enhancing confidence before selecting integrative intervention, thus providing the scientific basis for treating subclinical depression. Compared to traditional brain network models, the HFBN model highlights a sensitive and accurate ability to identify subtle FC changes and brain network connective mode alterations in the early stages of depression, enabling a more precise early diagnosis of the disease and predicting the effectiveness of non-pharmacological treatments. HFBN is designed to construct and analyze hierarchical FBNs within an attention-based deep network that mimics the brain's natural hierarchical architecture from neurons to neural networks. This hierarchical ideation enables the joint analysis of complementary information from multi-scale FBNs to facilitate prediction accuracy. However, this study only considers hierarchical structure and sparsity. To improve network design, future work should investigate additional brain properties, such as modularity and small-worldness, to enhance the effectiveness of deep models for FBN construction and analysis.
Limitations and future directionsThis study has several limitations that need to be acknowledged. Firstly, this study is limited by its focus on a sample of Chinese university students, which may affect the generalizability and external validity of the findings. University students possess unique characteristics in age, social experiences, and psychological traits, making it challenging to directly apply these results to individuals from other age groups, occupational backgrounds, or cultural contexts. Additionally, cultural differences may influence the expression, coping mechanisms, and social support systems related to depression, which can vary across countries and cultural backgrounds. As a result, relying solely on a sample of Chinese university students may not adequately represent other populations. This limitation suggests that caution should be exercised when interpreting these findings, particularly when generalizing to different groups or settings. Future research could address this limitation by expanding the sample to include a broader range of populations, incorporating individuals of diverse ages, occupational backgrounds, and cultural contexts. Future multicentre or cross-cultural comparative studies could provide a more comprehensive understanding of the mechanisms and intervention effects of depression, enhancing the applicability and robustness of the conclusions.
Secondly, to improve the predictive performance of the HFBN model for multiple non-pharmacological treatments, we chose to use a combined treatment approach, which underpowered the detection of the effects of each type of treatment. However, this study primarily aims to investigate the potential synergistic effects of non-pharmacological therapy, given that these two interventions are often combined in clinical practice (Gautam et al., 2017; Park et al., 2013). Simulating the complexity of real-world therapeutic scenarios could improve the model's generalizability and accuracy in predicting the effects of various non-pharmacological therapies. Focusing on single-treatment studies would limit model application scenarios and restrict its potential for clinical adoption. Disentangling the neural mechanisms underlying each treatment is an important challenge that may require further investigation through targeted studies focusing on each independently in the future.
Thirdly, the six-week intervention period is not considered a long treatment duration. However, it remains widely accepted as a key treatment time window for observing the initial effects of antidepressant interventions. According to guidelines from the American Psychological Association (APA) and the U.S. Food and Drug Administration (FDA), treatment modifications—whether through medication adjustments or the addition of non-pharmacological treatments—are typically recommended after 4 to 8 weeks (with six weeks serving as the median timeframe) (Al-Harbi, 2012; Rosenblat & McIntyre, 2017). In the case of this study, the six-week duration was chosen as it aligns with clinical practices for evaluating the onset of therapeutic effects, particularly in patients with subthreshold depression, whose symptoms are generally milder. Notably, the observed brain network alterations in 6 weeks provide relatively “pure” brain network signatures of treatment effect for constructing predictive models with real neuroimaging signatures. Extending the duration could introduce confounding factors that may dilute the distinct neural responses to treatment, thus complicating predictive model development, and diverging from the clinically established window for evaluating effectiveness of antidepressant interventions. Additionally, logistical challenges during the COVID-19 pandemic, including participant availability and adherence, posed constraints on extending the follow-up period. Future multimodal imaging and larger RCTs with longer follow-ups should be conducted to validate these findings and test each treatment effect, including EA, psychotherapy, and combined therapy to provide additional neuro-evidence for nondrug treatment effects of depression.
ConclusionNeuroimaging signatures associated with the sub-DMN have elucidated the antidepressant mechanisms of non-pharmacological treatments for subclinical depression, observed on two core levels: altered FCs within the sub-DMN and a modified brain networks connective mode. Compared to HCs, college students with subclinical depression displayed higher FCs within sub-DMN, ECN, and DAN. Moreover, the HFBN deep learning model demonstrated superior performance in early depression diagnosis and in predicting therapeutic outcomes of non-pharmacological interventions.
Registration of clinical trialsThis trial was registered on the website of the Chinese Clinical Trial Registry (No: ChiCTR1900028530).
The Ethics Committee of the First Affiliated Hospital of Guangzhou University of Chinese Medicine approved this rs-fMRI study (NO. ZYYECK [2019] 068).
Funding sourcesThis work was supported by grants from the National Natural Science Foundation of China (82204927, 81920108019, and 82330058, T2341014, 62471501, 62101611).
Science and Technology Plan Project of Guangzhou (202201011407 and 2023A04J1860).
Shenzhen Medical Research Fund (B2402030).
Guangdong Basic and Applied Basic Research Foundation (2023A1515012278).
Shenzhen Science and Technology Program (JCYJ20220530145411027, JCYJ20240813151026034).
The Open Research Fund of the State Key Laboratory of Brain-Machine Intelligence, Zhejiang University (BMI2400019).
China Scholarship Council (202108440345).
Innovation and Strong School Project of Education Department of Guangdong Province.
The Excellent Doctoral Dissertation Incubation Grant of First Clinical School of Guangzhou University of Chinese Medicine (YB201903).
We would like to express our sincere gratitude to Xiumin Jiang for their invaluable assistance in recruiting university students and administering electroacupuncture treatment for six weeks. The physicians at the school campus counseling room for their contribution in conducting counseling therapy for the participants. All the students who participated in the study for diligently completing the clinical questionnaires and undergoing two MRI scans (each lasting 40 min) during the challenging times of the COVID-19 pandemic.