In the last decade, socio-political violence in Colombia (South America) has created an environment of extreme/chronic stress. In this study, brain imaging technology (fMRI) and behavioral task performance were used to measure potential deficits in executive functioning for emotional processing in Colombian children.
MethodParticipants (22 Post-Traumatic Stress Disorder, PTSD and 22 neurotypical, NT) were asked to perform a word task with implicit emotional salience, which required them to report the color of the ink in which a positive, negative or neutral word was printed.
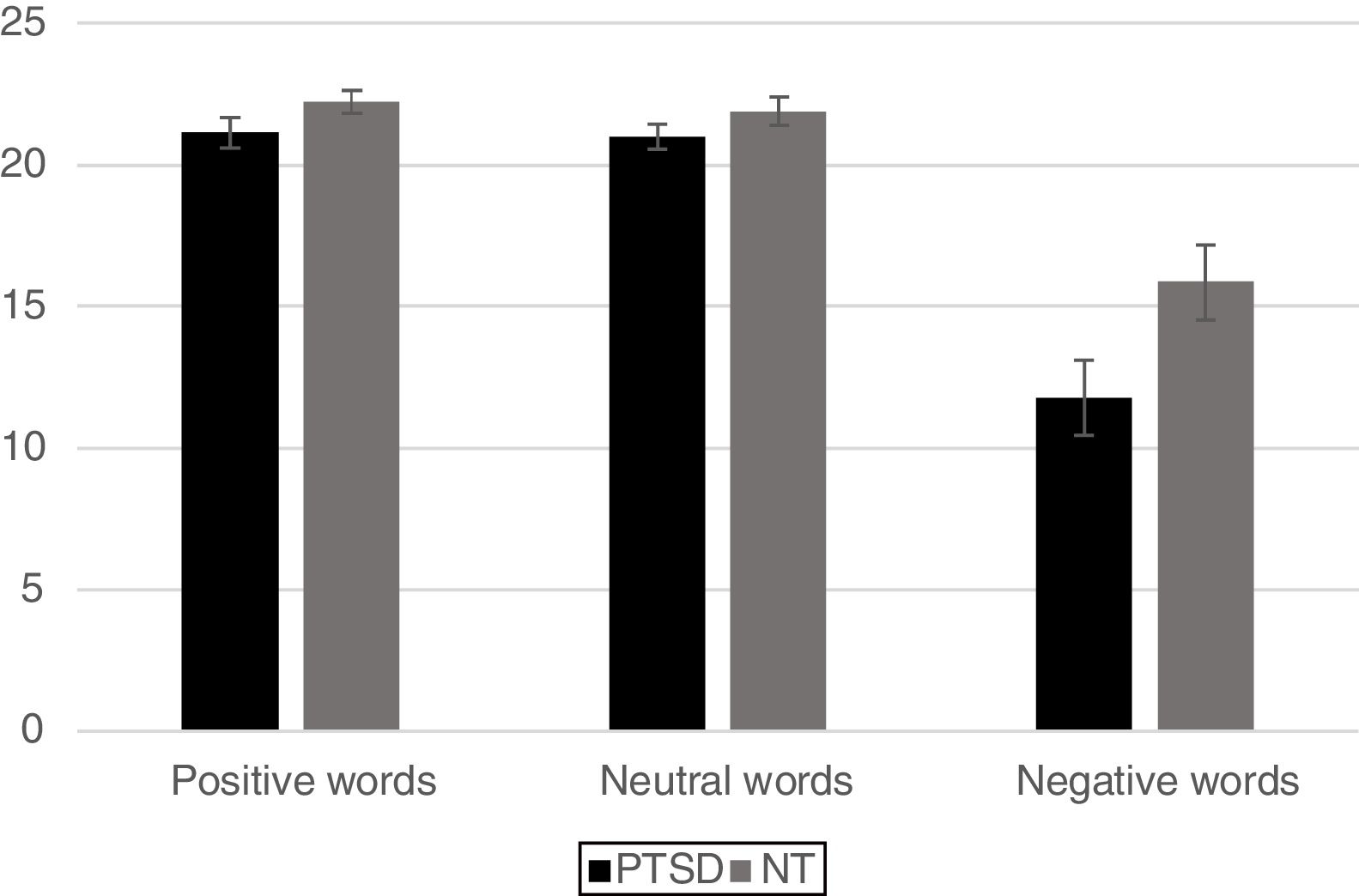
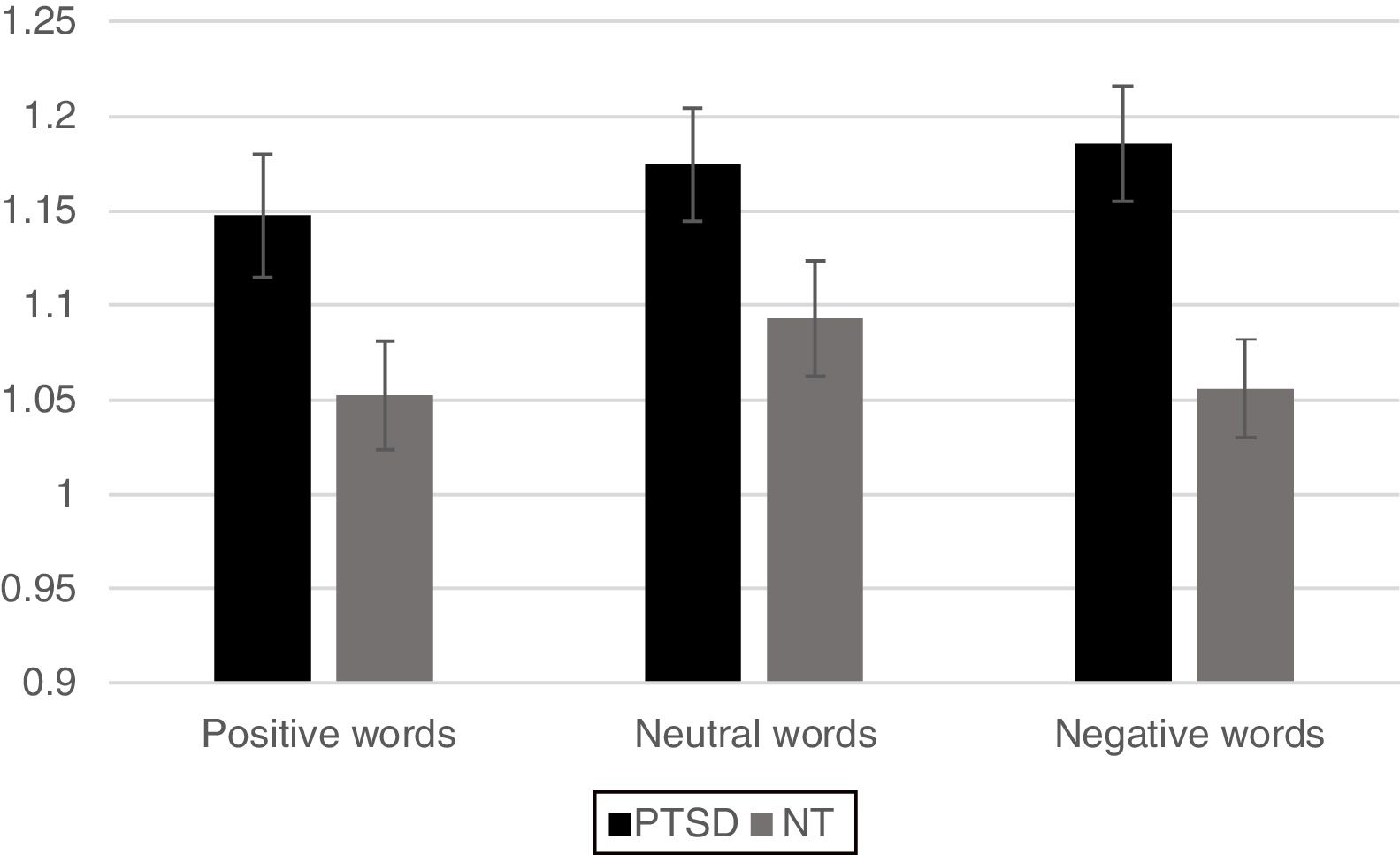
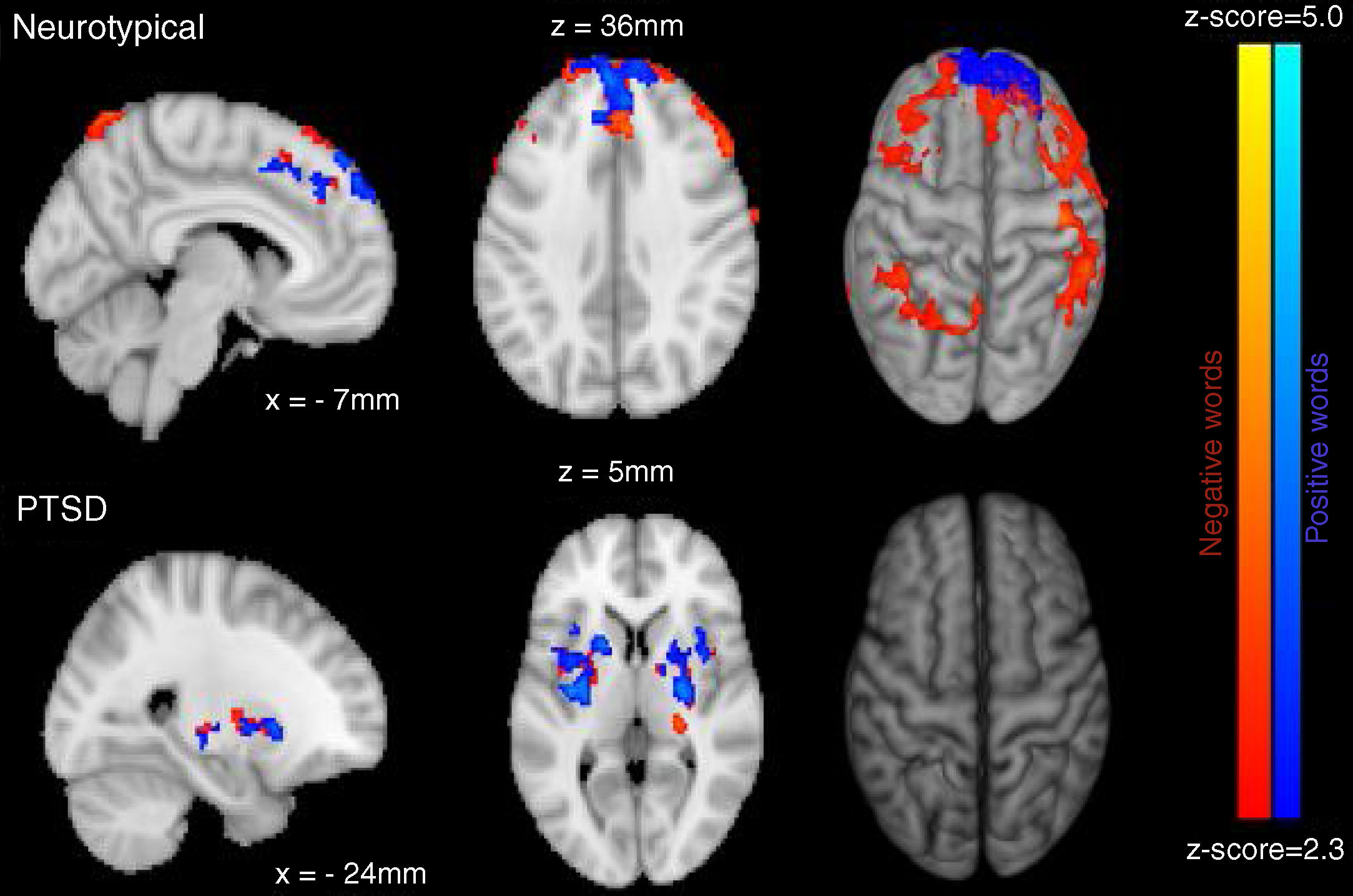
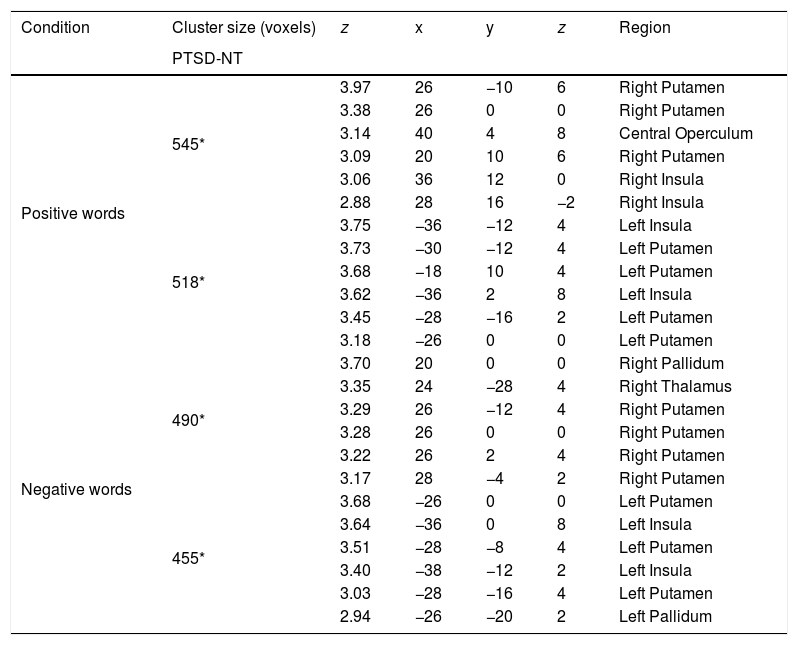
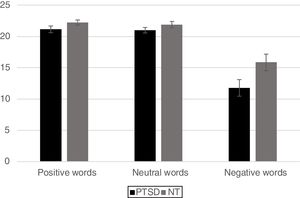
ResultsMixed design analysis of variance showed no group differences in accuracy for determining ink color when presented as a positive or neutral word. However, PTSD children were significantly less accurate (negative words) and notably slower (both positive and negative words) at determining ink color when presented in the context of an emotional word. PTSD processing of positive and negative words was associated with hypoactivation in the superior and middle frontal gyri of the right hemisphere in comparison to NT children.
ConclusionsThese results may reflect a deficit in executive functioning for emotionally laden stimuli, perhaps induced as a by-product of their traumatic experiences.
