Emerging research supports the idea that physical activity benefits brain development. However, the body of evidence focused on understanding the effects of physical activity on white matter microstructure during childhood is still in its infancy, and further well-designed randomized clinical trials are needed.
AimThis study aimed: (i) to investigate the effects of a 20-week physical activity intervention on global white matter microstructure in children with overweight or obesity, and (ii) to explore whether the effect of physical activity on white matter microstructure is global or restricted to a particular set of white matter bundles.
MethodsIn total, 109 children aged 8 to 11 years with overweight or obesity were randomized and allocated to either the physical activity program or the control group. Data were collected from November 2014 to June 2016, with diffusion tensor imaging (DTI) data processing and analyses conducted between June 2017 and November 2021. Images were pre-processed using the Functional Magnetic Resonance Imaging (MRI) of the Brain´s Software Library (FSL) and white matter properties were explored by probabilistic fiber tractography and tract-based spatial statistics (TBSS).
ResultsIntention-to-treat analyses were performed for all children who completed the pre-test and post-test DTI assessment, with good quality DTI data (N = 89). Of them, 83 children (10.06±1.11 years, 39 % girls, intervention group=44) met the per-protocol criteria (attended at least 70 % of the recommended sessions). Our probabilistic fiber tractography analysis did not show any effects in terms of global and tract-specific fractional anisotropy (FA) and mean diffusivity (MD) in the per-protocol or intention-to-treat analyses. Additionally, we did not observe any effects on the voxel-wise DTI parameters (i.e., FA and MD) using the most restricted TBSS approach (i.e., per protocol analyses and p-corrected image with a statistical threshold of p < 0.05). In the intention-to-treat analysis, we found that our physical activity program had a borderline effect (p-corrected image with a statistical threshold of p < 0.1) on 7 different clusters, including a cluster in the corpus callosum.
ConclusionWe conclude that a 20-week physical activity intervention was not enough to induce changes in global and tract-specific white matter during childhood. The effects of physical activity on white matter microstructure could be restricted to local changes in several white matter tracts (e.g., the body of the corpus callosum). However, our results were not significant, and more interventions are needed to determine whether and how physical activity affects white matter microstructure during childhood.
Childhood physical inactivity is a worldwide pandemic (Kohl et al., 2012). An inactive lifestyle not only increases the risk of becoming overweight or obese but also contributes to worse physical and mental health (Lee et al., 2012; Rodriguez-Ayllon et al., 2019). In this context and boosted by the rapid advances in brain imaging technologies, new studies have emerged to evaluate the impact of physical activity on brain health (Piercy et al., 2018). Specifically, there is a moderate grade of evidence to suggest that both acute and chronic moderate- to vigorous-intensity physical activity-based interventions might affect brain structure and function in children aged 6–13 (Erickson et al., 2019); however, results remain controversial (Ortega et al., 2022), and only a few studies have explored the extent to which physical activity programs benefits white matter microstructure (i.e., the neural architecture that forms bundles of myelinated nerve fibers, Fields 2008) during childhood (Chaddock-Heyman et al., 2018; Daniell, 2012; Schaeffer et al., 2014).
The two main indicators of white matter microstructure are fractional anisotropy (FA) and mean diffusivity (MD). Higher FA values are thought to reflect a higher degree of neuronal organization while higher MD indicates relatively unimpeded diffusion (i.e., it is negatively correlated with FA) (Giorgio et al., 2010). In over 2500 children from the Generation R Study, it was observed that total self-reported physical activity was positively associated with global fractional anisotropy (FA) and negatively associated with global mean diffusivity (MD) (Rodriguez-Ayllon et al., 2020a). Interestingly, these findings were confirmed in another sample of children with overweight or obesity from the ActiveBrains project (Rodriguez-Ayllon et al., 2020b). Although the effect sizes for these associations were, in both studies, relatively small, it was concluded that the association between unstructured physical activity and white matter microstructure seems more global than restricted to a particular set of white matter bundles. On the other hand, the few intervention studies that tested the effects of physical activity on white matter microstructure focused on a particular set of white matter tracts or regions of interest (i.e., the uncinate fasciculus, Schaeffer et al. 2014, the superior longitudinal fasciculus, Krafft et al. 2014a, the genu of the corpus callosum, Chaddock-Heyman et al. 2018). Since they only explored the effect of physical activity on white matter microstructure in these specific white matter tracts, it is unknown whether the effects of physical activity on white matter microstructure may be global, as observational studies indicated, or restricted to some particular white matter tracts.
