Introduction: COVID-19 pandemic, declared on March 11, 2020, constitute an extraordinary health, social and economic global challenge. The impact on people's mental health is expected to be high. This paper sought to systematically review community-based studies on depression conducted during the COVID-19 and estimate the pooled prevalence of depression. Method: We searched for cross-sectional, community-based studies listed on PubMed or Web of Science from January 1, 2020 to May 8, 2020 that reported prevalence of depression. A random effect model was used to estimate the pooled proportion of depression. Results: A total of 12 studies were included in the meta-analysis, with prevalence rates of depression ranging from 7.45% to 48.30%. The pooled prevalence of depression was 25% (95% CI: 18%−33%), with significant heterogeneity between studies (I2=99.60%, p<.001). Conclusions: Compared with a global estimated prevalence of depression of 3.44% in 2017, our pooled prevalence of 25% appears to be 7 times higher, thus suggesting an important impact of the COVID-19 outbreak on people's mental health. Addressing mental health during and after this global health crisis should be placed into the international and national public health agenda to improve citizens’ wellbeing.
Introducción: La pandemia de COVID-19, declarada el 11 de marzo de 2020, representa un reto global extraordinario a nivel sanitario, social y económico. Se espera un impacto alto en la salud mental de las personas. Este artículo tiene como objetivo realizar una revisión sistemática de estudios transversales basados en muestras comunitarias que proporcionaban la prevalencia de depresión durante la crisis del COVID-19. Método: Se realizó una búsqueda de estudios comunitarios publicados en Pubmed y Web of Science desde el 1 de enero del 2020 al 8 de mayo del 2020 y que informaron sobre la prevalencia de depresión. Se usó un modelo de efectos aleatorios para estimar la proporción agrupada de depresión. Resultados: Un total de 12 estudios fueron incluidos en el meta-análisis, con prevalencias de depresión que oscilaban entre 7,45% y 48,30%. La prevalencia agrupada de depresión fue de 25% (95% CI: 18%-33%), con heterogeneidad significativa entre estudios (I2=99,60%, p<0,001). Conclusiones: En comparación con una estimación global de depresión en 2017 del 3,44%, nuestra prevalencia agrupada del 25% es 7 veces mayor, sugiriendo un impacto importante del brote de COVID-19 en la salud mental de las personas. El abordaje de la salud mental durante y después de esta crisis global sanitaria debe ser parte de las agendas de salud pública nacionales e internacionales para mejorar el bienestar de los ciudadanos.
The novel coronavirus disease (COVID-19) was declared a pandemic by the World Health Organization (WHO) on March 11, 2020 (World Health Organization, 2020b). Since its identification in a wet market in Wuhan, China, in December 2019 (Lu et al. 2020), to this date (May 14th, 2020), there has been a total of 4,248,389 confirmed cases worldwide. Among them, 294,046 have died (World Health Organization, 2020a). This pandemic, and the public health measures implemented to slow it, have profoundly changed people's lifestyle and is thought to be a threaten for physical and mental wellbeing.
The unpredictable nature of the disease, the loss of control and personal freedoms, the conflicting messages from authorities, sudden changes in plans for the immediate future, or concern for one's own health and well-being and that of one's relatives are examples of sources of stress associated with these outbreaks and pandemics (Huremović, 2019). With the COVID-19 pandemic, this has been followed by home confinement for indefinite periods and substantial and growing financial losses. A recent systematic review on the psychological impact of previous confinement due to several pandemic such as Ebola, H1N1 influenza pandemic, Middle East respiratory syndrome and equine influenza found negative psychological effects including post-traumatic stress symptoms, anger and confusion (Brooks et al., 2020). According to the authors, factors such as long duration of quarantine, fears for infection, inadequate information, stigma, or financial loss were related to higher negative psychological impact. These major stressors can be expected to lead to an increased risk of psychopathology such as anxiety or depression (Huremović, 2019; Pfefferbaum & North, 2020).
To date there is not systematic review or meta-analysis assessing the prevalence of depression in the general population. Two reviews have provided data on psychopathology related to COVID-19, but while one covers epidemic outbreaks since 2007 (Fardin, 2020), another (Rajkumar, 2020) offers only one study (Wang, Di, et al. 2020) that examines depression in the general population. Given the increasing number of papers addressing mental health and COVID-19 published in the last month from various countries, we conducted a systematic review and meta-analysis of available studies investigating depression in the general population during the COVID-19 outbreak in order to obtain a more global perspective.
MethodThis study was conducted in accordance with the PRISMA guidelines for reporting systematic reviews and meta-analysis (Moher et al., 2009; Perestelo-Pérez, 2013). Appendix A.
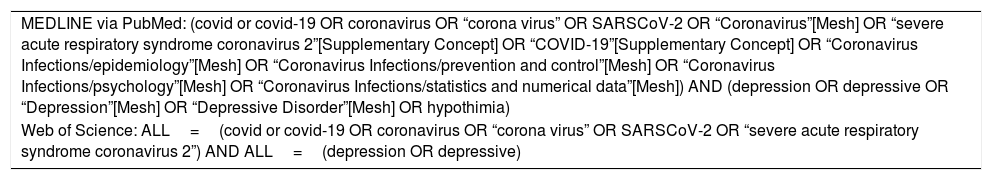
Search strategyTwo researchers searched for cross-sectional studies reporting the prevalence of depression published from January 1, 2020 to May 8, 2020 using MEDLINE, via PubMed, and Web of Science. The Pubmed and Web of Science search strategies are shown in Appendix B. No language restriction was made. References from selected articles were inspected to detect additional potential studies. We then performed a manual search of the “grey literature” (e.g., medRxiv) to detect other potentially eligible investigations. Inter-rater reliability analysis showed high levels of agreement between the reviews (Cohen's kappa (κ) ranged from .88 to .94). Any disagreement was resolved by consensus among a third and fourth reviewers.
Inclusion and exclusion criteriaStudies were included if: (1) reported cross-sectional data on the prevalence of depression during the COVID-19 outbreak; (2) they were focused on community-based samples; (3) they described the methods used to assess or diagnose depression; (4) the full-text was available. We excluded studies focusing on specific samples (e.g., medical professionals, patients, adolescents), and review articles.
