Background/objective: Sluggish Cognitive Tempo (SCT) is an attentional disorder characterized by the symptoms of slowness in behavior or thinking, a lack of en.ergy, difficulty initiating and sustaining effort, daydreaming, and drowsiness. The aim of the present study was to investigate the distinctive attentional characteristics of SCT as compared to Attention-Deficit/Hyperactivity Disorder (ADHD). Method: A total of 110 adults were recruited and divided into four groups: SCT+ADHD, SCT, ADHD, and healthy controls. The Revised version of Attention Networks Test was used to investigate each group’s attentional profile. Results: The results revealed that the two SCT groups (SCT+ADHD and SCT) showed a significantly weaker orienting network due to the problems of engaging and disengaging attention than the other two groups. Additionally, the two ADHD groups (SCT+ADHD and ADHD) showed a significantly weaker executive control network than the other two groups. Conclusions: The findings demonstrate an attentional distinction between the SCT and the ADHD groups with a greater dysfunction in the orienting network in the SCT group as compared to the ADHD group. Furthermore, a greater executive control dysfunction was observed in the ADHD group as compared to the SCT group.
Antecedentes/Objetivo: El Tiempo Cognitivo Lento (TCL) es un trastorno atencional caracterizado por síntomas de lentitud en el comportamiento o pensamiento, falta de energía, dificultad para iniciar y mantener el esfuerzo, soñar despierto y somnolencia. El propósito de este estudio es investigar las características únicas de la atención de TCL en comparación con el Trastorno por Déficit de Atención/Hiperactividad (TDAH). Método: Se reclutaron 110 participantes y se dividieron en cuatro grupos: TCL+TDAH, TCL, TDAH y controles sanos. Se empleó la versión revisada del Attention Networks Test para investigar el perfil de atención de cada grupo. Resultados: Los dos grupos de TCL (TCL+TDAH y TCL) mostraron una red de orientación significativamente más débil debido a los problemas de atraer y desconectar la atención que los otros dos grupos. Los grupos de TDAH (TCL+TDAH y TDAH) mostraron una red de control ejecutivo significativamente más débil que los otros dos grupos. Conclusiones: Se demuestra una distinción atencional entre los grupos TCL y TDAH con mayor disfunción en la red de orientación en TCL en comparación con TDAH. Además, se observó una mayor disfunción del control ejecutivo en el grupo TDAH en comparación con el grupo TCL.
Sluggish Cognitive Tempo (SCT) is a kind of attentional disorder characterized by symptoms of slowness in behavior or in thinking, difficulty initiating and sustaining effort, hypoactivity, daydreaming, forgetfulness, and confusion in thinking (Barkley, 2016; Becker, 2017). A growing body of evidence demonstrates that the SCT symptom dimension is empirically distinct from other psychopathology symptoms, such Attention Deficit/Hyperactivity Disorder (ADHD; Becker, Luebbe, & Joyce, 2015; Becker & Willcutt, 2018; Burns & Becker, 2019; Jarrett et al., 2017). Early studies yield three types of ADHD with two symptom dimensions (i.e., inattention, hyperactivity-impulsivity): the Predominantly Inattentive Type (I), the Predominantly Hyperactive-Impulsive Type (HI), and the Combined Type (C), respectively (Barkley, 2012; Rodríguez et al., 2018). However, as this categorization is pointed out not to be very reliable and useful, the concept of SCT has been gradually emphasized (Barkley, 2012; Bauermeister et al., 2012).
Dysfunction in attention, one of the key symptoms of SCT, plays a critical role in cognitive deficits affecting the impairment in several domains of life activities of individuals with SCT. An extensive body of research on ADHD, which is most relevant to attentional problem, has focused on the executive function problem and widely ranging cognitive impairments such as response inhibition and response variability in general (Bauermeister et al., 2012; Krieger et al., 2019; Ruiz-Herrera et al., 2020; Willcutt et al., 2014).
However, these theories of executive function deficits could account only for ADHD, but not for SCT. It remains unclear which kind of the cognitive profile might be unique to individuals with SCT (Baytunca et al., 2018; Becker & Barkley, 2018). Given that attention is a complex and multi-componential construct, attentional difficulties in individuals with SCT should be investigated within an empirically based neurocognitive framework of attention.
One plausible and well-validated theory is the attentional network that conceptualizes attention as three basic components, each subtended by a different set of brain structures: alerting, orienting, and executive control (Petersen & Posner, 2012). In this theory, the alerting network responsible for the ability to maintain a state of wakefulness and phasically increase response readiness to external warning inputs. Furthermore, the orienting network is responsible for the ability to selectively attend to specific information from various sensory, to disengage attention from its current focus, and to shift it to another target or modality. Finally, the executive control network is responsible for the ability to inhibit automatic responses and to resolve conflicts among competing mental processes in order to control thoughts or behaviors. These three basic networks of attention are separate systems distributed within the brain; yet they interact and integrate to work together. In addition, the underlying processes may have a hierarchical structure, with alerting and orienting being located at the lower level of processing, such as encoding of sensory input in primary sensory cortices driving specific attention, while executive control is placed at a higher level of processing, such as coordinating thoughts and integrating information across modalities (Spagna et al., 2015).
Although the underlying mechanism in SCT remains unknown, it has been suggested that SCT is not primarily a disorder of executive functioning, but rather is associated with poor efficiency in orienting network (Barkley, 2016; Becker, Luebbe et al., 2015; Becker & Willcutt, 2018). In support of this hypothesis, a neuroimaging study found that an increase of SCT symptoms was related to less activity in the left superior parietal lobe, associated with reorientation or shifting of attention (Becker et al., 2016; Fassbender et al., 2015). Furthermore, the continuous model of activity suggests that hyperactivity and hypoactivity are better explained as a single continuum of the activity level, rather than separated symptoms, with a conjecture that inattention may have an inverted U-shape relationship with the activity level (Miller & Prevatt, 2017). Therefore, individuals with SCT who have hypoactivity symptoms will show a poor performance of attention in relation to the low activity level. Therefore, it would seem that SCT is associated with impairments in orienting network due to the slowness in engagement, disengagement, and shift.