En la última década, la violencia socio-política en Colombia, ha propiciado un ambiente generador de situaciones de estrés crónico/extremo. El presente estudio empleó resonancia magnética funcional, junto con tareas conductuales, para medir posibles déficits en el funcionamiento ejecutivo en una tarea de palabras con contenido emocional en una muestra de niños colombianos.
MétodoA los participantes (22 TEPT y 22 controles), se les pidió indicar el color de la palabra impresa, omitiendo el contenido emocional implícito positivo, neutro o negativo.
ResultadosEl análisis de varianza de diseño mixto, no arrojó diferencias entre los grupos, en número de aciertos al determinar el color en que estaban impresas las palabras positivas o neutras. Sin embargo, los niños con TEPT tuvieron más errores con las palabras de contenido emocional negativo y fueron más lentos que los controles con palabras de valencia positiva o negativa. En cuanto a las palabras positivas y negativas, el grupo con TEPT se asoció a hipoactivación de los giros superior y medio frontal del hemisferio derecho, al compararlos con los controles.
ConclusionesEstos resultados sugieren déficits en el funcionamiento ejecutivo para estímulos con contenido emocional, quizás como consecuencia de las experiencias traumáticas vividas por el grupo de TEPT.
Childhood maltreatment is a major risk factor for psychopathology (Ohashi et al., 2019) with recent research (Malarbi, Abu-Rayya, Muscara, & Stargatt, 2017) indicating that chronic exposure to such trauma during childhood has negative implications for neurodevelopmental outcomes. For example, Cisler et al. (2014) collected longitudinal data from a representative sample of United States adolescents and found a strong correlation, such that risk for PTSD as well as depression, binge drinking, cigarette smoking, and delinquent behavior, all increased as a function of chronic exposure to early traumatic experiences.
In Colombia (South America), early traumatic experiences are closely related to instances of maltreatment, abuse, and neglect in both children and adolescents. During 2017, a total of 20,663 children and adolescents were victims of sexual abuse, another 10,385 suffered from domestic violence and 14,454 lived through multiple episodes of social violence (Instituto Nacional de Medicina Legal y Ciencias Forenses, 2017). Moreover, despite the implementation of the current Colombian peace process, new forms of violence have emerged that have reinforced the vicious circle of violence begetting more violence. As the Director of the National Institute for Legal Medicine and Forensic Sciences has said, "In Colombia, violence does not disappear, it transforms” (Guerrero & Fandiño-Lozada, 2017, p. 9). This is particularly true for children chronically exposed to such violence, who when becoming adults, often exhibit a tendency to resolve their conflicts using violence as well (Felitti et al., 2019; Spano, Rivera, & Bolland, 2006).
In the United States, one of the more common clinical outcomes stemming from early traumatic experience is PTSD. Children and adolescences are particularly susceptible to developing PTSD with the incidence ranging from 20% to 80% (Jovanovic, 2017). In socio-political violence contexts like Colombia, PTSD is often associated with feelings of fear, helplessness, and/or horror in response to the chronic threat of injury or death (Yehuda & LeDoux, 2007). According to Hart et al. (2018) childhood PTSD, whatever the source, is a strong predictor of future mental health issues that negatively affect peer interactions, decrease or delay academic achievements, and creates a reoccurring source of emotional distress for all family members.
From a physiological perspective, early traumatic experiences have been shown to disrupt experience-dependent brain development during critical periods. These disruptions are thought to adversely affect neural structure and function, as well as creating a delay in the overall course of neurodevelopment (Malarbi et al., 2017). Particularly affected by trauma are the frontal lobes, which are some of the last brain regions to fully develop (Gogtay & Thompson, 2010; Sinnamon, 2019). Indeed, it has been suggested that the cognitive processing difficulties, behavior abnormalities, and deficits in affect regulation and other executive functions as exhibited by children with PTSD may be partially due to the dysfunction of the dorsolateral prefrontal, orbitofrontal, and anterior cingulate cortices (Carrion & Weems, 2017; Li et al., 2019).
Currently, there are mixed results regarding activation (or not) of the aforementioned regions during emotion processing in PTSD children as well as in adults (Carrion & Weems, 2017; McNab et al., 2008). Somewhat surprisingly, PTSD altered brain development and related deficits in executive functioning are underexplored in the pediatric population living in Colombia, despite the fact that the latter country reports an extremely high prevalence of PTSD affected children (Barrera-Valencia, Calderón-Delgado, Trejos-Castillo, & O’Boyle, 2017). Thus, the current research aims to address this knowledge gap by specifically focusing on Colombian children, exploring their behavioral performance on an implicit emotional word processing task, while taking concomitant measures (using fMRI) of related brain activation.
