To examine subjective and objective sleep patterns in children with different Attention-Deficit/Hyperactivity Disorder (ADHD) presentations. Method: We assessed 92 children diagnosed with ADHD (29 ADHD-Inattentive [ADHD-I], 31 ADHD-Hyperactive/Impulsive [ADHD-H/I], and 32 ADHD-Combined [ADHD-C)]) aged 7–11 years. The Pediatric Sleep Questionnaire (PSQ), Pediatric Daytime Sleepiness Scale (PDSS), and a sleep diary were used as subjective sleep measures, and polysomnography was used to objectively assess sleep quantity, quality, and fragmentation. Results: Subjective data showed impaired sleep in 12.7% of the sample. No significant differences were found between ADHD presentations in any objective and subjective sleep variable. Nevertheless, data on sleep fragmentation suggested a worse sleep continuity for the ADHD-H/I group, and correlation analyses confirmed that sleep is affected by age. Conclusions: Children with ADHD may suffer from sleep breathing problems and daytime sleepiness, as reported by their parents, even when their total sleep time and sleep efficiency are not affected. It seems that sleep in this population does not largely vary as a function of the ADHD presentation. Sleep in children with ADHD evolves with age.
Examinar los patrones de sueño subjetivos y objetivos de niños con diferentes presentaciones del Trastorno por déficit de atención e hiperactividad (TDAH). Método: Se evaluaron 92 niños (29 TDAH-inatento [TDAH-I)], 31 TDAH-hiperactivo/impulsivo [TDAH-H/I] y 32 TDAH-combinado [TDAH-C]) de entre 7–11 años. El Pediatric Sleep Questionnaire (PSQ), la Pediatric Daytime Sleepiness Scale (PDSS) y un diario de sueño se utilizaron como medidas subjetivas de sueño. Para evaluar objetivamente la cantidad, calidad y fragmentación del sueño se utilizó la polisomnografía. Resultados: Los datos subjetivos mostraron alteraciones del sueño en el 12,7% de la muestra. No se observaron diferencias significativas en ninguna variable objetiva y subjetiva de sueño entre las diferentes presentaciones de TDAH. No obstante, los datos de fragmentación de sueño sugirieron una peor continuidad en el grupo con TDAH-H/I, y los análisis correlacionales confirmaron que el sueño se ve afectado por la edad. Conclusiones: Los niños con TDAH pueden experimentar problemas respiratorios durante el sueño y somnolencia diurna, según lo informado por sus padres, incluso cuando su tiempo total y eficiencia de sueño no se vean afectados. Parece que el sueño en el TDAH no varía en función de la presentación. El sueño en los niños con TDAH evoluciona con la edad.
Attention deficit hyperactivity disorder (ADHD) is one of the most prevalent neurodevelopmental disorders in children, affecting about 3.4% of the world population (Polanczyk, Salum, Sugaya, Caye, & Rohde, 2015). It can be classified into three clinical presentations: Inattentive (ADHD-I), hyperactive/impulsive (ADHD-H/I), and combined (ADHD-C) (American Psychiatric Association, 2013), which can be detected through the combined use of different assessment measures (see Morales-Hidalgo, Hernández-Martínez, Vera, Voltas, & Canals, 2017; Rodríguez, Areces, García, Cueli, & González-Castro, 2018). This disorder has been associated with numerous comorbid conditions (Barkley, 2006; Tung et al., 2016), including sleep problems (see reviews of Díaz-Román, Hita-Yáñez, & Buela-Casal, 2016; Hvolby, 2015), as well as with deficits in executive functioning (Krieger, Amador-Campos, & Peró-Cebollero, 2019).
According to previous studies, sleep in children with ADHD is characterized by a lower sleep quality and lower sleep efficiency than healthy children. These children may also present sleep breathing disorders and periodic limb movements, sleep fragmentation, and higher levels of daytime sleepiness compared to children without ADHD (see reviews of Cortese, Faraone, Konofal, & Lecendreux, 2009; Díaz-Román et al., 2016).