In contrast, according to the results of neurobiological and behavioral research on individuals with ADHD, ADHD was found to be associated with impairments in executive control, but not in orienting (Fabio & Urso, 2014; Mullane et al., 2011; Nigg, 2006). In addition, ADHD was found to be associated with deficits in the alerting network, so that individuals with ADHD have difficulties in maintaining a vigilant state (tonic alerting) and in readiness to react (phasic alerting) (Kofler et al., 2013; Lundervold, Adolfsdottir et al., 2011; Oberlin et al., 2005). This might be due to a deficit in executive functions in ADHD (Barkley, 2006). Indeed, ADHD-only group had greater executive functioning deficits than SCT-only group (Burns & Becker, 2019). Taken these studies into account, it can be inferred that ADHD is related to the overall degradation of EF, and SCT is related to the problem in the orienting network. Also, those who have ADHD and SCT problems together would suffer the most dysfunction.
The aim of the present study was to investigate the distinctive attentional characteristics of SCT as compared to ADHD. To this end, four different samples were used: SCT only, ADHD only, both SCT and ADHD, and healthy control groups. We evaluated and compared attentional networks in each group using the revised attention network test. It was hypothesized that the SCT only group and the SCT+ADHD group would show impairment in orienting network more than the other two groups. The ADHD only group and the SCT+ADHD group were expected to show a weaker executive control and alerting than the other two groups. Furthermore, it was hypothesized that the SCT only group would show impairment in the ability of attention engagement and disengagement in the orienting network.
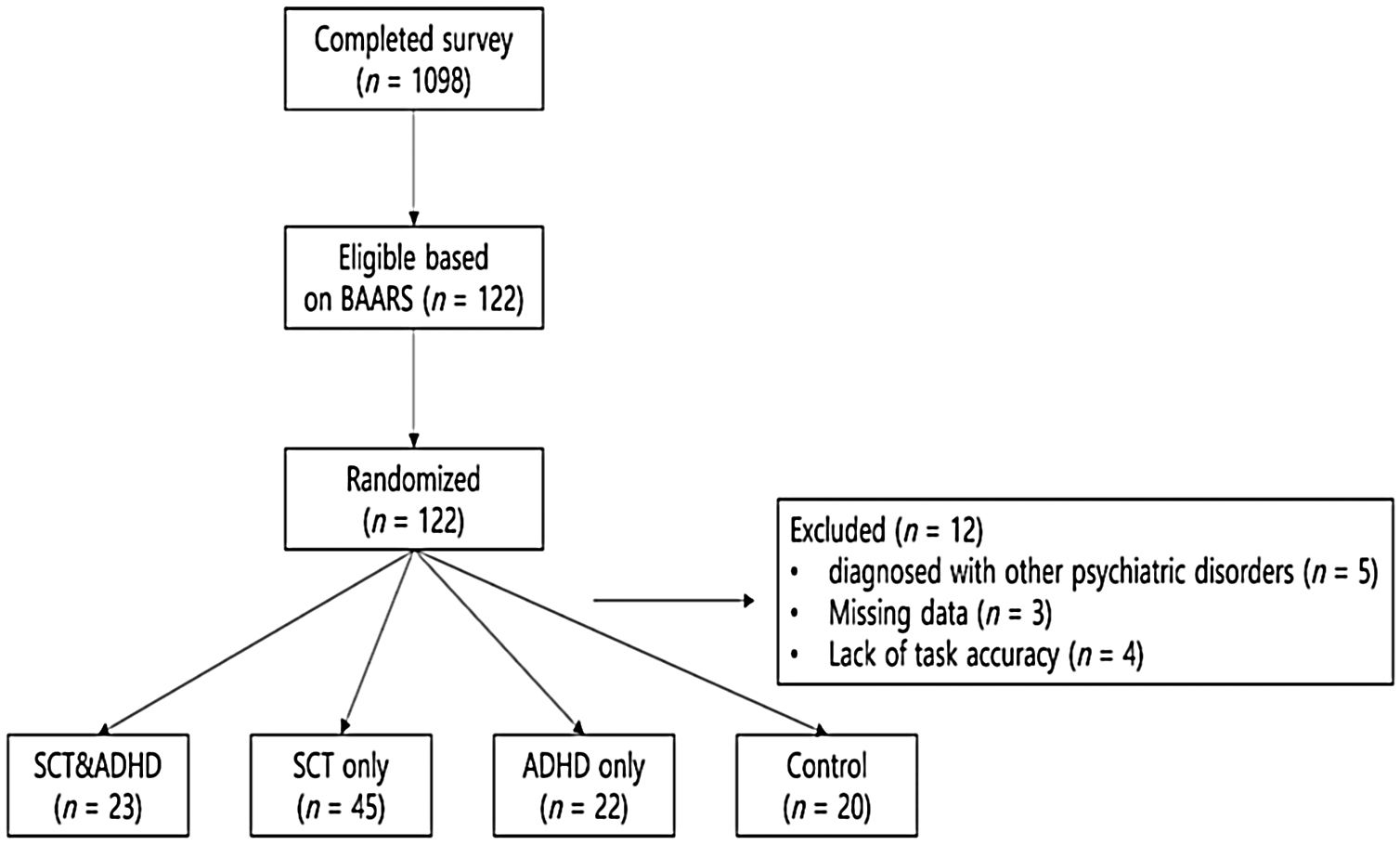
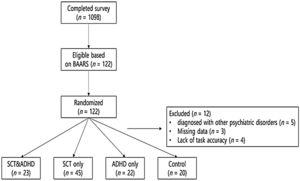
MethodParticipantsPrior to the experiment, candidate participants were recruited through advertisements in psychiatric clinics, online communities of individuals with attentional problems, and an internet bulletin board of several universities in Seoul, Korea (Figure 1). Participants were recruited from June to October 2018. As an initial screening for SCT and ADHD, a total of 1,098 adults completed the Barkley Adult ADHD Rating Scale IV (Barkley, 2011) and the Adult Concentration Inventory (Becker, Burns et al., 2015). Based on the previous recommendations concerning the inclusion criteria (Barkley, 2011, 2012; Becker & Barkley, 2018), a threshold corresponding to the 95 percentiles of five or more symptoms was used to identify SCT or ADHD. Healthy control participants were randomly selected among those who did not show ADHD symptoms (lower level of ADHD compared with the mean value on the inattention and hyperactivity-impulsivity subscale of Barkley Adult ADHD Rating Scale) and SCT symptoms (lower level of ADHD compared with the mean value on the SCT subscale of Barkley Adult ADHD Rating Scale and Adult Concentration Inventory). They did not report any functioning impairment due to attentional problems on BAARS-IV and were not diagnosed with psychiatric disorders by the interview that followed the experiment.