MethodParticipantsTwenty-two children with PTSD living in and around Medellin, Colombia (2 boys and 19 girls: ages 9-14), with scores > 24 (mean=46.3, SD=7.8) on the Child Scale of PTSD Symptoms (Bustos, Rincón, & Aedo, 2009) served as participants and were given individual psychiatric and psychological evaluations both by psychiatrists and psychologists from the Center of Mental Health services of CES University to confirm their PTSD diagnoses (were used the DSM-5 criteria diagnosis to do it). Each participant reported experiencing at least one specific traumatic event, either physical (e.g., sexual abuse) and/or psychological in nature (e.g., witnessing an incident of domestic violence or a life threating situation) in the prior six months. And, this PTSD symptomatology was chronically expressed throughout this period. Twenty-two NT children (11 boys and 11 girls: ages 9-14) from public schools (in the same area and of similar sociodemographic characteristics) served as controls (Scores < 8; mean=5.4; SD=3.5 on the Child Scale of PTSD Symptoms). All participants were right-handed with no history of previous psychiatric disorders, head injuries, or learning disabilities.
Instruments and procedureThe Mini-International Neuropsychiatric Interview for Kids (MINI Kids; Sheehan et al., 1998) was used to screen for anxiety. The Child Depression Inventory (Kovacs, 1992) was employed to monitor depression (PTSD mean=23.43; SD = 8.21/NT mean=6.61; SD=3.63). Note that while the two groups did differ on their CDI scores, clinical assessment deemed this difference to have been induced by their PTSD condition rather than reflecting a pre-existing difference in depression levels between the two groups.
Emotional Word Processing Task. Adapted from Thomaes et al. (2013), 72 emotional word trials (24 positive, 24 neutral, 24 negative words) were presented randomly via the scanner rear projection system for 2.5s with a jittered inter-stimulus interval between 1s–3s. Participants identified the ink color that each of the words was printed in via fiber optic button-press using their right hand: a word printed in red (left button, index finger), word in green (middle button; middle finger), word in blue (right button, ring finger). Figure 1.
Imaging Acquisition. All images were acquired via a 3-Tesla Siemens MRI scanner (Skyra) with a 20-channel head coil. T1-weighted sagittal MPRAGE was used to acquire anatomical brain scans using the following parameters: Total volumes=176; repetition time=2.3s; voxel size=.98×.98 x 0.98mm; echo time =3s; field of view=250mmx250mm; flip angle=9°. fMRI data (47 axial slices) were acquired using gradient echo-planar imaging (EPI) with the following parameters: Total volumes=184; repetition time=2.5s; voxel size=2.5×2.5×3mm; echo time=20s; field of view=200mmx200mm; flip angle=70°.
Imaging Data Processing and Analysis. FSL software was used for the pre-processing and analysis of all anatomical and functional images. After brain extractions using BET, slice-timing correction was employed to temporally align the fMRI slices. Motion correction of the data was also conducted via MCFLIRT, and spatial smoothing with FWHM of 5mm was used to reduce the effects of noise. For each participant, functional images were initially linearly registered to his/her own structural image, followed by a transformation from their native space into MNI-152 standard space. A first-level general linear model (GLM) analysis was conducted using a double gamma convolution to correct for delay between the EPI signal and the hemodynamic response function (HRF). A higher-level GLM with fixed effects (see Beckmann, Jenkinson, & Smith, 2003; Woolrich, 2008; Woolrich, Behrens, Beckmann, Jenkinson, & Smith, 2004) was conducted on the blood oxygenated (BOLD) responses to the word stimuli as a function of group membership (i.e., PTSD vs. NT). Whole brain maps with clusters of significantly activated voxels as determined by family-wise t-tests were analyzed, with activation non-parametrically set at z > 2.30 with a threshold of p< .05. Gaussian Random Field Theory was used to correct for multiple comparisons (Worsley, Taylor, Tomaiuolo, & Lerch, 2004).
The study was carried out according to the latest version of the Declaration of Helsinki, and the Ethics Committee of CES University gave its approval for the study. Based on the protocol of the Institutional Human Subjects Review Board of CES University, all 44 participants signed an assent form and their parents/guardians signed consent forms.
ResultsBehavioral dataFor analysis of the accuracy data a 2 Group (Between Subjects Factor: PTSD vs. NT) x 3 Word Type (Within Subjects Factor: positive, neutral, negative) mixed design Analysis of Variance (ANOVA) was conducted on the total number of correct ink-color determinations. This analysis revealed a significant main effect for Group [F (1, 42)=5.80, p < .02] with the NT group being significantly more accurate than the PTSD group. A main effect for Word Type was also significant [F (2, 84)=69.9 p < .0001]. Post-hoc comparisons using a Least Significant Difference test (LSD) revealed that negative words produced significantly less accurate responding (p< .05) compared to either positive or neutral words, while the latter two word types did not differ from each other. The Group x Word Type interaction was found to be marginally reliable [F (2, 84)=2.78, p< .067]. Pre-planned comparisons of this interaction using an LSD test revealed that compared to the NT, the PTSD were less accurate for ink color determinations involving negative words (p< .05), while the two groups did not differ in accuracy on positive or neutral word types (Figure 2).