Sleep problems in children with ADHD are associated with a worse neurocognitive functioning (Frye et al., 2018) and with emotional and behavioral conditions, such as oppositional defiant disorder and depressive symptoms (Becker, Cusick, Sidol, Epstein, & Tamm, 2018). Consequently, investigating their sleep characteristics may help to improve the neurocognitive functioning and mental health of these children, thus preventing potential emotional and behavioral problems already noted in general populations (Kazdin et al., 2018; Teismann et al., 2018). In this relation, sleep problems have been explored focusing on factors such as sex, age, comorbidity with other disorders, and pharmacological treatment (Becker et al., 2018; Vigliano et al., 2016). Nevertheless, the results of these studies have been inconsistent (see Cortese et al., 2009; Díaz-Román et al., 2016, for reviews).
Discrepancies in previous findings on sleep in ADHD should be interpreted in the light of some differences across studies. Firstly, while some studies have recorded sleep information through objective measures (i.e., Polysomnography [PSG] and actigraphy), other studies have used questionnaires based on parent or child reports and self-reports (see Cortese et al., 2009, for a review). Only few studies have used both objective and subjective measures to assess sleep in this population (Huang et al., 2004; Wiebe, Carrier, Frenette, & Gruber, 2013; Wiggs, Montgomery, & Stores, 2005), even if the use of a single instrument may be not enough to achieve a full understanding of the complex interactions between sleep and ADHD (Hvolby, 2015). Secondly, the notable heterogeneity within the disorder remarks the importance of investigating sleep problems accounting for the different ADHD presentations, but there are few studies in this regards and their results are inconsistent. Some studies have reported differences in some sleep features as a function of the presentation, such as more presence of chronic snoring for ADHD-H/I than ADHD-C, shorter sleep latencies, and more fragmented sleep for ADHD-H/I than in other two presentations (LeBourgeois, Avis, Mixon, Olmi, & Harsh, 2004; Ramos, Vela, Espinar, & Kales, 1990). In addition, greater daytime sleepiness (LeBourgeois et al., 2004; Mayes et al., 2009) or hypersomnia symptoms like daytime inadvertent napping (Chiang et al., 2010) and shorter sleep-onset latencies (Lecendreux, Konofal, Bouvard, Falissard, & Mouren-Simeoni, 2000) have been found to be grater in ADHD-I than in the other presentations. However, other studies have failed to show any difference in sleep characteristics between ADHD presentations (Virring, Lambek, Thomsen, Møller, & Jennum, 2016; Wiggs et al., 2005).
Therefore, the goal of this study was to examine sleep characteristics in three different groups of children with ADHD (ADHD-I, ADHD-H/I, ADHD-C) through objective (i.e PSG) and subjective (i.e., sleep diary and questionnaires) measures. Considering previous findings (LeBourgeois et al., 2004), we expected to find higher scores in snoring variable for the ADHD-H/I group and a higher daytime sleepiness for the group ADHD-I in parents’ questionnaires. We also expected to observe more fragmented sleep and less sleep efficiency in the ADHD-H/I group compared to the other two groups (Ramos et al., 1990).
MethodParticipantsThe sample consisted of 92 children with ADHD from Granada, Spain (29 ADHD-I, 31 ADHD-H/I and 32 ADHD-C) aged 7-11 years (Table 1). The sample was recruited with the collaboration of public and private schools and with the cooperation of the Child and Youth Mental Health Department of the Virgen de las Nieves Hospital Center, Granada. The parent(s) of each participant gave informed written consent before the start of the study and actively participated by completing questionnaires on the subjective quality of sleep and daytime sleepiness. The study was evaluated and approved by the ethics committee of the University of Granada and the Andalusian Biomedical Research Ethics, Coordinating Committee. The anonymity of the participants and the confidentiality of the data were guaranteed.