Data extractionA pre-designed data extraction form was used to extract information on the following variables: country, sample size, prevalent rates of depression, proportion of women, average age, instruments used to assess depression, response rate, and sampling methods.
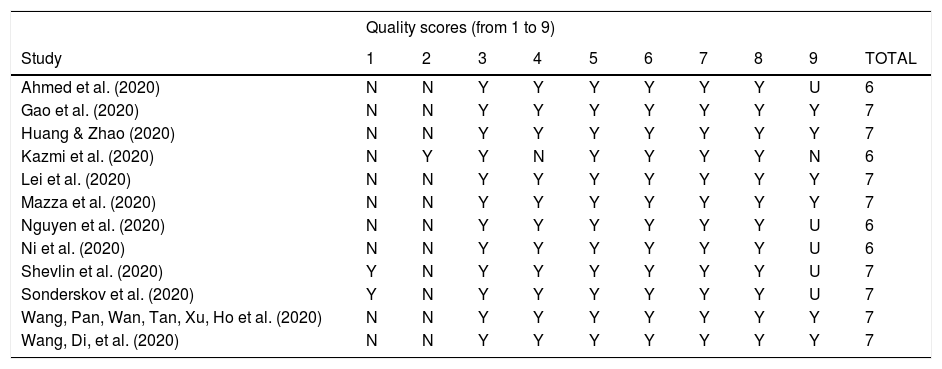
Methodological quality assessmentArticles selected for retrieval were assessed by two independent reviewers for methodological validity before they were included in the review using the Joanna Briggs Institute (JBI) standardized critical appraisal instrument for prevalence studies (Moola et al., 2017). Inter-rater reliability analysis showed high levels of agreement between the reviews (intra-class correlation coefficient=.85, 95% CI=.51-.95). Any disagreements that arose between the reviewers were resolved through discussions, or by further discussion with a third reviewer (PGG).
Statistical analysisA generic inverse variance method with a random effect model was used (DerSimonian & Laird, 1986). Freeman and Tukey's double arcsine transformation of prevalence to stabilize the variance was applied (Freeman & Tukey, 1950). The Hedges Q statistic was reported to check heterogeneity across studies, with statistical significance set at p<.10. Following the recommendations for a small number of studies (Higgins & Green, 2011), the I2 statistic and 95% confidence interval was also used to quantify heterogeneity (von Hippel, 2015). I2 values between 25%-50% are considered as low, 50%-75% as moderate, and 75% or more as high (Higgins et al., 2003). Heterogeneity of effects between studies occurs when differences in results for the same exposure-disease association cannot be fully explained by sampling variation. Sources of heterogeneity can include differences in study design or in demographic characteristics. We performed meta-regression and subgroup analyses (Thompson & Higgins, 2002) to explore the sources of heterogeneity expected in meta-analyses of observational studies (Egger et al., 1998). We conducted a sensitivity analysis to determine the influence of each individual study on the overall result by omitting studies one by one. Publication bias was determined through visual inspection of a funnel plot and Egger (Egger et al., 1997) and Begg (Begg & Mazumdar, 1994) tests (p values <.10 indicate publication bias).