A total of 122 adults who met the inclusion criteria or were selected as the control group participated in the experiment and completed the structured clinical interview for DSM-5 (SCID-5; First et al., 2016) by clinical psychologists to determine a diagnosis and their eligibility to participate. Exclusion criteria in the present study were as follows: (1) problems with intellectual ability; (2) history of head injury; (3) history of drug exposure; (4) diagnosis with other neurological or psychiatric disorders; and (5) participating in other non-pharmacological treatment interventions for ADHD (Tamm et al., 2013). Additionally, participants currently on medication for ADHD treatment were asked not to take medication on the day of participation for better measurement of their dysfunctions.
Of all participants, 12 participants were missed–five participants diagnosed with other psychiatric disorders, three participants who had almost half of the data missing due to the task error, and four participants whose overall accuracy rate of the ANT-R was less than 2SD from the mean. Finally, the following four groups were formed: (a) SCT+ADHD (at least five or more of symptoms of both SCT and ADHD); (b) SCT only (at least five or more of symptoms of SCT but not ADHD); (c) ADHD only (at least five or more of symptoms of ADHD but not SCT); and (d) healthy controls with low levels of both SCT and ADHD.
The sample included 110 adults with ages ranging between 18 to 31 years (M = 21.80, SD = 2.87); 52 (47.30%) of them were male and 58 (52.70%) were female. The SCT+ADHD group consisted 23 participants (age: M = 22.74, SD = 3.41; 52% males) and the SCT only group consisted of 45 participants (age: M = 21.11, SD = 2.32; 38% males). The ADHD only group consisted of 22 participants (age: M = 22.36, SD = 3.08; 41% males), and the control group consisted of 20 participants (age: M = 21.65, SD = 2.87; 70% males).
InstrumentsThe Barkley Adult ADHD Rating Scale IV (BAARS-IV) contains 18 items with a four-point that assess the levels of ADHD and SCT (Barkley, 2011). Internal consistency (Cronbach’s α) of the subscales were as follows: ADHD Inattention = .90; ADHD Hyperactive-Impulsive = .80; SCT = .90 (Barkley, 2012). In the present study, Cronbach’s α values were .90, .80, and .90 for the ADHD inattention, ADHD hyperactive-impulse, and SCT, respectively.
The Adult Concentration Inventory (ACI), developed for a new adult self-report measure of SCT, includes 10 items identified in a recent meta-analysis as optimal for the assessment of SCT symptoms (Becker, Burns et al., 2015). Cronbach’s α of 10-item ACI scale was .89 in the validation study (Becker et al., 2018) and Cronbach’s α was .89 in the present study.
The Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) measures general intellectual functioning (Wechsler, 2008). In the present study, we used the Arithmetics and Information subtests of the WAIS-IV, as these subtests were reported to have the strongest correlation with the full scale intelligence quotient (IQ) in the Korean WAIS-IV as a screening measure of intelligence (Choe et al., 2014; Hwang et al., 2012). Cronbach’ α values in the present study were .83 for the Arithmetics and .96 for the Information subscale. The full-scale IQ was estimated using regression equations [54.762 + (2.330 × AR) + (2.151 × IN)] suggested elsewhere (Choe et al., 2014).
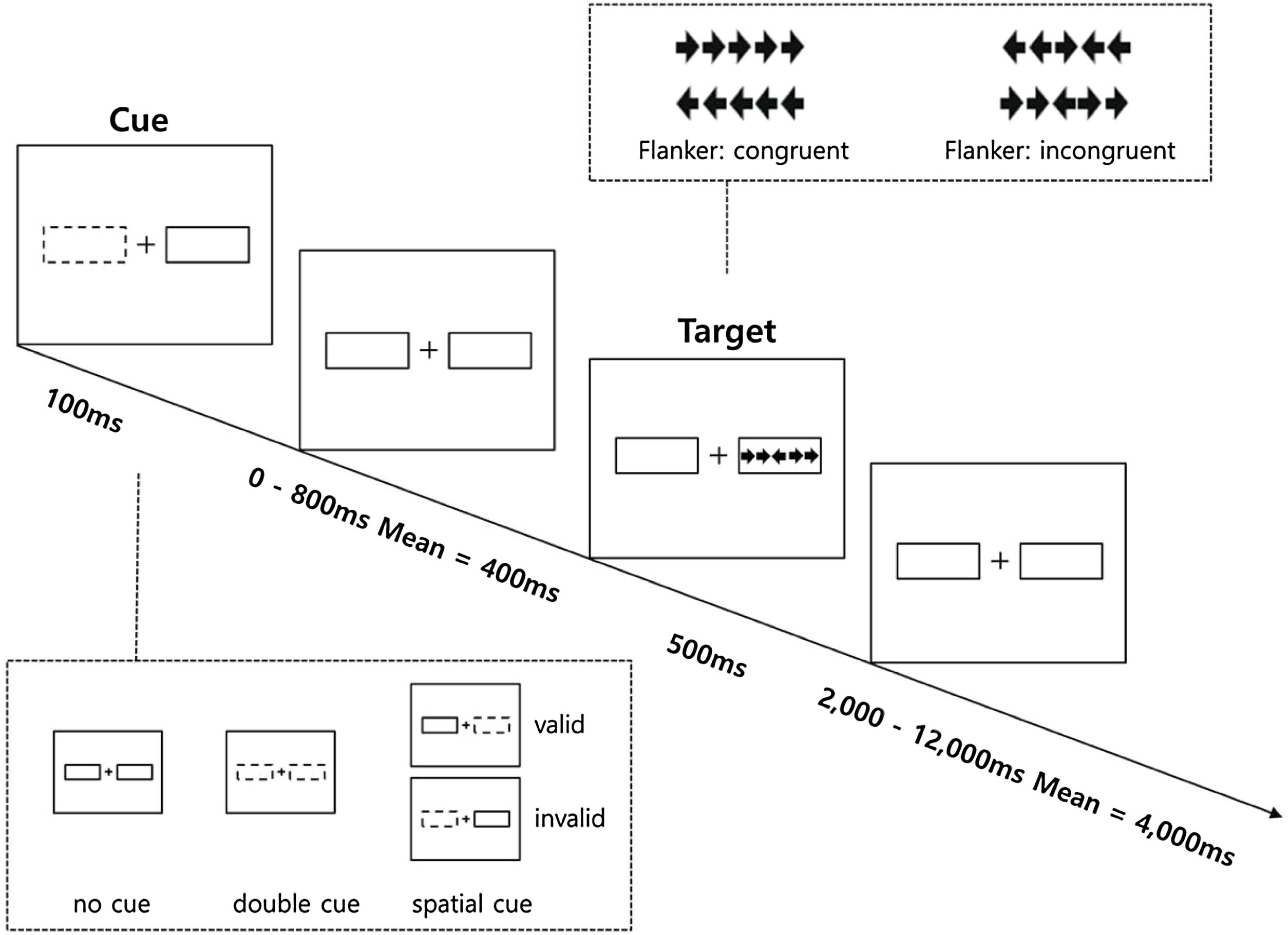
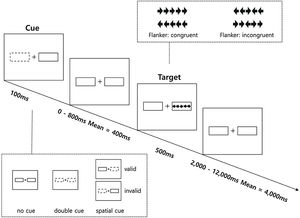
The Revised Attention Network Test (ANT-R) was developed based on the original ANT and was optimized to draw distinction between attentional networks and to more specifically investigate orienting network than the original ANT (Fan et al., 2009). The ANT-R is a computerized task consisting of three cue conditions (no-cue, double-cue, and spatial-cue) and two target conditions (congruent, incongruent). Further details on the ANT-R is provided in Figure 2. The task consisted of 4 runs, each with 72 test trials, resulting in a total of 288 trials in four different categories of conditions: 48 trials in the no cue condition, 48 trials in the double cue condition, 48 trials in the invalid cue condition, and 144 in the valid cue condition. Prior to each task, a total of 24 trials were administered as practice trials. The practice continued until the participants reached at least 90% accuracy. Three networks of attention were considered for the ANT-R in the present data analysis. First, the alerting network represents the benefit of the target response speed by calculating the difference between the no cue and double cue conditions. Second, in the ANT-R, the orienting c network could be separately measured as: (1) the engaging index (orienting network in the original ANT) represents the benefit of target response under valid cue condition because of orienting and engaging is measured by the difference between double cue and valid cue conditions; (2) the disengaging index represents the cost of disengaging from invalid cue and is measured by the difference between the invalid cue and double cue condition; (3) the validity index represents the cost of disengaging, and move operation is measured by difference between invalid cue and valid cue conditions. Third, the executive control network represents the flanker conflict effect measured by the difference between incongruent and congruent conditions.