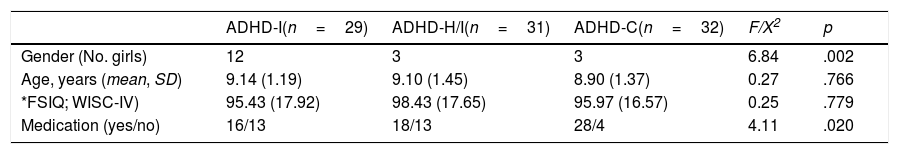
Sociodemographic Data of the Total Sample (N=92).
| ADHD-I(n=29) | ADHD-H/I(n=31) | ADHD-C(n=32) | F/X2 | p | |
|---|---|---|---|---|---|
| Gender (No. girls) | 12 | 3 | 3 | 6.84 | .002 |
| Age, years (mean, SD) | 9.14 (1.19) | 9.10 (1.45) | 8.90 (1.37) | 0.27 | .766 |
| *FSIQ; WISC-IV) | 95.43 (17.92) | 98.43 (17.65) | 95.97 (16.57) | 0.25 | .779 |
| Medication (yes/no) | 16/13 | 18/13 | 28/4 | 4.11 | .020 |
Note. ADHD-I=Attention Deficit Hyperactivity Disorder inattentive presentation; ADHD-H/I=Attention Deficit Hyperactivity Disorder hyperactive/impulsive presentation; ADHD-C=Attention Deficit Hyperactivity Disorder combined presentation; FSIQ=Full Scale Intelligence Quotient; WISC-IV=Wechsler Intelligence Scale for Children. *Scores in FSIQ WISC-IV were no obtained for one ADHD-I and one ADHD-C children.
The participants had a clinical diagnosis of ADHD as primary disorder, established by a healthcare professional according to the criteria of the International Classification of Diseases 10th revision (ICD-10; World Health Organization, 1992). Psycho-educational reports provided by the educational psychologist of children’ school were considered equivalent to clinical diagnosis when children had been registered as children with ADHD into the SENECA platform of the Spanish Ministry of Education and Science (Spanish Organic Law 8/2013, 9th December, for the improvement of the educational quality [Ley Orgánica 8/2013, de 9 de diciembre, para la mejora de la calidad educativa]). Specifically, children with ADHD can be considered children with ‘specific educational support needs’ with no significant curricular adaptation. Eighty-eight percent of the participants had a clinical diagnosis, and the rest of children were registered into SENECA as meeting criteria for ADHD in school environment, waiting for clinical assessment. We did not include any child with a total score less than 70 in the Wechsler Intelligence Scale for Children–Four Edition (WISC-IV; Wechsler, 2010), so three children were excluded from the sample. This score was not obtained for two children but they remained in the study since it was explicitly reported that they have no significant curricular adaptation for intellectual disability. Other exclusion criteria were having been diagnosed with any severe organic disease with sleep impairment (including severe respiratory-related diseases); having sensory deficits and/or generalized developmental disorders; having a body mass index>30.
Instruments and measuresSocio-demographic data was obtained through a non-structured interview for parents. Data related to exclusion criteria, age, gender, diagnosis, and medical consume were obtained in this interview.
Pediatric Sleep Questionnaire (PSQ)The PSQ (Tomás, Miralles, & Beseler, 2007) is a questionnaire compiled by parents and it allows detecting sleep-disordered breathing (α=.81), sleepiness (α=.63), and behavioral problems (α=.86). It consists of 22 items divided into three parts and its response format into the first two parts (α part A=.81; α part B=.63) is yes/no/do not know and the third part (α part C=.86) from 0 (never) to 3 (most of the time). A higher PSQ score means worse sleep quality, and it has been clinically validated (cutoff score≥.33 reflects clinical problems; Chervin, Hedger, Dillon, & Pituch, 2000).
Pediatric Daytime Sleepiness Scale (PDSS)The PDSS is a questionnaire filled in by parents to assess the child's level of daytime sleepiness and related school outcomes (α=.80). It consists of 8 items with a response scale from 0 (never) to 4 (always). The last item is inverse and the global score goes from 0–32 (higher score, higher levels of sleepiness). A score >20 indicates clinical levels of excessive daytime sleepiness (Drake et al., 2003).
Sleep diaryAn 8-items sleep log was compiled by children’s parents during seven days to collect sleep habits of the child. Specifically, we collected information about bedtime and wake time, total sleep time (TST), number of awakenings and sleep efficiency.