For the analysis of reaction time data a similar 2 Group x 3 Word Type mixed ANOVA revealed a significant main effect for Group, [F(1,42)=6.63, p < .05] with the PTSD responding more slowly than the NT group. A main effect was also found for Word Type [F (2, 84)=4.02, p < .05] such that all participants exhibited significantly faster responses for positive words compared to neutral words (p< .05) and marginally faster responses compared to negative words (p< .10), but response times between negative and neutral words did not differ from each other. The Group x Word Type interaction was not significant (p< .12). However, based on the finding of a marginally reliable interaction for the accuracy data, pre-planned comparisons of the interaction for the reaction time data using an LSD test revealed that the PTSD group responded more slowly to both positive and negative words compared to the NT (p < .05 and p < .01, respectively). Reaction time to identify the ink color of neutral words was not significantly different between the two Groups (Figure 3).
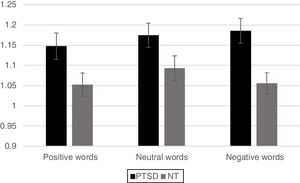
Neuroimaging imaging dataSignificant differences in levels of activation in various brain locations for both the NT>PTSD and the PTSD>NT contrasts appear in the top and bottom of Figure 4, respectively. Table 1 and Table 2 present the cluster activations and peak coordinates for the NT>PTSD and PTSD>NT contrasts, respectively. As can be seen in Figure 4 (top) for the NT >PTSD contrast, greater activation was prominent for the NT group in both anterior and posterior regions of the brain. Specifically, for positive words, selective activation of these regions included the left and right frontal poles and bilateral activation of the superior frontal gyri. For negative words, NT participants exhibited significantly greater activation bilaterally in the middle and superior frontal gyri, which represent dorsolateral and medial prefrontal cortices, respectively. Note that peak coordinates were obtained for the right prefrontal cortices, but not the left. Greater NT activation was also observed in a variety of other regions, including the angular gyrus bilaterally, the right pre- and post-central gyri, the right paracingulate cortex, the left supramarginal gyrus, the left superior parietal lobe, the left superior temporal gyrus, and the left inferior and superior occipital lobes. Note that for the PTSD group, significant activations were generally restricted to internal brain regions, and the pattern was similar for both positive and negative word types. The selective regions of greater activation in the PTSD group included the thalamus (right; negative words only), central operculum (positive words only), pallidum (left, negative words only) and the insula and putamen bilaterally for both positive and negative word types (bottom of Figure 4).
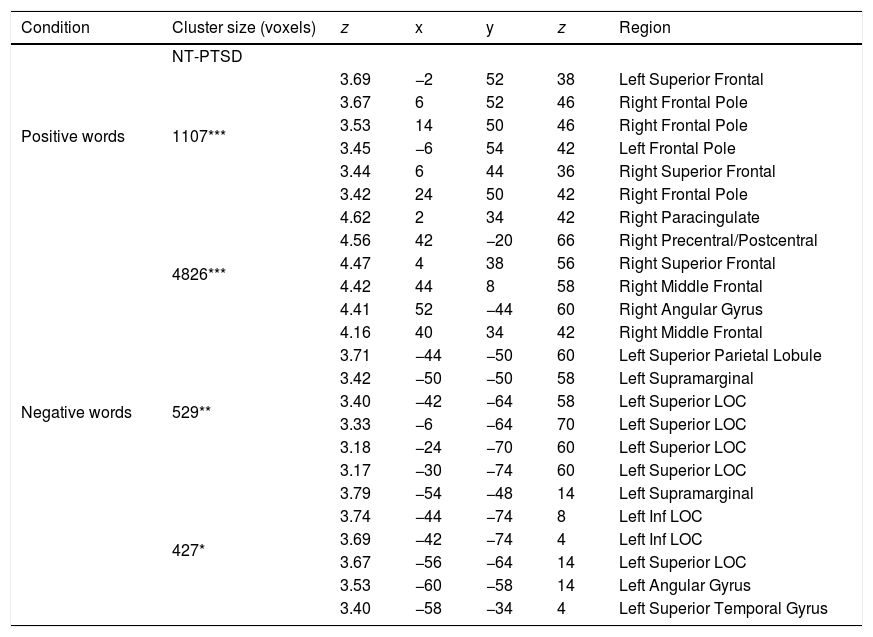
Brain regions (and coordinates) showing selectively greater activation for the NT participants compared to the PTSD for positive and negative words.