Statistical analyses were conducted by JS and run with the metaprop package (Nyaga et al., 2014), STATA statistical software (version 10.0; College Station, TX, USA).
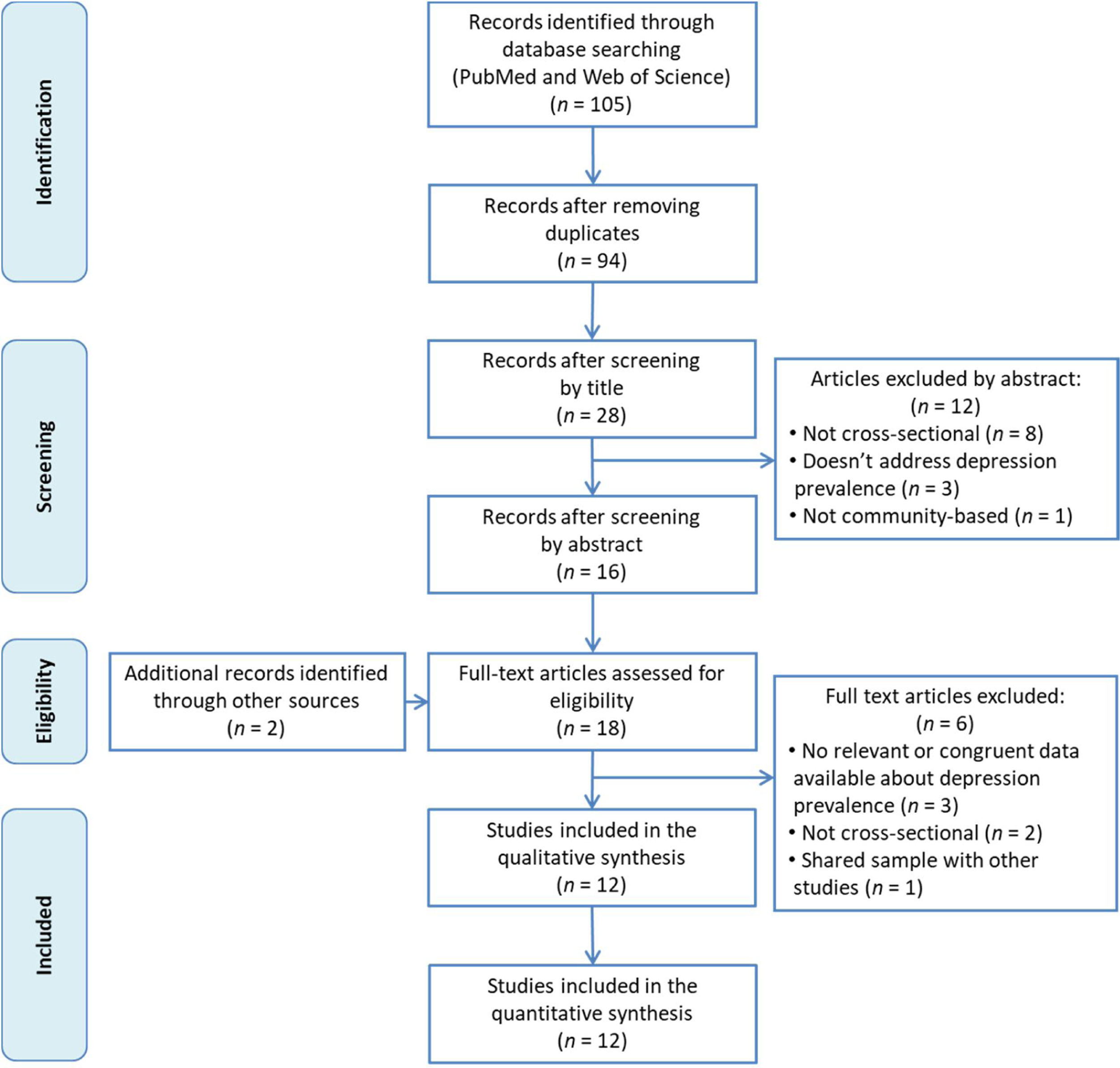
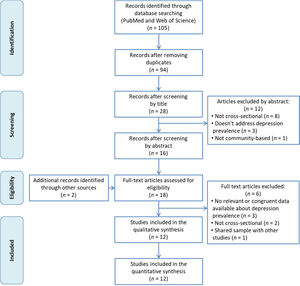
ResultsFigure 1 shows the flow chart of the literature search strategy and the study selection process. Initially, 105 potential records were identified, of which 85 were retrieved from PubMed and 20 from Web of Science. After removing duplicates, the titles of the remaining 94 articles were read and 66 of them were excluded for not meeting inclusion criteria. Subsequently, the abstracts of the remaining 28 articles were read and 8 articles were removed for not being cross-sectional studies, 3 for not analyzing the prevalence of depression and one for not being a community-based study. We added 2 more articles found by manual search of other databases and reference lists. After reading these 18 articles in full, we finally included 12 in our systematic review.
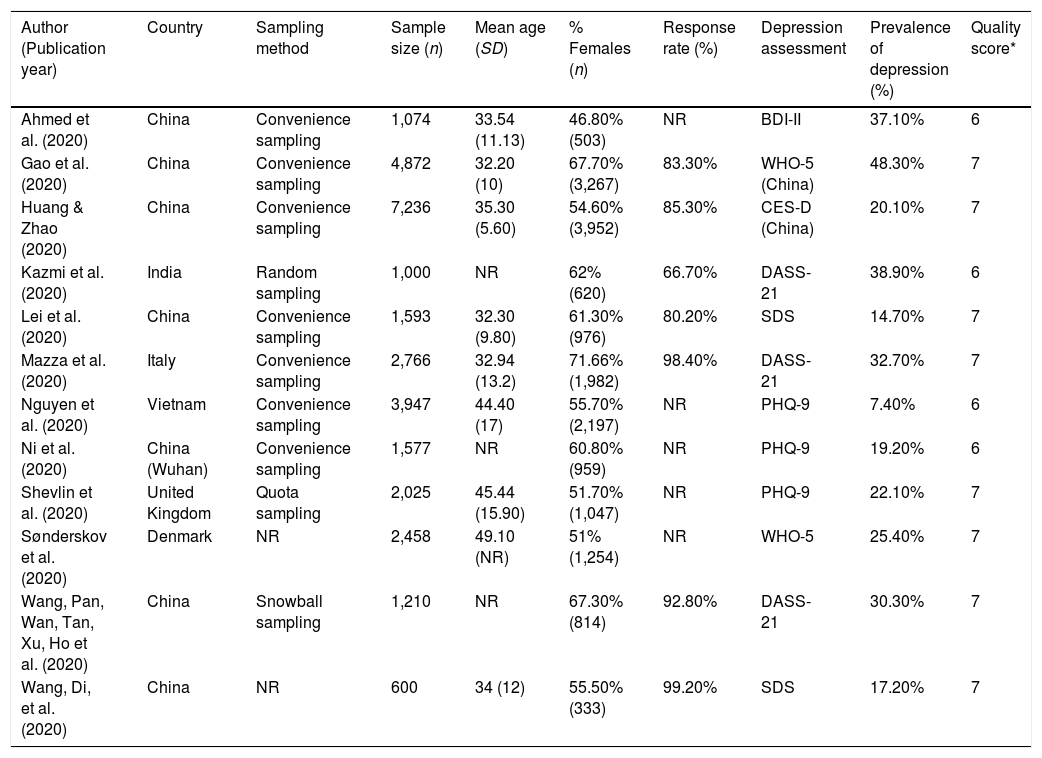
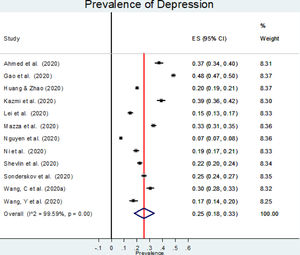
Table 1 summarizes the characteristics of the included studies (Ahmed et al., 2020; Gao et al., 2020; Huang & Zhao, 2020; Kazmi, Hasan, Talib, & Saxena, 2020; Lei et al., 2020; Mazza et al., 2020; Nguyen et al., 2020; Ni et al., 2020; Sønderskov, Dinesen, Santini, & Østergaard, 2020; Shevlin et al., 2020, Wang, Pan, Wan, Tan, Xu, Ho et al., 2020; Wang, Di, et al. 2020), 7 of which were from China, 1 from Vietnam, 1 from India, and three from Europe (Italy, Denmark and the United Kingdom). The sample size ranged from 600 to 7,236 participants, and the mean age ranged from 32.20 to 49.10 years in the nine studies reporting it. All studies included both men and women, and the percentage of women ranged from 46.80% to 71.66%, with a majority of women in most of them. All studies were conducted using online questionnaires, and, of those who reported it, all but one used non-random sampling methods. The response rate was reported by 7 studies and ranged from 66.66% to 99.17%. All studies measured depression using standardized scales, the most common being the Depression, Anxiety and Stress Scale (DASS) and the Patient Health Questionnaire (PHQ). The studies reported highly diverse values of depression prevalence, ranging from 7.45% to 48.30%.