PSGA PSG recording was performed at the children’s home, using SomnoScreen® Plus. The recording electrodes were placed following the 10-20 International System: Electroencephalography (five channels: two frontals (Fpz, Fz), one central (Cz), one parietal (Pz), one occipital (Oz)) and the left auricular as a reference (A1); electrooculogram (from the left and right outer canthi of the eye); electromyogram (EMG) in submentalis muscle; EMG of the right and left anterior tibial muscles. We recorded the sleep breathing pattern with nasal airflow thermistors and thoracic and abdominal respiratory effort calibrator. Oxygen saturation was also evaluated with pulse oximetry. Signals were sampled at 256Hz and stored for further analysis with DOMINO light version 10.04, and the sleep phases were scored in 30-second epochs following the criteria of Rechtschaffen and Kales (1968). Two trained researchers performed the sleep scoring independently.
The following sleep parameters were computed: Time in bed (TIB, time from lights off to lights on), sleep period time (SPT, time from sleep onset to final awakening), TST (total time spent in a sleep stage), sleep onset latency (time from wakefulness to stage 1 sleep), sleep efficiency (TST/TIB×100), proportion of the TST spent in each sleep stage (including slow wave sleep (SWS), i.e. stages 3+4 sleep), REM latency (time spent from the start of sleeping and the start of REM sleep), arousal index (frequency of arousals per hour of sleep; number of rapid changes in the electroencephalography frequency of 3 or more seconds and preceded by a minimum of 10 continuous seconds of sleep), and index of periodic limb movements (number of periodic limb movements per hour of sleep). In addition, we extracted information about sleep continuity (number of awakenings/hour <2min), sleep stability (number of stage shifts, considering stages 3 and 4 sleep as a single stage), and sleep organization (number of sleep cycles, not interrupted by periods >2min of wake/stage 1 sleep) (see Conte et al., 2014, for further information about these constructs).
Epochs containing technical artifacts or extremely high muscle activity causing saturation of amplifiers were carefully detected and marked for their exclusion from the analysis.
ProcedureFirstly, parents gave written consent for their children to participate in the study, conducted the interview on sociodemographic data and were administered questionnaires on subjective sleep quality and daytime sleepiness. The intelligence test was carried out as long as it was not already available (maximum two years before). This first phase of the procedure was used to assess the inclusion criteria of participants.
In the second phase, we performed the PSG recordings at the participants’ home. Consumption of stimulant beverages (i.e. caffeinated) during the day of the PSG was not allowed. Consume of methylphenidate, atomoxetine, guanfacine, and lisdexamphetamine was controlled as specific medication for ADHD (and it was withdrawn 36hours before the sleep study).
Statistical analysisThe sample size was estimated using d=0.34 (it corresponded to t=3.3; Mayes et al., 2009), α=.05, power=.80. Based on these data, the required estimated sample was 87 participants.
Analysis of variance (ANOVA) and chi-square test were performed for age and sex (respectively) group comparisons. T-student analyses were performed to compare possible differences in sleep variables between children with and without medication. Scores on PSQ and PDSS, and sleep parameters measured through PSG were compared with one factor ANOVA. Independence of observations was verified through Durbin-Watson statistic, the normality of the distribution by Kolmogorov-Smirnov, and the homogeneity of the variances using the Levene Test. No significant violations were observed in any of the variables. Also, Mahalanobis distance was used, not detecting any outlier. Whenever statistically significant differences were observed in any analysis, other comparisons were made with Fisher's least-significant difference (LSD) test.
Furthermore, using a Bayesian approach (Wagenmakers, 2007), we estimated the probability that the alternative hypothesis (Pr (H1|D) be true as a function of the approximate Bayes Factor (BF10), with largeBF10 values supporting the alternative hypothesis (H1), and small BF10 values supporting the null hypothesis (H0). The Bayesian analysis was implemented using JASP 0.9.1 (JASP Team, 2018).
ResultsSample descriptionTable 1 presents the demographics and medication data for the three groups. A higher proportion of males were noted in the three ADHD presentations (p<.05). There were no statistical differences in age and full-scale IQ. There were significant differences in the medication use (p<.05) between the three groups, with a higher medication use among children with the ADHD-C presentation. However, when compared children with and without medication, no significant results were found in any sleep variable (all p-values≥.09).