| Condition | Cluster size (voxels) | z | x | y | z | Region |
|---|---|---|---|---|---|---|
| NT-PTSD | ||||||
| Positive words | 1107*** | 3.69 | −2 | 52 | 38 | Left Superior Frontal |
| 3.67 | 6 | 52 | 46 | Right Frontal Pole | ||
| 3.53 | 14 | 50 | 46 | Right Frontal Pole | ||
| 3.45 | −6 | 54 | 42 | Left Frontal Pole | ||
| 3.44 | 6 | 44 | 36 | Right Superior Frontal | ||
| 3.42 | 24 | 50 | 42 | Right Frontal Pole | ||
| Negative words | 4826*** | 4.62 | 2 | 34 | 42 | Right Paracingulate |
| 4.56 | 42 | −20 | 66 | Right Precentral/Postcentral | ||
| 4.47 | 4 | 38 | 56 | Right Superior Frontal | ||
| 4.42 | 44 | 8 | 58 | Right Middle Frontal | ||
| 4.41 | 52 | −44 | 60 | Right Angular Gyrus | ||
| 4.16 | 40 | 34 | 42 | Right Middle Frontal | ||
| 529** | 3.71 | −44 | −50 | 60 | Left Superior Parietal Lobule | |
| 3.42 | −50 | −50 | 58 | Left Supramarginal | ||
| 3.40 | −42 | −64 | 58 | Left Superior LOC | ||
| 3.33 | −6 | −64 | 70 | Left Superior LOC | ||
| 3.18 | −24 | −70 | 60 | Left Superior LOC | ||
| 3.17 | −30 | −74 | 60 | Left Superior LOC | ||
| 427* | 3.79 | −54 | −48 | 14 | Left Supramarginal | |
| 3.74 | −44 | −74 | 8 | Left Inf LOC | ||
| 3.69 | −42 | −74 | 4 | Left Inf LOC | ||
| 3.67 | −56 | −64 | 14 | Left Superior LOC | ||
| 3.53 | −60 | −58 | 14 | Left Angular Gyrus | ||
| 3.40 | −58 | −34 | 4 | Left Superior Temporal Gyrus | ||
Note. The cluster size is presented as the number encompassing voxels with statistical significance: ***p < .001, **p < .01, *p < .05. Also included are the intensity (z-scores), location (MNI coordinates), and region of the peak voxels (maximum intensity).
Brain regions (and coordinates) showing selectively greater activation for the PTSD compared to neurotypical participants for positive and negative words.
| Condition | Cluster size (voxels) | z | x | y | z | Region |
|---|---|---|---|---|---|---|
| PTSD-NT | ||||||
| Positive words | 545* | 3.97 | 26 | −10 | 6 | Right Putamen |
| 3.38 | 26 | 0 | 0 | Right Putamen | ||
| 3.14 | 40 | 4 | 8 | Central Operculum | ||
| 3.09 | 20 | 10 | 6 | Right Putamen | ||
| 3.06 | 36 | 12 | 0 | Right Insula | ||
| 2.88 | 28 | 16 | −2 | Right Insula | ||
| 518* | 3.75 | −36 | −12 | 4 | Left Insula | |
| 3.73 | −30 | −12 | 4 | Left Putamen | ||
| 3.68 | −18 | 10 | 4 | Left Putamen | ||
| 3.62 | −36 | 2 | 8 | Left Insula | ||
| 3.45 | −28 | −16 | 2 | Left Putamen | ||
| 3.18 | −26 | 0 | 0 | Left Putamen | ||
| Negative words | 490* | 3.70 | 20 | 0 | 0 | Right Pallidum |
| 3.35 | 24 | −28 | 4 | Right Thalamus | ||
| 3.29 | 26 | −12 | 4 | Right Putamen | ||
| 3.28 | 26 | 0 | 0 | Right Putamen | ||
| 3.22 | 26 | 2 | 4 | Right Putamen | ||
| 3.17 | 28 | −4 | 2 | Right Putamen | ||
| 455* | 3.68 | −26 | 0 | 0 | Left Putamen | |
| 3.64 | −36 | 0 | 8 | Left Insula | ||
| 3.51 | −28 | −8 | 4 | Left Putamen | ||
| 3.40 | −38 | −12 | 2 | Left Insula | ||
| 3.03 | −28 | −16 | 4 | Left Putamen | ||
| 2.94 | −26 | −20 | 2 | Left Pallidum |
Note. The cluster size is presented as the number encompassing voxels with statistical significance: ***p < .001, **p < .01, *p < .05. Also included are the intensity (z-scores), location (MNI coordinates), and region of the peak voxels (maximum intensity).