Characteristics of the studies included in the meta-analysis.
| Author (Publication year) | Country | Sampling method | Sample size (n) | Mean age (SD) | % Females (n) | Response rate (%) | Depression assessment | Prevalence of depression (%) | Quality score* |
|---|---|---|---|---|---|---|---|---|---|
| Ahmed et al. (2020) | China | Convenience sampling | 1,074 | 33.54 (11.13) | 46.80% (503) | NR | BDI-II | 37.10% | 6 |
| Gao et al. (2020) | China | Convenience sampling | 4,872 | 32.20 (10) | 67.70% (3,267) | 83.30% | WHO-5 (China) | 48.30% | 7 |
| Huang & Zhao (2020) | China | Convenience sampling | 7,236 | 35.30 (5.60) | 54.60% (3,952) | 85.30% | CES-D (China) | 20.10% | 7 |
| Kazmi et al. (2020) | India | Random sampling | 1,000 | NR | 62% (620) | 66.70% | DASS-21 | 38.90% | 6 |
| Lei et al. (2020) | China | Convenience sampling | 1,593 | 32.30 (9.80) | 61.30% (976) | 80.20% | SDS | 14.70% | 7 |
| Mazza et al. (2020) | Italy | Convenience sampling | 2,766 | 32.94 (13.2) | 71.66% (1,982) | 98.40% | DASS-21 | 32.70% | 7 |
| Nguyen et al. (2020) | Vietnam | Convenience sampling | 3,947 | 44.40 (17) | 55.70% (2,197) | NR | PHQ-9 | 7.40% | 6 |
| Ni et al. (2020) | China (Wuhan) | Convenience sampling | 1,577 | NR | 60.80% (959) | NR | PHQ-9 | 19.20% | 6 |
| Shevlin et al. (2020) | United Kingdom | Quota sampling | 2,025 | 45.44 (15.90) | 51.70% (1,047) | NR | PHQ-9 | 22.10% | 7 |
| Sønderskov et al. (2020) | Denmark | NR | 2,458 | 49.10 (NR) | 51% (1,254) | NR | WHO-5 | 25.40% | 7 |
| Wang, Pan, Wan, Tan, Xu, Ho et al. (2020) | China | Snowball sampling | 1,210 | NR | 67.30% (814) | 92.80% | DASS-21 | 30.30% | 7 |
| Wang, Di, et al. (2020) | China | NR | 600 | 34 (12) | 55.50% (333) | 99.20% | SDS | 17.20% | 7 |
Note. * Quality score based on the Joanna Briggs Institute (JBI) standardized critical appraisal instrument for prevalence studies (Moola et al., 2017; see Appendix C). SD=standard deviation; NR=not reported; BDI-II=Beck depression inventory-second edition; WHO-5=World Health Organization-five well-being index; CES-D=Center for Epidemiologic Studies-Depression scale; DASS-21=Depression, Anxiety and Stress scales; PHQ-9=Patient Health Questionnaire; SDS=Sheehan Disability Scale.
The risk of bias scores ranged from 6 to 7 out of a possible total of 9, with a mean score of 6.4 (Appendix C). The most common limitations were: (a) recruitment of participants not appropriate (11 studies) or sample not clearly representative of the population (10 studies), and (b) response rate not reported, or large number of non-responders (6 studies).
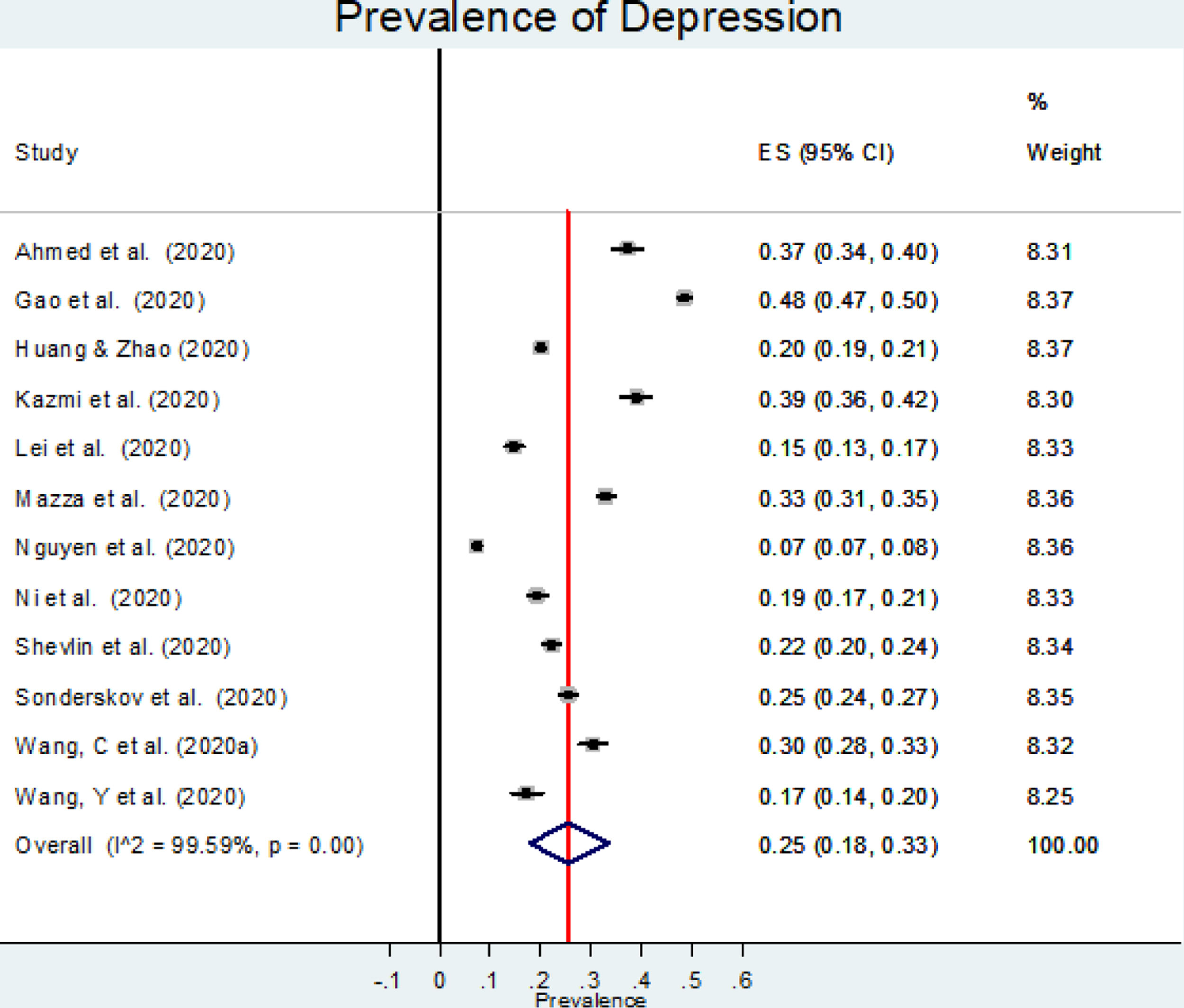
The estimated overall prevalence of depression was 25% (95% CI: 18%−33%; Figure 2), with significant heterogeneity between studies (I2=99.60%, p<.001).