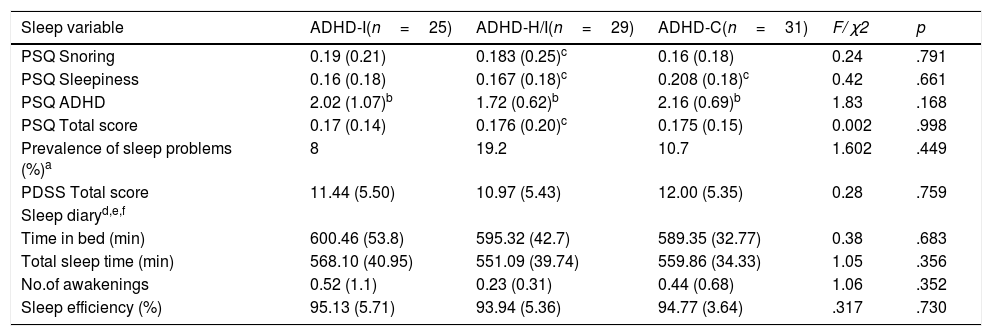
Sleep descriptionSubjective measuresTable 2 summarizes the subjective measures data analysis. Data obtained with PSQ questionnaire revealed no significant differences in snoring, daytime sleepiness, ADHD symptomatology, or total score between the three groups, as also shown by the Bayesian analysis (all the posterior probabilities being lower than Pr (H1|D)≈14% and all BF10s<0.16). According to the PSQ snoring scores, 17.7% of the sample presented problems, with the inattentive presentation obtaining the highest percentage (20.8%). In the PSQ sleepiness scores, it was the 17.4% of the sample who exhibited problems, and the ADHD-H/I presentation showed the highest scores (20%). The total score of PSQ in the sample was 12.7%, with the ADHD-H/I presentation showing the higher frequency of sleep problems. No differences between the three ADHD presentations were observed for the PDSS score (Pr (H1|D)≈15% and all BF10≈0.17). Nevertheless, the ADHD-C presentation showed the higher scores. Similarly, no differences were found in the sleep diary parameters (Pr (H1|D<21% and all BF10s<0.26). Overall, subjective measures did not reveal any difference between ADHD presentations.
Total scores and subscale scores on PSQ, PDSS, and sleep diary completed by parents in the ADHD-I, ADHD-H/I and ADHD-C groups (N=85).
| Sleep variable | ADHD-I(n=25) | ADHD-H/I(n=29) | ADHD-C(n=31) | F/ χ2 | p |
|---|---|---|---|---|---|
| PSQ Snoring | 0.19 (0.21) | 0.183 (0.25)c | 0.16 (0.18) | 0.24 | .791 |
| PSQ Sleepiness | 0.16 (0.18) | 0.167 (0.18)c | 0.208 (0.18)c | 0.42 | .661 |
| PSQ ADHD | 2.02 (1.07)b | 1.72 (0.62)b | 2.16 (0.69)b | 1.83 | .168 |
| PSQ Total score | 0.17 (0.14) | 0.176 (0.20)c | 0.175 (0.15) | 0.002 | .998 |
| Prevalence of sleep problems (%)a | 8 | 19.2 | 10.7 | 1.602 | .449 |
| PDSS Total score | 11.44 (5.50) | 10.97 (5.43) | 12.00 (5.35) | 0.28 | .759 |
| Sleep diaryd,e,f | |||||
| Time in bed (min) | 600.46 (53.8) | 595.32 (42.7) | 589.35 (32.77) | 0.38 | .683 |
| Total sleep time (min) | 568.10 (40.95) | 551.09 (39.74) | 559.86 (34.33) | 1.05 | .356 |
| No.of awakenings | 0.52 (1.1) | 0.23 (0.31) | 0.44 (0.68) | 1.06 | .352 |
| Sleep efficiency (%) | 95.13 (5.71) | 93.94 (5.36) | 94.77 (3.64) | .317 | .730 |
Note. ADHD-I=Attention Deficit Hyperactivity Disorder, inattentive subtype; ADHD-H/I=ADHD hyperactive/impulsive subtype; ADHD-C=ADHD combined subtype; PSQ=Pediatric Sleep Questionnaire; PDSS=Pediatric Daytime Sleepiness Scale. Data are presented as mean and (standard deviation).
ADHD-H/I sample for snoring values was 27, for sleepiness was 26 and 27 for the total score; ADHD-C sample for the sleepiness score was 23.