ProcedureThe study met ethical standards of the Declaration of Helsinki. Upon arrival, the participants were given a brief instruction regarding the procedure and their rights as research participants; then, the participants signed an informed consent form approved by the institutional review board of Chung-Ang University (No. 1041078-202001-HR-010-01). Afterwards, the participants were asked to complete the ANT-R in the experimental room. The task was performed by four master's students of clinical psychology under the supervision of clinical psychologists. After the task, the participants completed a clinical interview and were asked to perform other psychological measurements. Finally, the participants were debriefed about the experiment and received the reward of 15,000 won (ca. 15 USD). All participants were individually asked not to share any information with anyone who might participate in the experiment after them.
Data analysisThe descriptive statistics for the variables were computed including means, standard deviations. Skewness and kurtosis were also examined independently for each group for each variable because behavioral data often have non-normal distributions. Since there were no variables with skewness and kurtosis value greater than 2, we assumed the normal distribution and proceeded with the next analysis. Prior to the analysis of the ANT-R, mean RT and error rate for each condition were calculated. If the overall accuracy of ANT-R is less than 2 SD on average, the data were considered not performed properly and excluded from the analysis. Next, a one-way analysis of variance (ANOVA) was conducted to compare the differences between the four groups on each attentional network in order to investigate a distinction of attentional network across all groups. When significant group differences were observed, the pairwise group contrasts using Scheffé post-hoc tests were performed. All statistical data were analyzed using SPSS 17.0 for Windows.
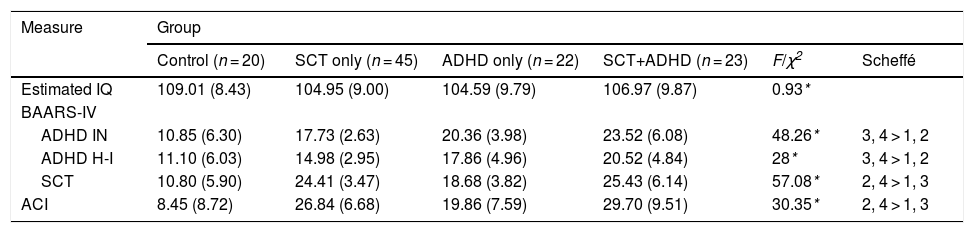
ResultsClinical characteristicsTable 1 shows the characteristics of the participants analyzed in the present study. According to the selection criteria, there were significant effects of groups for ADHD inattention [F (3, 104) = 48.26, p < .01, η2 = .58], ADHD hyperactive-impulsive [F (3, 104) = 28, p < .01, η2 = .45], SCT [F (3, 104) = 57.08, p < .01, η2 = .62] in BAARS-IV, and ACI [F (3, 104) = 30.35, p < .01, η2 = .47]. As expected from the inclusion criteria, the two SCT groups (SCT+ADHD, SCT only) had significantly higher SCT symptoms than did the other two groups.
Clinical characteristic of each group.
| Measure | Group | |||||
|---|---|---|---|---|---|---|
| Control (n = 20) | SCT only (n = 45) | ADHD only (n = 22) | SCT+ADHD (n = 23) | F/χ2 | Scheffé | |
| Estimated IQ | 109.01 (8.43) | 104.95 (9.00) | 104.59 (9.79) | 106.97 (9.87) | 0.93* | |
| BAARS-IV | ||||||
| ADHD IN | 10.85 (6.30) | 17.73 (2.63) | 20.36 (3.98) | 23.52 (6.08) | 48.26* | 3, 4 > 1, 2 |
| ADHD H-I | 11.10 (6.03) | 14.98 (2.95) | 17.86 (4.96) | 20.52 (4.84) | 28* | 3, 4 > 1, 2 |
| SCT | 10.80 (5.90) | 24.41 (3.47) | 18.68 (3.82) | 25.43 (6.14) | 57.08* | 2, 4 > 1, 3 |
| ACI | 8.45 (8.72) | 26.84 (6.68) | 19.86 (7.59) | 29.70 (9.51) | 30.35* | 2, 4 > 1, 3 |
Note: Mean (standard deviation); *p < .01; SCT: Sluggish Cognitive Tempo; ADHD: Attention-Deficit/Hyperactivity Disorder; BAARS-IV: Barkley Adult Attention-deficit/Hyperactivity Disorder Rating Scale IV; IN: Inattentive; H-I: Hyperactive and impulsive; ACI: Adult Concentration Inventory; Estimated IQ: Intelligence Quotient estimated by Wechsler Adult Intelligence Scale- IV.