The focus of the present study was to investigate how chronic stress and exposure to trauma, affected the brain activity and executive functioning of Colombian children with PTSD compared to NT children. To that end, our results show that those children with PTSD were significantly less accurate than their NT counterparts when asked to determine the ink color of presented words expressing negative affect. Additionally, PTSD children were slower than NTs when asked to determine the ink color of words expressing positive or negative affect. Note that ink color determination does not require the participant to actually process the meaning of the word, and even if such processing for word meaning does occur, its influence is implicit and indirect at best as participant attention is focused solely on processing the ink color of the word. Yet, even under such implicit and indirect conditions, the emotional valence of the word affected processing, resulting in reduced accuracy and increased latency for the PTSD children. This was particularly evident for words expressing negative emotions. Thus, even at a non-explicit level, stimuli that evoke emotion (particularly negative emotions although positive emotions appear affected as well) appear to have unusual influence on the cognitive functioning of children with PTSD. This is important because mental health professionals treating PTSD children need to be aware of the saliency of stimuli exhibiting emotional valence (particularly negative emotions), even when/if such exposure is implicit. Thus, there is a need to develop and tailor clinical interventions accordingly, i.e., evaluating and remediating both the explicit as well as the implicit impact of emotional stimuli on these PTSD children. It is worth mentioning that processing of positive words also implicitly impacted children with PTSD. Note that in the DSM-5 there is a cluster of symptoms defined as “emotional numbing”, which is characterized by emotional response deficits, feelings of detachment from others, and markedly diminished interest or participation in significant activities. This restricted range of affect has been reported by Kashdan, Elhai, and Frueh (2006), and thought to reflect a deficit in the ability to experience or process positive emotions. Recently, Fonzo (2018) highlighted the role of similar brain structures when processing positive and negative emotions. Thus, it may be that the PTSD children in the present study are experiencing a similar sense of emotional detachment.
The brain imaging results presented here indicate that PTSD children exhibit selectively less activation of the frontal regions, known to play an important role in mediating executive functions. Thus, compared to NT children, those with PTSD may experience a deficit in the ability to regulate the processing of emotion-laden stimuli, particularly for those conveying negative affect. Furthermore, children with PTSD showed hypo-activation of the dorsolateral prefrontal cortex (i.e., middle frontal gyri) during presentation of negative words, a pattern that has been empirically linked to reappraisal of emotionally salient stimuli (Golkar et al., 2012). Emotional reappraisal demands (i.e., neutralizing one’s feelings) may be greater when processing the ink color of words expressing negative affect compared to words expressing more neutral affect. Therefore, children with PTSD incur executive processing deficits as reflected in less accuracy and slower reaction times compared to their NT counterparts, for tasks requiring modulation of emotional salience—particularly for those involving negative emotions.
Although the present study was limited to emotional word processing per se, it is also reasonable to speculate that other types of stimuli requiring similar emotion regulation and reappraisal, particularly those expressing negative affect, would also have special access to the cognitive systems of PTSD children. In fact, preliminary data from our own ongoing pilot study (Calderon et al., 2018) using positive, negative and neutral face stimuli suggests that the processing of such emotions expressed in human faces (particularly those expressing negative emotions) is also accompanied by atypical engagement of the frontal lobes in these same Colombian PTSD children.
The fact that selective brain activation of the PTSD children was restricted primarily to internal brain structures involved in emotion processing was somewhat anticipated. It is well documented that the insula, putamen, operculum and pallidum are all primary mediators of emotion processing (Mechias, Etkin, & Kalisch, 2010). Moreover, the fact that PTSD hyper-activation (relative to their NT counterparts) of these regions (along with the thalamus) was greater in response to the presentation of emotion laden words compared to neutral words suggests that their cognitive system has been sensitized to the processing of stimuli evoking emotion. Additional evidence of PTSD sensitivity to stimuli implicitly evoking negative emotion is observed in their difficulty in accurately processing the font color of such words. Indeed, the latter sensitivity may well have been induced as a byproduct of their exposure to an environment of chronic stress, and trauma.
Regarding study limitations, it should be noted that the PTSD group was disproportionally comprised of females, which reflects the actual male/female incidence ratio of PTSD in the Colombian pediatric population (Gaviria et al., 2016). We did consider conducting an analysis using sex as a covariate, however, given that there were only 2 male participants, such an analysis was deemed statistically inappropriate. Thus, further research with a more balanced sample is required to examine the extent to which biological sex of the individual selectively influences proneness to acquiring PTSD, as well as investigating the brain activation patterns and cognitive processes that impact PTSD male as compared to female children.
An additional factor that may have influenced the present results is the fact that participant IQ was different between the two groups (PTSD mean IQ=81.5, SD = 14.2; NT mean IQ=95.2, SD = 14.3). And while this difference was statistically reliable (p< .05), it is relatively small. Note that both groups were quite successful at completing the simple and rather automatic ink-color identification task required by the study. Importantly, both groups produced a high (and similar) percentage of correct ink-color determination for both positive and neutral word types – with a notable exception of those trials involving negative words, suggesting that IQ was not a significantly contributing variable to the obtained effect.
Finally, it should be noted that the type of trauma experienced by our PTSD sample was primarily due to chronic exposure to a context of socio-political violence. However, there are a number of collateral stressors that may accompany such exposure (e.g., sexual assault, etc.) and so the findings reported here should not be interpreted as specific to PTSD acquired solely in response to violence alone. Rather, the current results are likely to be a composite effect of violence exposure coupled with the impact of other associated traumatic stressors all acting in concert. Further research is required to tease apart the independent contributions of these associated traumatic stressors, as all are likely factors for acquiring PTSD in this population.
FundingThe present article is a product from a research project founded by the Administrative Department of Science, Technology and Innovation of the Colombian government (COLCIENCIAS) according to the contract 854/2015 code 122871149988.