ADHD-I sample for Time in bed was 20, for total sleep time 20, for No. of awakenings was 29, and for sleep efficiency was 18.
In Table 3, we present the means and SDs of the standard quantitative PSG measures of children with ADHD. Significant differences were not observed for any sleep variable. No differences between objective sleep parameters seem to be present between ADHD presentations. These observations were further corroborated by the Bayesian approach, which showed no support for the H1 hypothesis (i.e., all the posterior probabilities being lower than Pr (H1|D)≈34% and all BF10s<0.52).
Quantitative Sleep Parameters Obtained by Polysomnography.
| Sleep variable | ADHD-I(n=29) | ADHD-H/I(n=30) | ADHD-C(n=31) | F | p |
|---|---|---|---|---|---|
| Time in bed (min) | 550.78 (58.32) | 555.25 (59.11) | 554.04 (61.47) | 0.04 | .957 |
| Sleep period time (min) | 531.49 (60.20) | 530.48 (56.92) | 528.11 (58.59) | 0.03 | .974 |
| Total sleep time (min) | 508.70 (54.87) | 501.48 (57.13) | 485.42 (105.02) | 0.73 | .484 |
| Sleep onset latency (min) | 15.05 (7.78) | 18.65 (10.48) | 18.60 (14.35) | 0.99 | .377 |
| Sleep efficiency (%) | 92.39 (3.24) | 90.76 (4.62) | 90.79 (6.03) | 1.11 | .334 |
| Wake time (%) | 2.72 (2.77) | 3.82 (3.73) | 4.41 (5.78) | 1.18 | .312 |
| Stage 1 (%) | 2.20 (2.09) | 1.95 (1.75) | 4.12 (8.00) | 1.75 | .179 |
| Stage 2 (%) | 35.98 (5.99) | 36.35 (8.40) | 35.44 (8.35) | 0.11 | .897 |
| Stage 3 (%) | 13.83 (5.28) | 12.66 (4.62) | 13.66 (6.99) | 0.36 | .699 |
| Stage 4 (%) | 22.72 (4.42) | 24.61 (7.44) | 24.01 (5.16) | 0.81 | .448 |
| Slow wave sleep (%) | 36.56 (8.02) | 37.29 (10.00) | 37.68 (8.12) | 0.00 | .995 |
| REM (%) | 25.09 (6.40) | 24.37 (6.15) | 23.47 (5.23) | 0.56 | .572 |
| REM latency (min) | 149.13 (63.68) | 142.64(48.44) | 151.13 (56.99) | 0.18 | .835 |
| Arousal (index) | 0.62 (0.64) | 0.53 (0.92) | 1.31 (2.54) | 2.08 | .131 |
| Periodic limb movements (index) | 0.64 (1.20) | 0.63 (1.24) | 0.81 (1.26) | 0.21 | .812 |
Note. ADHD-I=Attention Deficit Hyperactivity Disorder, inattentive subtype; ADHD-H/I=ADHD hyperactive/impulsive subtype; ADHD-C=ADHD combined subtype; REM=rapid eye movement. Data are presented as mean and (standard deviation).
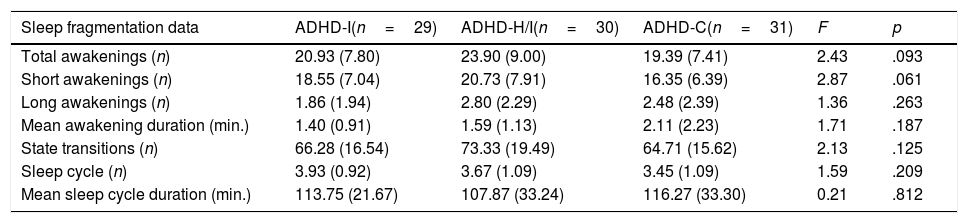
Overall, no significant differences were observed for sleep continuity variables. However, the data suggests that ADHD-H/I tended to have a more fragmented sleep as indexed by the higher number of awakenings (F2,87=2.44, p=.093, η2p=.05, Pr (H1|D)≈41%, BF10=0.68; Table 4). This effect was mainly driven by the number of short awakenings (less than 2min; F2,87=2.87, p=.062, η2p=.06, Pr (H1|D)≈49%, BF10=0.96) with a higher number of awakenings in the ADHD-H/I group compared to the ADHD-C (p=.018, BF10=1.73) and, not significantly, to the ADHD-I (p=.243, BF10=0.34). Instead, no differences were observed for the number of long awakenings (longer than 2min; F2,87=1.36, p=.263, η2p=.03, Pr (H1|D)≈22%, BF10=0.29) and for the average duration of the awakenings F2,87=1.71, p=.187, η2p=.04, Pr (H1|D)≈28%, BF10=0.38).