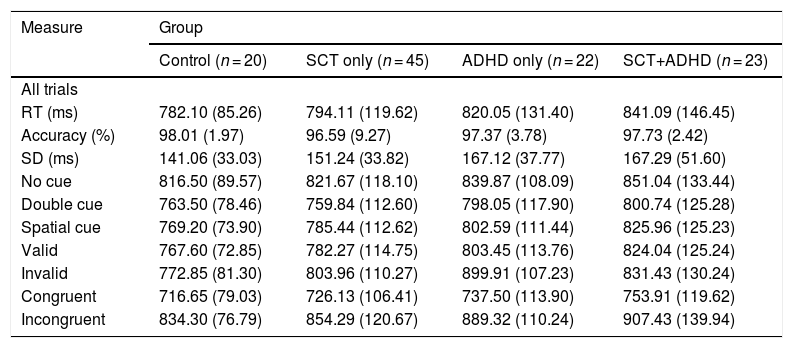
Table 2 shows the RT and accuracy rate for each experimental condition. Table 3 shows each attentional network effect and interaction related to orienting network in RT for the ANT-R. There were no significant differences in the overall RT [F (3, 106) = 1.11, n.s.] and the accuracy rate [F (3, 106) = .30, n.s.] between the groups. There was a marginally significant tendency for the overall SD [F (3, 106) = 2.41, p = .07, η2 = .06], indicating that participants showed difference in variability of RT among groups. Results of the post-hoc test represented that two ADHD groups (SCT+ADHD, ADHD only) showed higher overall SD in RT than the control group, indicating that ADHD was associated with a weaker tonic alerting than the control group. Tables 2 and 3.
Comparison of reaction time and accuracy between all groups on ANT-R.
| Measure | Group | |||
|---|---|---|---|---|
| Control (n = 20) | SCT only (n = 45) | ADHD only (n = 22) | SCT+ADHD (n = 23) | |
| All trials | ||||
| RT (ms) | 782.10 (85.26) | 794.11 (119.62) | 820.05 (131.40) | 841.09 (146.45) |
| Accuracy (%) | 98.01 (1.97) | 96.59 (9.27) | 97.37 (3.78) | 97.73 (2.42) |
| SD (ms) | 141.06 (33.03) | 151.24 (33.82) | 167.12 (37.77) | 167.29 (51.60) |
| No cue | 816.50 (89.57) | 821.67 (118.10) | 839.87 (108.09) | 851.04 (133.44) |
| Double cue | 763.50 (78.46) | 759.84 (112.60) | 798.05 (117.90) | 800.74 (125.28) |
| Spatial cue | 769.20 (73.90) | 785.44 (112.62) | 802.59 (111.44) | 825.96 (125.23) |
| Valid | 767.60 (72.85) | 782.27 (114.75) | 803.45 (113.76) | 824.04 (125.24) |
| Invalid | 772.85 (81.30) | 803.96 (110.27) | 899.91 (107.23) | 831.43 (130.24) |
| Congruent | 716.65 (79.03) | 726.13 (106.41) | 737.50 (113.90) | 753.91 (119.62) |
| Incongruent | 834.30 (76.79) | 854.29 (120.67) | 889.32 (110.24) | 907.43 (139.94) |