Our meta-regression showed that prevalence of depression was independent of the percentage of women, mean age at baseline, response rate, or methodological quality. Neither study location nor sampling method were significant moderators according to subgroup analysis (data not shown). The only significant finding was a lower prevalence of depression for studies using the SDS (Self-Rating Depression Scale) (15% [95% CI: 14%-17%]) or the PHQ-9 (16% [95% CI: 7%-27%]) compared to those using the DASS-21 (34% [95% CI: 30%-38%]) or the WHO-5 (World Health Organisation-Five Well-being index) (40% [95% CI: 39%-41%]) (p<.001). No comparison with BDI-II (Beck Depression Inventory–II) or CES-D (Center for Epidemiological Studies–depression) was performed since only one study using each one was found.
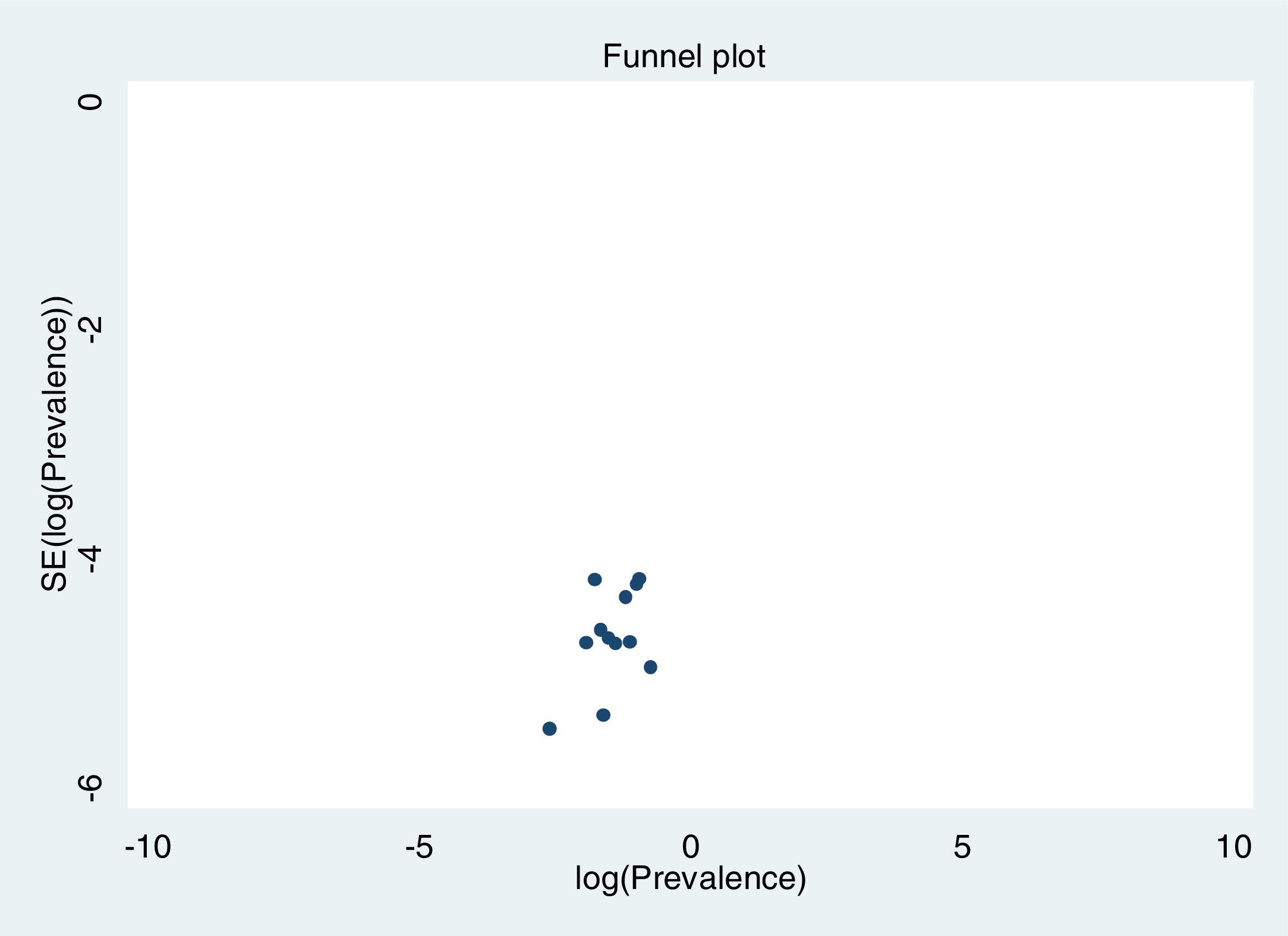
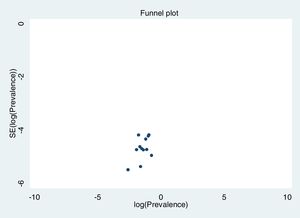
Excluding each study one-by-one from the analysis did not substantially change the pooled prevalence of depression, which varied between 23% (95% CI: 18%-30%), with Gao et al. (2020) excluded, to 27% (95% CI: 21%-35%), with Nguyen et al. (2020) excluded. This indicates that no single study had a disproportional impact on the overall prevalence. Visual inspection of the funnel plot (Figure 3) suggested no presence of publication bias for the estimation of prevalence, confirmed by non-significant results in the Begg's (p=.304) and Egger's (p=.126) tests. Publication bias may not be a problem in this meta-analysis, since the prevalence rate is the outcome measure and there are no significant levels that may have biased publications. The reasons for non-publication are more likely small studies with poor methods.