Sleep Continuity, Stability, and Organization.
| Sleep fragmentation data | ADHD-I(n=29) | ADHD-H/I(n=30) | ADHD-C(n=31) | F | p |
|---|---|---|---|---|---|
| Total awakenings (n) | 20.93 (7.80) | 23.90 (9.00) | 19.39 (7.41) | 2.43 | .093 |
| Short awakenings (n) | 18.55 (7.04) | 20.73 (7.91) | 16.35 (6.39) | 2.87 | .061 |
| Long awakenings (n) | 1.86 (1.94) | 2.80 (2.29) | 2.48 (2.39) | 1.36 | .263 |
| Mean awakening duration (min.) | 1.40 (0.91) | 1.59 (1.13) | 2.11 (2.23) | 1.71 | .187 |
| State transitions (n) | 66.28 (16.54) | 73.33 (19.49) | 64.71 (15.62) | 2.13 | .125 |
| Sleep cycle (n) | 3.93 (0.92) | 3.67 (1.09) | 3.45 (1.09) | 1.59 | .209 |
| Mean sleep cycle duration (min.) | 113.75 (21.67) | 107.87 (33.24) | 116.27 (33.30) | 0.21 | .812 |
Note. ADHD-I=Attention Deficit Hyperactivity Disorder, inattentive subtype; ADHD-H/I=ADHD hyperactive/impulsive subtype; ADHD-C=ADHD combined subtype. Data are presented as mean and (standard deviation).
No significant differences were observed for sleep stability. However, again the ADHD-H/I showed, although not significantly, a higher number of state transitions (F2,87=2.13, p=.125, η2p=.05, Pr (H1|D)≈35%, BF10=0.54, Table 4).
Sleep organizationNo significant differences were observed for sleep organization variables. Sleep cycles, here operationalized as an interrupted sequence (interleaved by a maximum of 2 continuous min of wake) of NREM-REM epochs lasting at least 10min each max, were 3.8±1.05 and lasted on average 112.66±29.90min (Table 4).
[Table 4 could be inserted here].
Exploratory correlationsSleep diary vs PSGSignificant associations were observed for the TIB score obtained by both PSG and diary (r=.83, p<.001, n=71) suggesting that both measures are efficient for estimate this. Also, we found correlation between number of awakenings and sleep efficiency obtained with the sleep diary (r=−.26, p<.04, n=63). However, we found no associations between the number of awakenings measured with the sleep diary and the time spent awake (r=−.41, p=.71, n=84) or the number of long awakenings measured with the PSG (r=.09, p=.38, n=84), and between sleep efficiency obtained through both measures (r=−.08, p=.52, n=64), suggesting that sleep diary can provide limited information about the sleep quality of the children of the current study.
Age and sleep parametersWe confirmed in our sample the inverse relationship between age and the proportion of SWS (r=−.23, p=.003, n=90). Interestingly, with increase age we also observed an increment of the number of awakenings (r=.22, p=.032, n=90), again driven by the number of short awakenings (r=.28, p=.007, n=90), and a concurrent reduction of their durations (r=.34, p=.001, n=90).
DiscussionThe present study investigated whether children diagnosed with attention-deficit/hyperactivity disorder (ADHD) experience different sleep patterns depending on the ADHD symptoms. We observed no differences in any sleep parameter between the three ADHD presentations. Medication did not affect these results, as no significant differences were either found in sleep variables between medicated and non-medicated children.