Note: Mean (standard deviation); in milliseconds (ms). RT: reaction time; SD: standard deviation; SCT: Sluggish Cognitive Tempo; ADHD: Attention-Deficit/Hyperactivity Disorder; ANT-R: Attention network test-revised version.
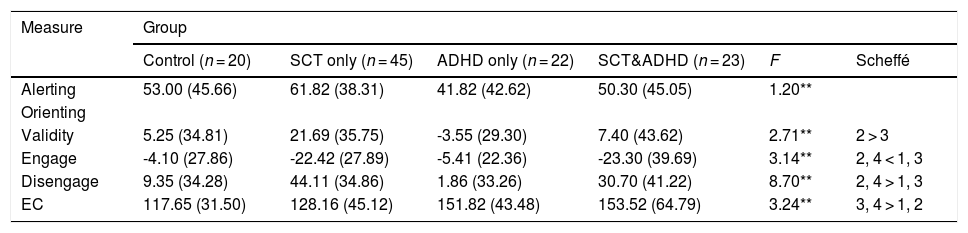
Comparison of attentional network index between all groups on ANT-R.
| Measure | Group | |||||
|---|---|---|---|---|---|---|
| Control (n = 20) | SCT only (n = 45) | ADHD only (n = 22) | SCT&ADHD (n = 23) | F | Scheffé | |
| Alerting | 53.00 (45.66) | 61.82 (38.31) | 41.82 (42.62) | 50.30 (45.05) | 1.20** | |
| Orienting | ||||||
| Validity | 5.25 (34.81) | 21.69 (35.75) | -3.55 (29.30) | 7.40 (43.62) | 2.71** | 2 > 3 |
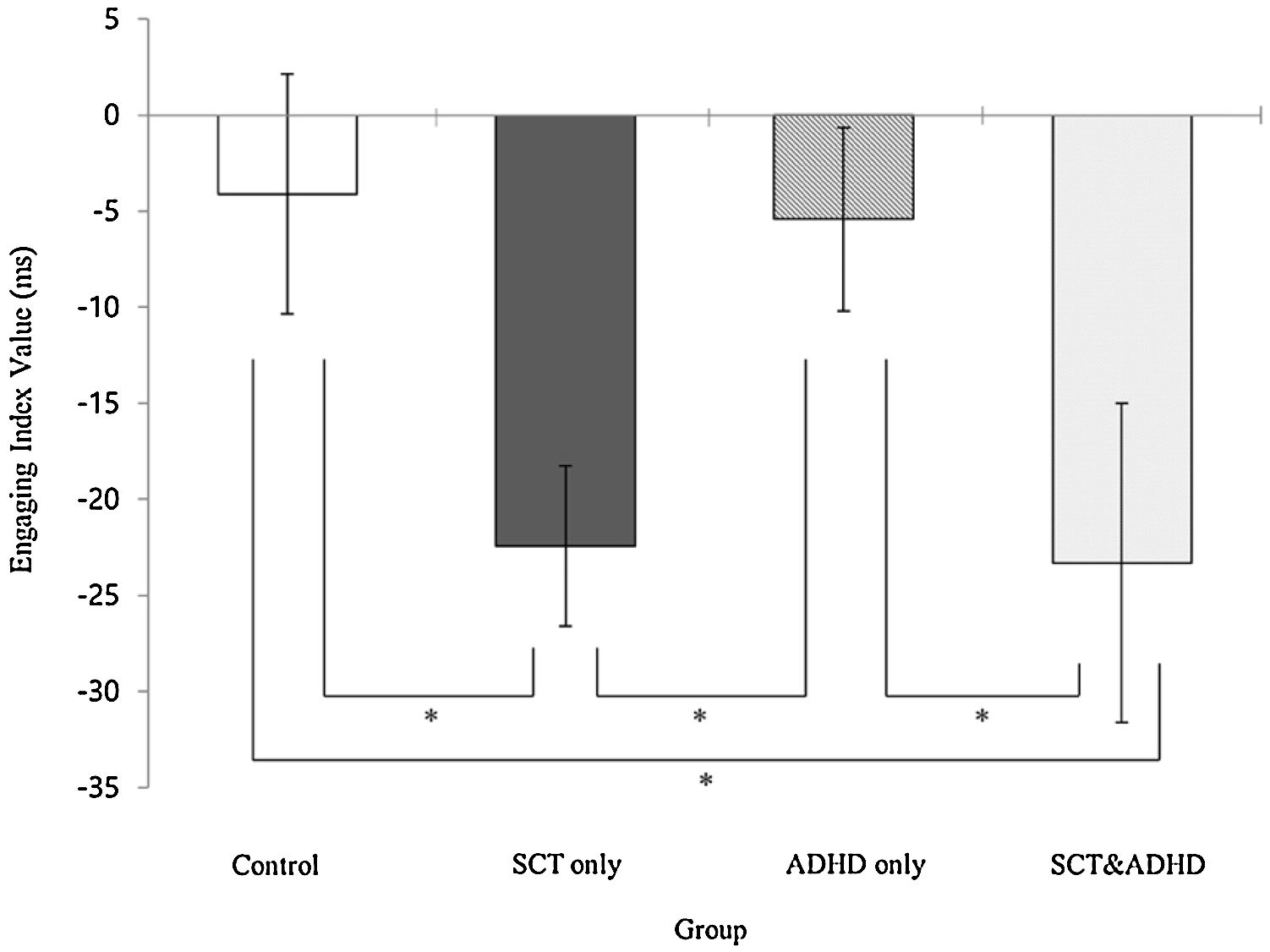
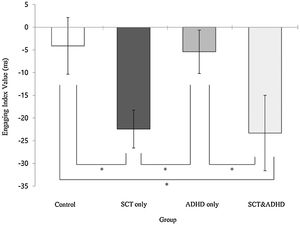
| Engage | -4.10 (27.86) | -22.42 (27.89) | -5.41 (22.36) | -23.30 (39.69) | 3.14** | 2, 4 < 1, 3 |
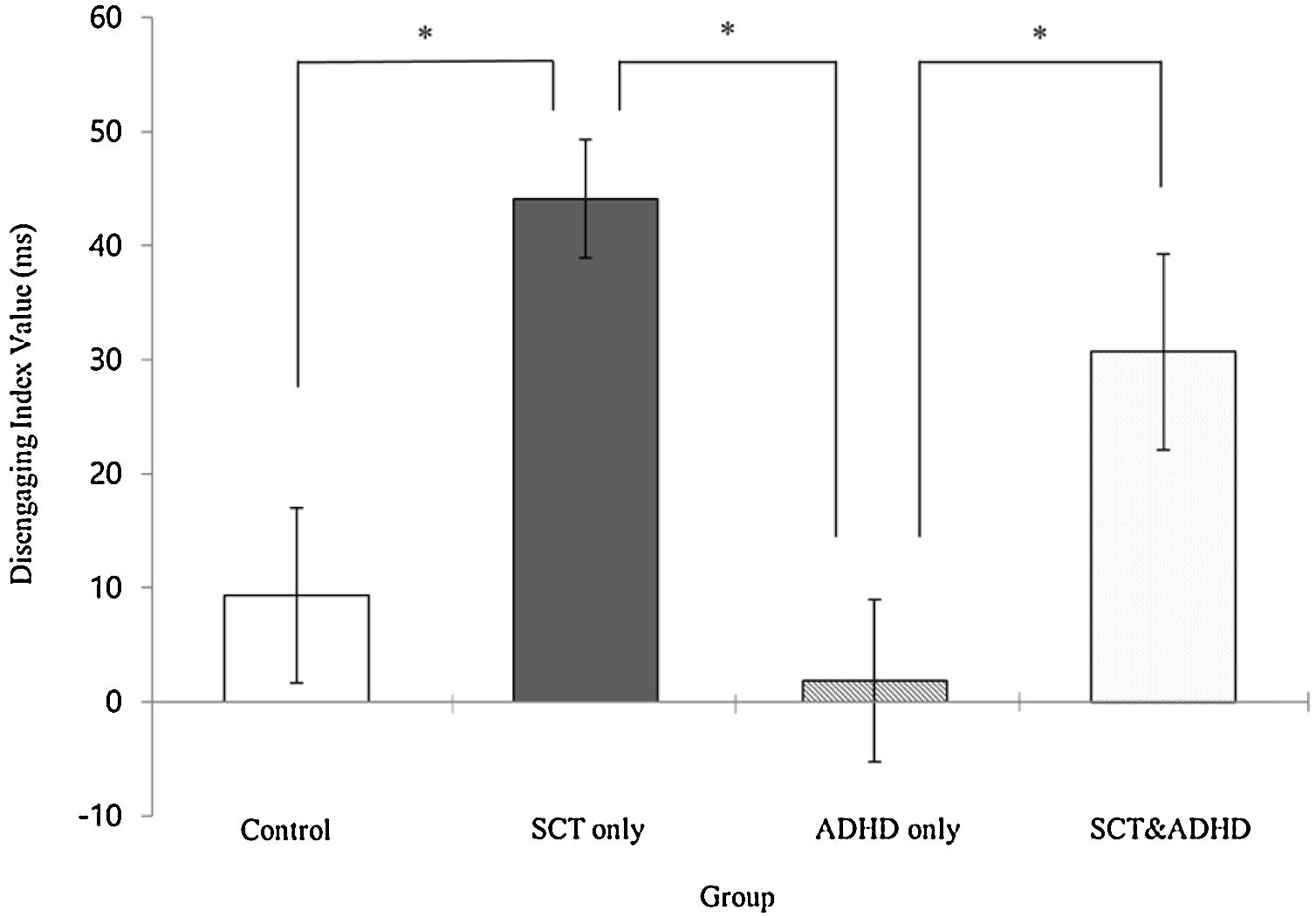
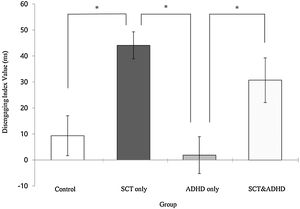
| Disengage | 9.35 (34.28) | 44.11 (34.86) | 1.86 (33.26) | 30.70 (41.22) | 8.70** | 2, 4 > 1, 3 |
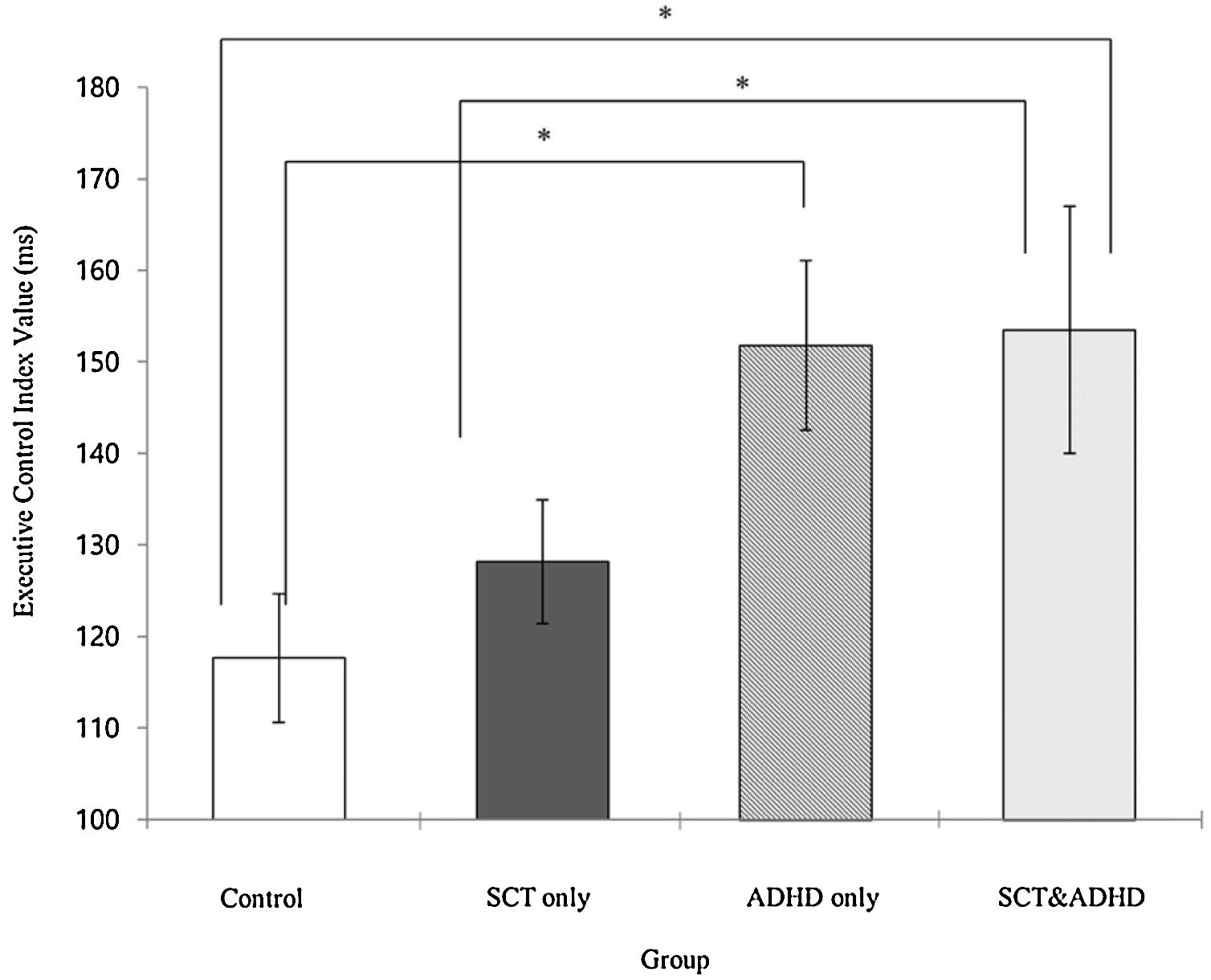
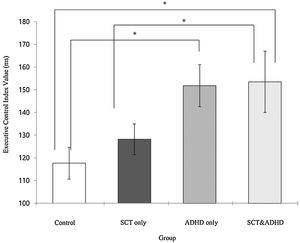
| EC | 117.65 (31.50) | 128.16 (45.12) | 151.82 (43.48) | 153.52 (64.79) | 3.24** | 3, 4 > 1, 2 |
Note: Mean (standard deviation); *p < .05; **p < .01; Mean (SD) in milliseconds (ms); SCT: Sluggish Cognitive Tempo; ADHD: Attention-Deficit/Hyperactivity Disorder.
There was no significant effect among groups for the alerting index [F (3, 106) = 1.20, n.s.], indicating that participants did not show difference in phasic alerting among the groups.
The validity index (Invalid cue - Valid cue)There was a significant effect for the validity index [F (3, 106) = 2.71, p < .05, η2 = .07], indicating that participants showed difference in the cost of disengaging and moving operation between the groups. Results of the post-hoc test represented that the SCT only group showed significantly higher validity index than the ADHD only group, indicating that the SCT-only group had significantly weaker orienting network than the ADHD only group.