Collectively, the body of evidence focused on the effects of physical activity on white matter microstructure during childhood is still in its infancy, and further well-designed randomized controlled trials (RCTs) are needed. Combining voxel-wise analysis with fiber tractography provides an alternative approach to exploring global and local changes in white matter microstructure after a physical activity-based intervention. Therefore, this study aimed to investigate the effects of a 20-week physical activity intervention on global white matter microstructure in children with overweight or obesity (primary aim). Secondly, to determine whether the effect of physical activity on white matter microstructure was indeed global or local, the effect of physical activity on tract-specific and voxel-based FA and MD was also explored (secondary aim). We further hypothesized that physical activity increases FA and decreases MD; however, based on previous literature (Chaddock-Heyman et al., 2018) and limited by the relatively short duration of the physical activity program (i.e., 4.5 months vs. 8 or 9 months), we expected the magnitude of these relationships to be relatively small.
MethodsTrial design and participantsThis study includes data from the ActiveBrains project, an RCT, whose primary aim was to explore the effects of a 20-week physical activity program on behavioral outcomes, including intelligence, executive function, and academic performance as well as on hippocampal volume as a primary region of interest in children with overweight or obesity (Cadenas-Sánchez et al., 2016; Ortega et al., 2022). As secondary outcomes, it also included physical and mental health as well as other specific brain structural and functional indicators (Migueles et al., 2023; Ortega et al., 2022), as white matter microstructure, which is the main outcome of this study. The ActiveBrains project was approved by the Human Research Ethics Committee of the University of Granada and was registered in ClinicalTrials.gov (identifier: NCT02295072). Data (at baseline and post-intervention) were collected between November 2014 and June 2016. Data processing and analyses were conducted between June 2017 and November 2021. Details of the ActiveBrains project methodology and the inclusion/exclusion criteria have been described elsewhere (Cadenas-Sánchez et al., 2016). In brief, the eligibility criteria were: (i) to be 8 to 11.9 years old; (ii) to be overweight or obese according to the World Obesity Federation cut-off points (Bervoets & Massa, 2014; Cole & Lobstein, 2012); (iii) not to have any physical disabilities or neurological disorders that hampered exercise; (iv) in the case of girls, not to have started menstruation at the baseline assessments; (v) not to use medications that influenced central nervous system function; (vi) to be right-handed (Oldfield, 1971); and (vii) not to report an attention-deficit hyperactivity disorder (ADHD) over the 85th percentile (Pappas, 2006). Details of: (i) randomization, (ii) power and sample size calculation, and (iii) intervention and control groups were described previously (Ortega et al., 2022). For feasibility reasons, the ActiveBrains project was conducted in 3 waves divided by the first academic year of the project (i.e., 2014–2015), the beginning of the following academic year (i.e., 2015–2016), and the later on the same academic year.
Intervention and controlThe program had a duration of 20 weeks. Participants could attend the program daily from Monday to Friday (i.e., 5 sessions/week), although the attendance criterion was set as a minimum of 3 times/week. The duration of the session was 90 min/session. Each session was structured in three parts: 5–10 min of warming-up, 60 min of aerobic multi-games at moderate-to-vigorous intensities with emphasis on high-intensity aerobic activities (i.e., above 80 % of maximal heart rate), 20 min of strength training that included muscle- and bone-strengthening game-based activities, and 5–10 min of cooling-down that included stretching and relaxation exercises.
Participants in the intervention and control groups were provided with information about healthy nutrition and recommendations for physical activity. Children allocated to the control group were advised to continue their daily routines, and they were offered the intervention program after the trial was completed.
Magnetic Resonance Imaging (MRI) procedureImage acquisitionMRI data were acquired with a 3.0 Tesla Siemens Magnetom Tim Trio scanner (Siemens Medical Solutions, Erlangen, Germany). DTI data were acquired using an echo planar imaging (EPI) sequence with the following parameters: TR = 3300 ms, TE = 90 ms, flip angle = 90, matrix=128×128, FOV= 230 mm x 230 mm, slice thickness= 4 mm, number of slices = 25 and voxel resolution= 1.8 × 1.8 × 4 mm3. One volume without diffusion weighting (b = 0 s/mm2) and 30 volumes with diffusion weighting (b = 1000s/mm2) were collected.