The lack of sleep differences, as assessed by the PSG, are in line with studies indicating a lack of differences in the sleep architecture between children with different ADHD presentations (Virring et al., 2016; Wiggs et al., 2005). Interestingly, although the study of Lecendreux et al. (2000) reported shorter sleep onset latencies for children with ADHD-H/I, the authors also showed no differences in nocturnal sleep between the groups, in line with the current results. Results on sleep fragmentation also revealed no significant differences for both sleep organization and sleep stability in ADHD presentations. However, the data obtained for sleep continuity indicated a tendency of the ADHD-H/I group to experience more short awakenings than the ADHD-C group, which is consistent with two previous studies (Becker et al., 2018; Ramos et al., 1990). This latter result is also in line with studies linking the presence of hyperactive-impulsive symptoms to more disrupted sleep (Chiang et al., 2010; Mayes et al., 2009).
Regarding to parents’ reports, on the one hand the lack of significant differences between ADHD presentations in terms of daytime sleepiness, sleep problems, TIB, TST, number of awakenings, or sleep efficiency are consistent with previous studies reporting no differences in subjective sleep measures between ADHD presentations (Virring, Lambek, Jørgen, Ruge, & Hove, 2017; Wiggs et al., 2005). On the other hand, the PDSS results seem to contradict the previously reported link between sleepiness and ADHD-I (LeBourgeois et al., 2004; Lecendreux et al., 2000; & Mayes et al., 2009). In addition, parents reported a high percentage of sleep-related breathing disorders (17.7%) among these children, as previously noted in research (Cortese et al., 2009), and without meaningful differences between presentations. Finally, our results in terms of general sleep problems are also inconsistent with prior research. Specifically, parents’ ratings in the PSQ indicated that 13% of the sample was suffering from sleep problems. Moreover, children with ADHD-H/I showed the highest percentage of sleep problems in our study, though non-significant, whereas the results of the study of Mayes et al. (2009)—including a larger sample of children—pointed to children with ADHD-C as those who have the greatest sleep problems.
The lack of significant differences in sleep patterns between ADHD presentations in our sample might be also explained by commonalities in symptomatology. In this respect, the results of some studies noted a relationship between severity of ADHD symptomatology and sleep disturbances (Hysing, Lundervold, Posserud, & Sivertsen, 2016; Vélez-Galarraga, Guillén-Grima, Crespo-Eguílaz, & Sánchez-Carpintero, 2016). However, no significant differences in severity of ADHD symptomatology, as reported by children’s parents using the PSQ, were revealed between the three groups of children in our study. Consequently, the absence of differences in sleep variables might be derived from the similar degree of symptoms between the groups.
Results obtained through correlations indicate a low agreement between measures for assessing sleep in ADHD, which also supports the notion that objective and subjective sleep measures actually assess different constructs and generate a high inconsistency between their results (Cortese et al., 2009). However, data related to age confirmed that sleep naturally evolves with age also in children with ADHD. Specifically, a decrease in the amount of SWS and an increase in the number of awakenings are observed with age, which is consistent with previous research (Schwarz et al., 2017).
Interestingly, children’s subjective and objective sleep efficiency was not affected in our sample (>90%), in contrast with most of the findings present in research (see Cortese et al., 2009, for a review). In addition, TST of children in this study was within the range suggested as appropriate (7–12hours) according to the recommendations of the National Sleep Foundation (Hirshkowitz et al., 2015). Finally, the percentage of sleep problems measured by PSQ (13% of the total sample) was lower that what previously reported (25% to 50%; Corkum, Tannock, & Moldofsky, 1998).
The current results should be interpreted in light of some limitations. Firstly, we collected the sleep diary data for one week instead of 15 days, as recommended by Bioulac, Micoulaud-Franchi, and Philip, (2015). Additionally, objective sleep assessment was based on a single night, so it is possible that some differences between ADHD presentations would have not been captured by a single PSG recording (Hvolby, 2015).
In conclusion, there seem to be no differences in subjective or objective sleep variables between ADHD presentations. Discrepancies with respect to previous studies reporting such differences may be due to factors such as age (e.g. LeBourgeois et al., 2004; Lecendreux et al., 2000), or diagnostic criteria (e.g. Ramos et al., 1990). Therefore, further research is needed to disentangle the complex relationship between sleep and ADHD symptoms, and to resolve the discrepancies arisen in this regard.
FundingThis work was supported by the Spanish Ministry of Economy and Competitiveness [PSI2014-58046-P].