The engaging index (Double cue - Valid cue)There was a significant effect for the engaging index [F (3, 106) = 3.14, p < .05, η2 = .08], indicating that that participants showed difference in the benefit of target response under valid cue condition because of engaging attention between groups. Results of the post-hoc test represented that two SCT groups (SCT+ADHD, SCT only) showed significantly lower engaging effect than the other two groups (ADHD only, control), indicating that both the SCT+ADHD and the SCT only group had significantly weaker orienting network due to difficulties in engaging attention than other two groups Figure 3.
The disengaging index (Invalid cue - Double cue)There was a significant effect for the disengaging index [F (3, 106) = 8.70, p < .01, η2 = .20] indicating that participants showed difference in the cost of target response under an invalid cue condition because of disengaging attention between the groups. Results of the post-hoc test represented that the two SCT groups (SCT+ADHD, SCT only) showed significantly
higher the disengaging index than the ADHD only and control group, and especially the SCT only group showed significantly higher disengaging index than control group Figure 4.
The executive control network index (Incongruent – Congruent)There was a significant effect for the executive control index [F (3, 106) = 3.24, p < .05, η2 = .08], indicating that participants showed difference in the cost of target response because of the flanker conflict effect between groups. Results of the post-hoc test represented that two ADHD groups (SCT+ADHD, ADHD only) showed significantly higher the executive control network index than the control group, and especially SCT+DHD group showed significantly higher executive control network index than SCT only group Figure 5.