Image preprocessingImage preprocessing was conducted using the Functional MRI of the Brain´s Software Library (FSL) (Gorgolewski et al., 2011; Jenkinson et al., 2012). Images were first adjusted for minor head motion (Andersson & Sotiropoulos, 2016) using eddy, a tool to correct for eddy current-induced distortions and participants' movements. Then, a Gaussian process for outlier replacement (Andersson et al., 2016) was used. To account for rotations applied to the image data (Jones & Cercignani, 2010; Leemans & Jones, 2009), the resulting transformation matrices were used to rotate the diffusion gradient direction table. Non-brain tissue was removed using the FSL Brain Extraction Tool (Smith, 2002). Then, the diffusion tensor was fit, and common scalar maps (e.g., FA, MD) were subsequently computed.
Image quality assuranceRaw image quality was assessed via visual inspection (Muetzel et al., 2017). The sum-of-squares error (SSE) maps from the tensor estimation were calculated and visually inspected for structured noise. Image quality was rated using a 4-point scale, with 1= “excellent”, 2= “minor”, 3= “moderate”, and 4= “severe”. Datasets determined to be of insufficient quality (i.e., moderate and severe) for statistical analyses were excluded (n = 4). Lastly, probabilistic tractography data were inspected visually. First, the native space FA map registration was inspected, to ensure images were all properly aligned to the template (masks were properly mapped to native space). Second, all tracts were visualized to ensure accurate path reconstruction. Any participants were excluded after tractography visualization.
Probabilistic fiber tractographyDiffusion data were first processed using the Bayesian Estimation of Diffusion Parameters Obtained using Sampling Techniques (BEDPOSTx), accounting for two fiber orientations at each voxel (Behrens et al., 2003, 2007). Next, for each participant, the FA map was aligned to the FMRIB-58 FA template image with the FSL nonlinear registration tool (FNIRT). The inverse of this nonlinear warp field was computed and applied to a series of predefined seed, target, exclusion, and termination masks provided by the AutoPtx plugin (de Groot et al., 2015).
Probabilistic fiber tracking was then performed with the FSL Probtrackx module using these supplied tract-specific masks (i.e., seed, target, etc.) that were warped to the native diffusion image space of each subject (Behrens et al., 2007). The resulting path distributions were normalized to a scale from 0 to 1 using the total number of successful seed-to-target attempts and were subsequently thresholded to remove low-probability voxels likely related to noise.
After the tracts were thresholded (de Groot et al., 2015), average FA and MD values were then computed for each fiber bundle. Connectivity distributions were estimated for 7 large fiber bundles (i.e., corticospinal tract, superior longitudinal fasciculus, inferior longitudinal fasciculus, uncinate fasciculus, cingulate gyrus part of cingulum, forceps minor, and forceps major) selected based on previous reports (Muetzel et al., 2017; Navas-Sánchez et al., 2014; Schmithorst et al., 2005). A depiction of the tracts used in this study can be found in Muetzel et al. (2017). The average of FA and MD in the left and right hemisphere was calculated in those tracts present in both hemispheres (i.e., corticospinal tract, superior longitudinal fasciculus, inferior longitudinal fasciculus, uncinate fasciculus, and cingulate gyrus part of cingulum).
Lastly, to assess whether exposures were related to global measures of white matter integrity (i.e., global FA, MD), selected tracts (i.e., corticospinal tract, superior longitudinal fasciculus, inferior longitudinal fasciculus, uncinate fasciculus, cingulate gyrus part of cingulum, forceps minor, and forceps major) (Muetzel et al., 2017) were combined into a single factor (“global factor”). The global factor was computed by averaging all tracts and weighting this average by the size (volume) of the tracts (to ensure small regions do not contribute equally to larger regions, which is common practice in the neuroimaging literature, in particular, cortical morphology studies). This method was based on previous work (Bolhuis et al., 2019; Mulder et al., 2019; Rodriguez-Ayllon et al., 2019).
Tract-based spatial statisticsTract-based spatial statistics (TBSS) was used to perform voxel-wise statistical analyses of the DTI data (HERE) (Smith et al., 2006). Pre and post-mean FA and mean MD images were computed by averaging all the FA and MD images respectively in standard space. Then, for each participant, we calculated two different images by subtracting the post-image from the pre-image (i.e., post-pre), one for FA and one for MD. All different images were visually inspected. To exclude those voxels not belonging to white matter, a skeleton image was created by thresholding the FA and MD mean images with a value of 0.2. FA and MD difference maps of each participant were then projected onto the skeleton. To classify the white matter structures where physical activity had an effect, we used two atlases: (i) JHU ICBM-DTI-81 White-Matter Labels, which identify white matter regions, and (ii) JHU White-Matter Tractography Atlas, which identify some of the most well-known tracts. Notably, we first used the JHU ICBM-DTI-81 White-Matter Labels. When it was not able to identify the regions, we completed the information using the JHU White-Matter Tractography Atlas.