DiscussionThe present meta-analysis of twelve large studies suggests that the pooled prevalence of depression in the general population during the COVID-19 outbreak is 25% (95% CI: 18%-33%). The main source of heterogeneity in the prevalence rates of depression among the studies included in this meta-analysis was the scale used for its analysis, with the highest prevalence rates in studies using the WHO-5 and DASS-21 scales, and the lowest in those using the PHQ-9 and SDS scales. In addition, the use of self-reported data may imply the presence of biases such as social desirability bias (Ahmed et al., 2020), or have less efficacy than standardized clinical interviews, so that ultimately the sensitivity of the different scales, even standardized, differs greatly (Dunstan et al., 2017).
The latest global estimated prevalence of depression is from 2017 and shows a proportion of 3.44% (ranging between 2 and 6%) (Ritchie & Roser, 2018). This estimation, based on the Global Burden of Disease data, includes both dysthymia and major depressive disorder and it is based on studies reporting depression prevalence rates based on medical, epidemiological data, surveys and meta-regression modelling. Our results suggest that rates of depression in the general population might be 7 times higher during the COVID-19 outbreak. However, cautious is needed when interpreting these results, since the type of instruments and criteria used to ascertain depression might widely differ as well as the number of studies and countries included in the estimates. This is especially true for meta-analyses that combine data from studies using different assessment tools, such as diagnostic interviews and screening, self-reported tests. Levis et al. (2019), for example, found that prevalence estimates of depression based on rating tools were on average 14% greater than estimates based on diagnostic interviews. Previous meta-analysis reporting point prevalence rates of depression from epidemiological studies that used both symptoms scales and diagnostic tools, showed a global prevalence of depression of 4.70% (95% CI=4.40–5%) in 2010, when accounting for methodological differences (Ferrari et al., 2013). However, a meta-analysis combining data from 30 countries from 1994 and 2014 and using only community studies using self-reported instruments found a prevalence of 17.30% (95% CI=15-19.90%) (Lim et al., 2018). Thus, and despite methodological challenges when comparing results with previous data, our findings still suggest that the prevalence rate of depression during confinement and COVID-19 seems to have considerably increased.
The reported rates of depression in the general population during previous epidemic outbreaks (SARS and Ebola) are between 3% and 73.10% (Chew et al, 2020), and most of them are lower than the rate of depression during the COVID-19 outbreak we have identified here. These past epidemics were contained faster and, despite a higher mortality rate, infection rates were lower, which may explain the prevalence of lower rates of depressive symptoms (Huremović, 2019). Moreover, Hawryluck et al. described that the length and uncertainty of the lockdown contributed to higher levels of depression during the SARS outbreak in Canada (Hawryluck et al., 2004). Thus, the current lockdown measures imposed all around the world could also explain the higher rates of depressive symptoms observed during the COVID-19 outbreak.
Our study supports the need for integration of mental health considerations into COVID-19 care, including the monitoring of psychological symptoms and social needs within the general population (Pfefferbaum & North, 2020). Depression is a normal reaction to a sudden worsening in living circumstances, involving separation and uncertainty (Huremović, 2019). When people are exposed to uncontrollable events, they exhibit helplessness and lack of motivation, with depression as a consequence (Seligman, 1972). In this sense, subjects with depression are less prone to seek help either for physical or mental symptoms (Lei et al., 2020); thus, and similar to anxiety (Asmundson & Taylor, 2020), depression can become a barrier to rational medical and mental health interventions during pandemics (Wang, Pan, Wan, Tan, Xu, Ho et al., 2020). Mental health of the general population should be placed within the national and international public health agenda, with appropriate psychological support provided by governments or community agencies (Lei et al., 2020).
The papers we reviewed report associations between several variables and increased rates of depression in the general population. Associations with some variables, such as suspected COVID symptoms (Nguyen et al., 2020), having a contact infected by COVID (Mazza et al., 2020; Ni et al., 2020), fatality rates of COVID reported in the areas where respondents belong to (Ahmed et al., 2020), poorer self-rated health status (Gao et al., 2020; Lei et al., 2020; Wang, Di, et al. 2020), and/or history of chronic illness (Mazza et al., 2020; Shevlin et al., 2020; Wang, Di, et al. 2020) are expected. Additionally, increased rates of depression were consistently found to be associated with non-health related variables, such as younger ages (Ahmed et al., 2020; Gao et al., 2020; Huang & Zhao, 2020; Shevlin et al., 2020). In fact, some studies found higher rates of depression specifically among students (Lei et al., 2020; Wang, Pan, Wan, Tan, Xu, Ho et al., 2020). Young population could be more vulnerable to uncertainty about the future of jobs, careers and economic crisis (Kazmi et al., 2020) and they are also more exposed to social media. Interestingly, despite the fact that a regular update on health information related to COVID seems to decrease depression (Nguyen et al., 2020; Wang, Di, et al. 2020), it is also suggested that the exposure to social media is associated with depression (Ni et al., 2020) and mixed anxiety and depression (Gao et al., 2020). Social media can generate an immediate flooding of fear during the rapid spread of a disease, independently of real risk (Ofri, 2009) and fostered by popularity which is quickly reached by post with inaccurate information (“fake news”) (Sommariva et al., 2018). Socio-economic factors such as unemployment (Kazmi et al., 2020; Mazza et al., 2020), low social status (Nguyen et al., 2020), lack of social support (Ni et al., 2020) and economic losses (Lei et al., 2020) can also contribute to higher rates of depression.