DiscussionThe present study evaluated and compared attentional network in the SCT only, ADHD only, SCT+ADHD, and healthy control groups using ANT-R in order to investigate which kind of attention dysfunction might be unique in SCT. The major finding of the present study is that two SCT groups (SCT+ADHD, SCT only) showed a poorer efficiency in the orienting network than other two groups. This supports our hypothesis which we formulated based on previous research (Baytunca et al., 2018; Fassbender et al., 2015)—namely, that SCT is associated with impairment in the orienting network. This result may reflect that individuals with SCT are impaired in both engaging and disengaging attention by examining detailed elements of orienting network. Therefore, this supports that SCT may have dysfunction in the early information processing or visual selective attention, which is not typical for ADHD (Baytunca et al., 2018; Huang-Pollack et al., 2005).
Although the mechanism of the orienting network deficit in SCT is yet unknown, there are two possible explanations for these results. First, previous studies suggested that the orienting network is related to brain activity in the superior parietal lobe (Morein-Zamir et al., 2014), and that an increase in the SCT symptoms would be associated with hypoactivity in the left superior parietal lobe during the Flanker task (Fassbender et al., 2015). Therefore, although the present research has not been able to address this issue satisfactorily, it can be inferred that there could be impairment in the orienting network due to less activity in the left superior parietal lobe among individuals with SCT. Second, according to the continuous model of activity, hyperactivity and hypoactivity could be explained as a single continuum of activity level, with a conjecture that attentional problems may have an inverted U-shaped relationship with the activity level (Miller & Prevatt, 2017). SCT is characterized by hypoactivity, and a slower processing or motor speed has been linked to specifically hypoactivity (Lundervold, Posserud et al., 2011). Although further research is needed, it can be inferred that individuals with SCT may show impairments of the orienting network due to the slow engagement, disengagement, and shift in attention.
The unique pattern of the results found in the SCT group may raise a question of whether or not attentional difficulties in individuals with SCT are the result of difficulties on the information-processing side or the motor preparatory and execution side, or both (Jacobson et al., 2018). Although the term “sluggish” implies that there is the core cognitive deficit (e.g., slowness in behavior or in thinking), there were controversial findings in terms of whether or not SCT remains associated with a slower motor or processing speed after controlling for ADHD (Bauermeister et al., 2012; Willcutt et al., 2014). This result can provide possible explanations for the underlying mechanism in key symptoms of SCT. In the present study, attentional difficulties in the individuals with SCT were not found to be associated with the overall slow motor speed but were found to be related to the results of dysfunction with specific information-processing. Specifically, individuals with SCT showed impairments of the orienting network due to being slow to engage, disengage, and to shift attention. Therefore, as individuals with SCT have difficulty paying attention to certain stimuli or moving attention away from one stimulus to another one, it seems that they are either head-in-air or drowsy, rather than slow in the overall motor speed. By contrast, ADHD is primarily a disorder of the executive control network, which entails difficulties in problem solving and response inhibition, rather than problems with input in information processing.
Another important finding of the present study is that two ADHD groups (SCT+ADHD, ADHD only) showed a poorer efficiency in the executive control network than other two groups. This supports our hypothesis that ADHD is associated with impairment in executive control network (cf. Fabio & Urso, 2014; Mullane et al., 2011). The slower performance of ADHD in the incongruent condition of the ANT-R may reflect that the participants were vulnerable to distractors and experienced difficulty in filtering out distracting information during goal-directed behavior. In line with previous findings, our results of ADHD participants showing a poor performance on flanker interference tasks may reflect the deficit of attentional control or dysfunction in a general behavior monitoring system of individuals with ADHD (Patros et al., 2019; Rommel et al., 2019). That is, ADHD is associated with a primary dysfunction of executive functions, such as interference control and problem solving for future goal attainment (Castellanos and Proal, 2012; Neely et al., 2017). Unlike ADHD, SCT appears to be related to the orienting network, suggesting that SCT is dissociable from ADHD according to the differences in attention networks.
However, in the results of the present study, we found no difference in the alerting network among the groups, while there were differences in the overall SD in RT between the two ADHD groups and the other groups. These results provide little support for the hypothesis that two ADHD groups (SCT+ADHD, ADHD only) would show a poorer efficiency in the alerting network than the other two groups. The higher variability in the RT of ADHD may reflect that individuals with ADHD experience difficulties in maintaining the alert state during the sustained attention task. Although the findings regarding whether or not ADHD is associated with the alerting network were mixed (Fabio & Urso, 2014; Kofler et al., 2013; Lundervold, Adolfsdottir et al., 2011; Oberlin et al., 2005), these results are consistent with previous result showing that individuals with ADHD have a problem with the tonic or internal aspects of alerting (e.g., maintaining vigilant state), but still preserve the ability to use cues to phasic alerting (e.g., readiness to react) (Kofler et al., 2013). These data may also support the theories of ADHD which emphasize the problem of arousal regulation, whereby ADHD individuals were found to have difficulties in regulating arousal, which is not typical of individuals with SCT. These results indicate that the cognitive profile of SCT is different from ADHD. In addition, the SCT+ADHD group showed a poor efficiency in orienting and executive control networks of attention that the other groups. As the SCT+ADHD group had both SCT and ADHD symptoms, they may also have dysfunctions in those attentional networks. Therefore, SCT may be a distinct attention disorder that frequently occurs together, but is nonetheless distinct from ADHD.
The present study has several limitations. First, since we focused on adult individuals with SCT, our conclusions might not be directly applicable to individuals with SCT of other age groups. Therefore, there is a clear need to investigate cognitive characteristics of children or adolescent individuals with SCT. In addition, the sample size of the control group regarding the ADHD or SCT group was relatively small. Second, in order to extend understanding of the attentional profile of SCT, additional studies should be carried out with diverse measures, such as multi-modal attentional network, and neuroimaging measures. Finally, our results do not clarify the attentional differences between SCT and ADHD subtypes, particularly with regard to ADHD of the predominantly inattentive type (ADHD-I). Although SCT is a separate symptom dimension distinct from ADHD-I and other dimensions of psychopathology in the factor analytic research (Becker et al., 2016), additional research is necessary to draw a clear demarcation line between SCT and ADHD-I.
This research was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2018R1D1A1B07050534). This paper is a condensed version of the first author's doctoral thesis. The abstract of this paper was presented at the 7th World Congress on ADHD: From Child to Adult Disorder as a poster presentation with interim findings.