Statistical analysisAll analyses, except TBSS analyses, were performed using the Statistical Package for Social Sciences (IBM SPSS Statistics for Windows, version 22.0, Armonk, NY, p set at < 0.05). The characteristics of the study sample were presented as mean and standard deviations (SD) or percentages.
Overall, the effects of the physical activity intervention on the DTI outcomes were tested according to the per-protocol principle (to complete the post-intervention assessments and, for the intervention group, to attend at least 70 % of the required three sessions per week). We also tested intention-to-treat analysis including those who completed the baseline and the post-evaluations but did not meet the per-protocol criteria differed in the main study variables from the participants who did it.
For tractography analyses, we ran analysis of covariance (ANCOVA) using post-intervention data (i.e., global DTI measures, corticospinal tract, superior longitudinal fasciculus, inferior longitudinal fasciculus, uncinate fasciculus, cingulate gyrus part of cingulum, forceps minor, and forceps major) as dependent variables, group (i.e., intervention vs. control) as fixed factor, and baseline data as covariates. The z-scores for each outcome at post-intervention were also formed by dividing the difference of the post-raw score of each participant from the baseline mean by the baseline standard deviation (i.e., (post-raw score – baseline mean) / baseline standard deviation). This effect size can be interpreted as approximately 0.2 SDs is considered a small effect size, 0.5 SDs a medium effect size, and 0.8 SDs a large effect size. Tractography analyses were adjusted for multiple comparisons using a false discovery rate (FDR) based on the Benjamini-Hochberg method (Benjamini & Hochberg, 1995).