Mazza et al. (2020) was the only study that focused on the influence of personality traits in depression rates during COVID-19 outbreak, reporting higher rates of depression in individuals that scored higher in negative affect and detachment. They also found higher vulnerability for individuals with a history of stressful situations (Mazza et al., 2020). The association between depression and anxiety was also frequently observed in two studies (Gao et al., 2020; Wang, Di, et al. 2020).
Depression that appears under these circumstances may rarely require pharmacological treatment, at least in the short term. COVID-19 outbreak and lockdown situation constitutes an extraordinary circumstance that requires significant personal and social adjustments. Thus, depression linked to this specific context could be best addressed with supportive interventions, such as reassurance and provision of accurate information, and by empowering individuals to make right decisions and helping them to establish an activity schedule to maintain a mental and physical equilibrium (Huremović, 2019). Moreover, it is suggested that healthy behaviors during the quarantine, such as having more physical activity and eating healthier, could also help counteract depression (Nguyen et al., 2020).
To our knowledge, this is the first systematic review of all available studies of depression in the general population during the COVID-19 outbreak, and the first one to implement meta-analytic procedures. Meta-analysis confers greater power than individual studies to estimate more accurate rates of depression, by considering a much larger population drawn from different countries.
However, limitations should be considered when interpreting our results. First, and due to the fact that studies were conducted during the COVID-19 outbreak, they had particular constraints. For example, randomization of the sample was not possible in some cases, and data had to be collected via online surveys, which might have introduced selection biases such as oversampling younger and more educated people (Wang, Di, et al. 2020). Second, we found that the use of different scales to assess depression was a major source of the heterogeneity. Third, the included studies were all cross-sectional, thus making difficult the establishment of casual associations between the pandemic and depression. Forth, the studies did not consider preexisting psychiatric conditions that might be related to higher risk of depression (Shigemura et al., 2020). Fifth, depression was not assessed at different stages of the epidemic and duration of quarantine was not considered into account. Only Wang, Pan, Wan, Tan, Xu, McIntyre, et al. 2020 investigated the psychological impact of COVID-19 during the initial outbreak and four weeks later, during the epidemic's peak, and found no significant difference in depression levels. Finally, our meta-analysis focuses on studies including general population. The impact of COVID-19 on the psychological wellbeing of vulnerable groups, such as health workers, outpatients or elderly people is expected to be high. Thus, future epidemiological studies conducted within these subpopulations as well as systematic reviews pooling the evidence are specially needed to adapt public health interventions.
Taking into account that the overall global prevalence of depressive disorders is estimated to be around 3.44%, our results seem to suggest that the proportion of depression in the general population is 7 times higher during the COVID-19 outbreak. This implies a substantial impact of the current pandemic situation on mental health that should be targeted by individual and population-level strategies. This evolving situation requires jointly efforts from the scientific community to contribute to the population surveillance during quarantine and the COVID-19 outbreak and to investigate the negative impact on psychological wellbeing in the short and long term. In this respect, new studies are continuing to be published and the number of them is expected to increase in the coming months (e.g., Brailovskaia & Margraf, 2021).
FundingThis work has been supported by grants from the Fondo de Investigación Sanitaria, Instituto de Salud Carlos III, Spanish Ministry of Economy and Competitiveness, Madrid, Spain [grants 94/1562, 97/1321E, 98/0103, 01/0255, 03/0815, 06/0617, G03/128 and 19/01874] and from the Fondo Europeo de Desarrollo Regional (FEDER) of the European Union and Gobierno de Aragón [grant B15_17R]. BO's work is supported by the PERIS program 2016-2020 “Ajuts per a la Incorporació de Científics i Tecnòlegs” [grant number SLT006/17/00066], with the support of the Health Department from the Generalitat de Catalunya.
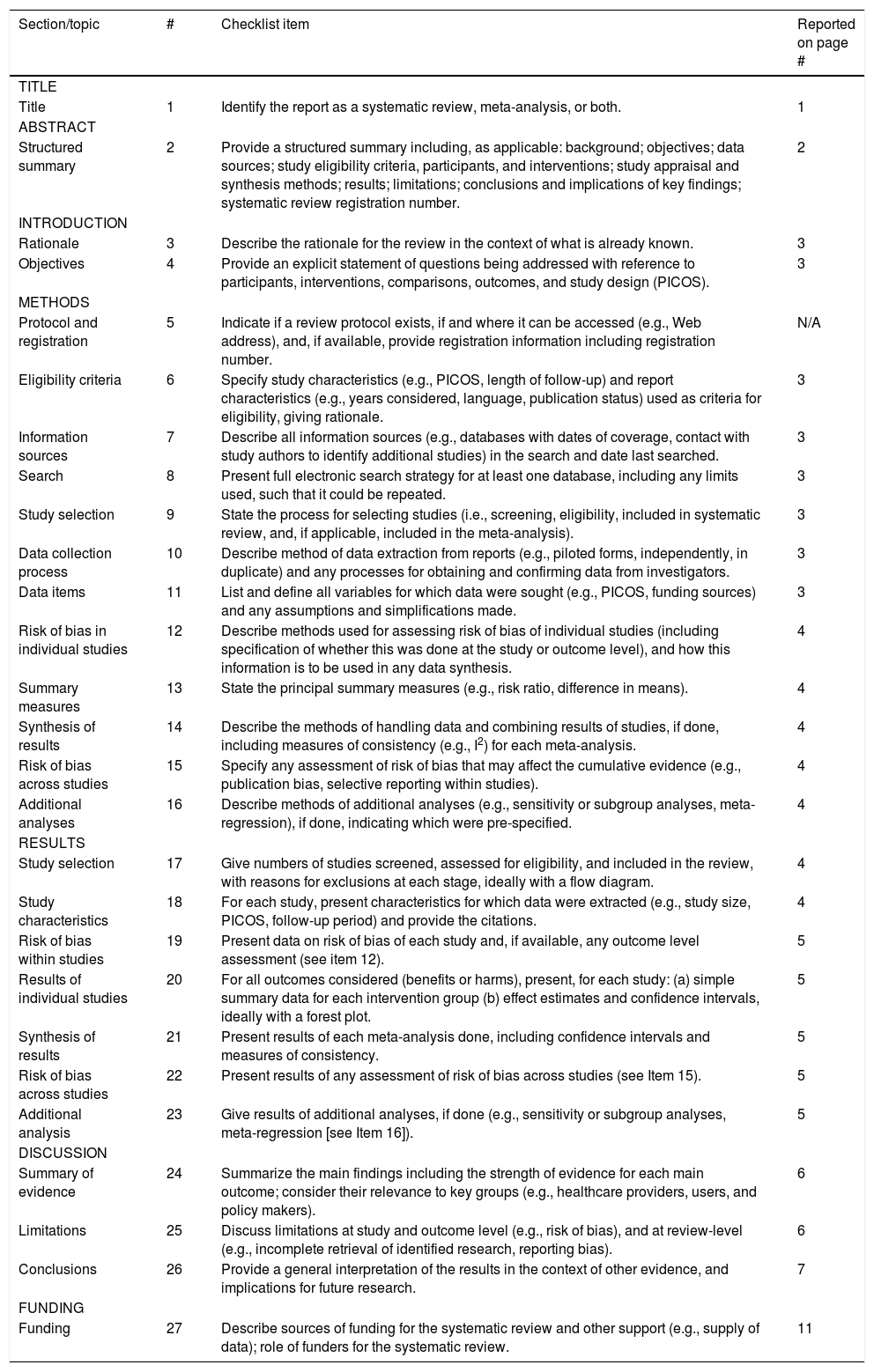
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both. | 1 |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number. | 2 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known. | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS). | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g., Web address), and, if available, provide registration information including registration number. | N/A |
| Eligibility criteria | 6 | Specify study characteristics (e.g., PICOS, length of follow-up) and report characteristics (e.g., years considered, language, publication status) used as criteria for eligibility, giving rationale. | 3 |
| Information sources | 7 | Describe all information sources (e.g., databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched. | 3 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated. | 3 |
| Study selection | 9 | State the process for selecting studies (i.e., screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis). | 3 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g., piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators. | 3 |
| Data items | 11 | List and define all variables for which data were sought (e.g., PICOS, funding sources) and any assumptions and simplifications made. | 3 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis. | 4 |
| Summary measures | 13 | State the principal summary measures (e.g., risk ratio, difference in means). | 4 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g., I2) for each meta-analysis. | 4 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g., publication bias, selective reporting within studies). | 4 |
| Additional analyses | 16 | Describe methods of additional analyses (e.g., sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified. | 4 |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram. | 4 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g., study size, PICOS, follow-up period) and provide the citations. | 4 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12). | 5 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot. | 5 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency. | 5 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15). | 5 |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g., sensitivity or subgroup analyses, meta-regression [see Item 16]). | 5 |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g., healthcare providers, users, and policy makers). | 6 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g., risk of bias), and at review-level (e.g., incomplete retrieval of identified research, reporting bias). | 6 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research. | 7 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g., supply of data); role of funders for the systematic review. | 11 |
| MEDLINE via PubMed: (covid or covid-19 OR coronavirus OR “corona virus” OR SARSCoV-2 OR “Coronavirus”[Mesh] OR “severe acute respiratory syndrome coronavirus 2”[Supplementary Concept] OR “COVID-19”[Supplementary Concept] OR “Coronavirus Infections/epidemiology”[Mesh] OR “Coronavirus Infections/prevention and control”[Mesh] OR “Coronavirus Infections/psychology”[Mesh] OR “Coronavirus Infections/statistics and numerical data”[Mesh]) AND (depression OR depressive OR “Depression”[Mesh] OR “Depressive Disorder”[Mesh] OR hypothimia) |
| Web of Science: ALL=(covid or covid-19 OR coronavirus OR “corona virus” OR SARSCoV-2 OR “severe acute respiratory syndrome coronavirus 2”) AND ALL=(depression OR depressive) |
| Quality scores (from 1 to 9) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | TOTAL |
| Ahmed et al. (2020) | N | N | Y | Y | Y | Y | Y | Y | U | 6 |
| Gao et al. (2020) | N | N | Y | Y | Y | Y | Y | Y | Y | 7 |
| Huang & Zhao (2020) | N | N | Y | Y | Y | Y | Y | Y | Y | 7 |
| Kazmi et al. (2020) | N | Y | Y | N | Y | Y | Y | Y | N | 6 |
| Lei et al. (2020) | N | N | Y | Y | Y | Y | Y | Y | Y | 7 |
| Mazza et al. (2020) | N | N | Y | Y | Y | Y | Y | Y | Y | 7 |
| Nguyen et al. (2020) | N | N | Y | Y | Y | Y | Y | Y | U | 6 |
| Ni et al. (2020) | N | N | Y | Y | Y | Y | Y | Y | U | 6 |
| Shevlin et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | U | 7 |
| Sonderskov et al. (2020) | Y | N | Y | Y | Y | Y | Y | Y | U | 7 |
| Wang, Pan, Wan, Tan, Xu, Ho et al. (2020) | N | N | Y | Y | Y | Y | Y | Y | Y | 7 |
| Wang, Di, et al. (2020) | N | N | Y | Y | Y | Y | Y | Y | Y | 7 |
Note: * Quality score based on the Joanna Briggs Institute (JBI) standardized critical appraisal instrument for prevalence studies (Moola et al., 2017). N: No; U: Unclear; Y: Yes; 1: Was the sample frame appropriate to address the target population?; 2: Were study participants recruited in an appropriate way?; 3: Was the sample size adequate?; 4: Were the study subjects and setting described in detail?; 5: Was data analysis conducted with sufficient coverage of the identified sample?; 6: Were valid methods used for the identification of the condition?; 7: Was the condition measured in a standard, reliable way for all participants?; 8: Was there appropriate statistical analysis?; 9: Was the response rate adequate, and if not, was the low response rate managed appropriately?