For TBSS analyses (HERE), we fed the projected FA and MD difference images (post-pre difference data) into general linear model (GLM) modeling and thresholding to find voxels that correlated with our model (intervention vs. control group). Then, we loaded the corrected p-value stats image in FSLeyes and set the display range to 0.95:1, which corresponds to thresholding the results at p < 0.05. For sensitivity analyses, we set the display range to 0.90 to detect the possibility of a type II error in our results (a false negative).
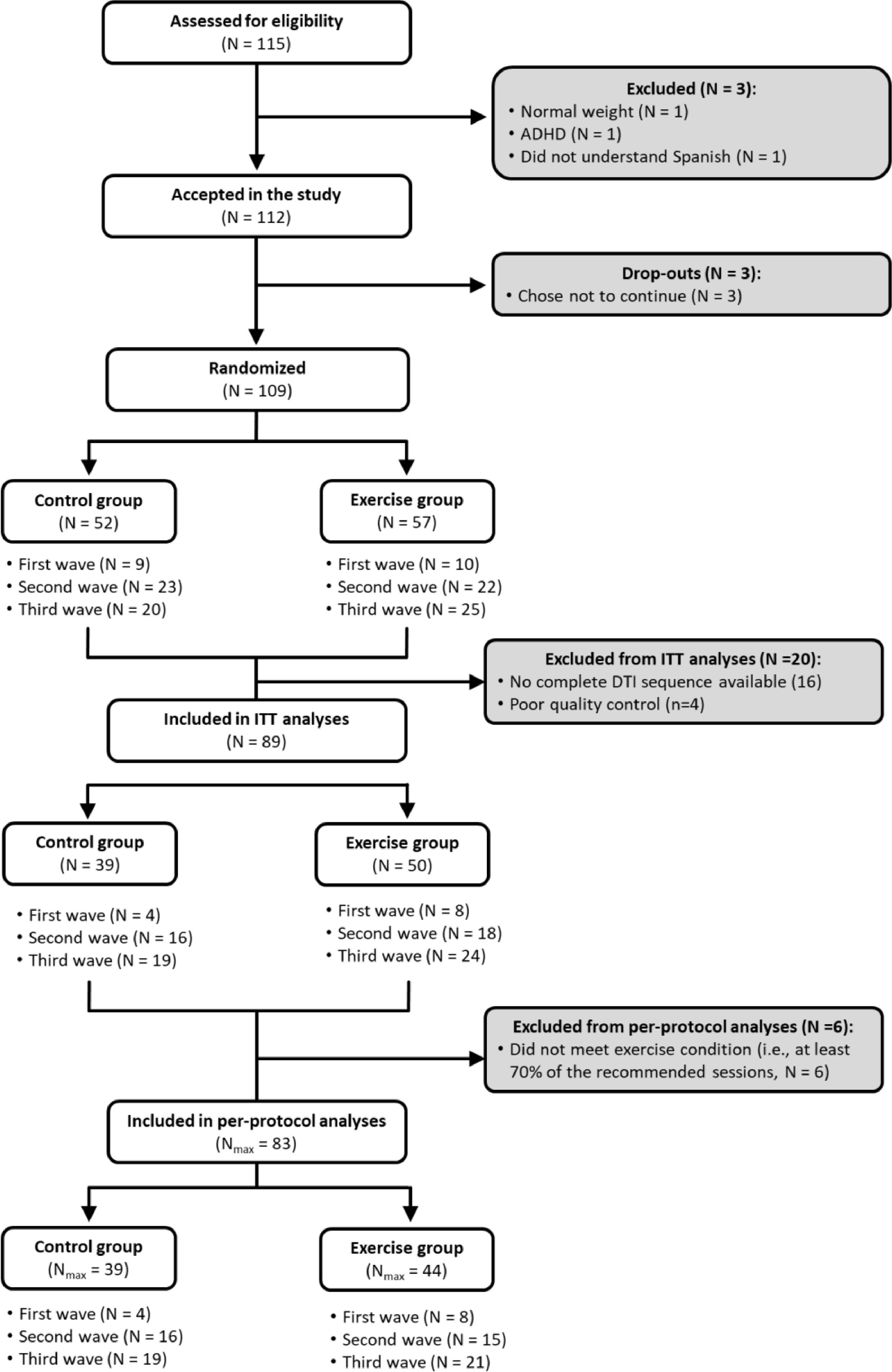
ResultsIn total, 112 children with overweight/obesity who met the inclusion/exclusion criteria participated in this study. Of them, 109 were randomly allocated to a physical activity group, which participated in the physical activity program, or to a wait-list control group. Four children were excluded from analyses due to visible motion on the reconstructed DTI data, and 16 were excluded due to the incomplete DTI sequence available. Then, intention-to-treat analyses were performed for all children who completed the pre-test and post-test DTI assessment, with good quality DTI data (N = 89). Of them, 83 children with overweight or obesity met the per-protocol criteria (see Fig. 1).
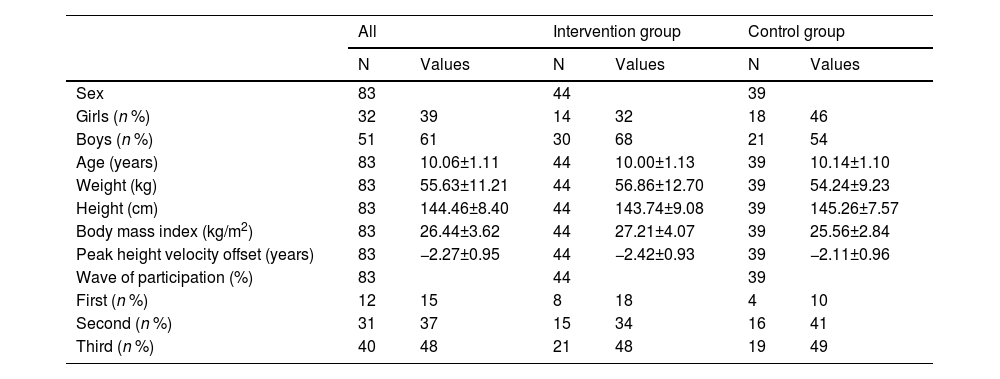
All the baseline characteristics of the study sample are presented in Table 1. The study sample had an average age of 10.06 years (SD = 1.11), a biological maturation age of −2.27 years (SD = 0.95) before the age at which the maximum peak height velocity occurs, and an average body mass index of 26.44 kg/m2 (SD = 3.62) at baseline. Descriptive baseline characteristics of the intervention groups meeting (n = 44) and not-meeting (n = 50) the per-protocol criteria are presented in Table S1. Baseline data on global and trac-specific white matter microstructure of the ActiveBrains participants meeting per-protocol criteria have been presented in Table S2.
Descriptive baseline characteristics of the ActiveBrains participants meeting per-protocol criteria (n = 83).
Values are expressed as means ± standard deviations (SD), unless otherwise indicated.
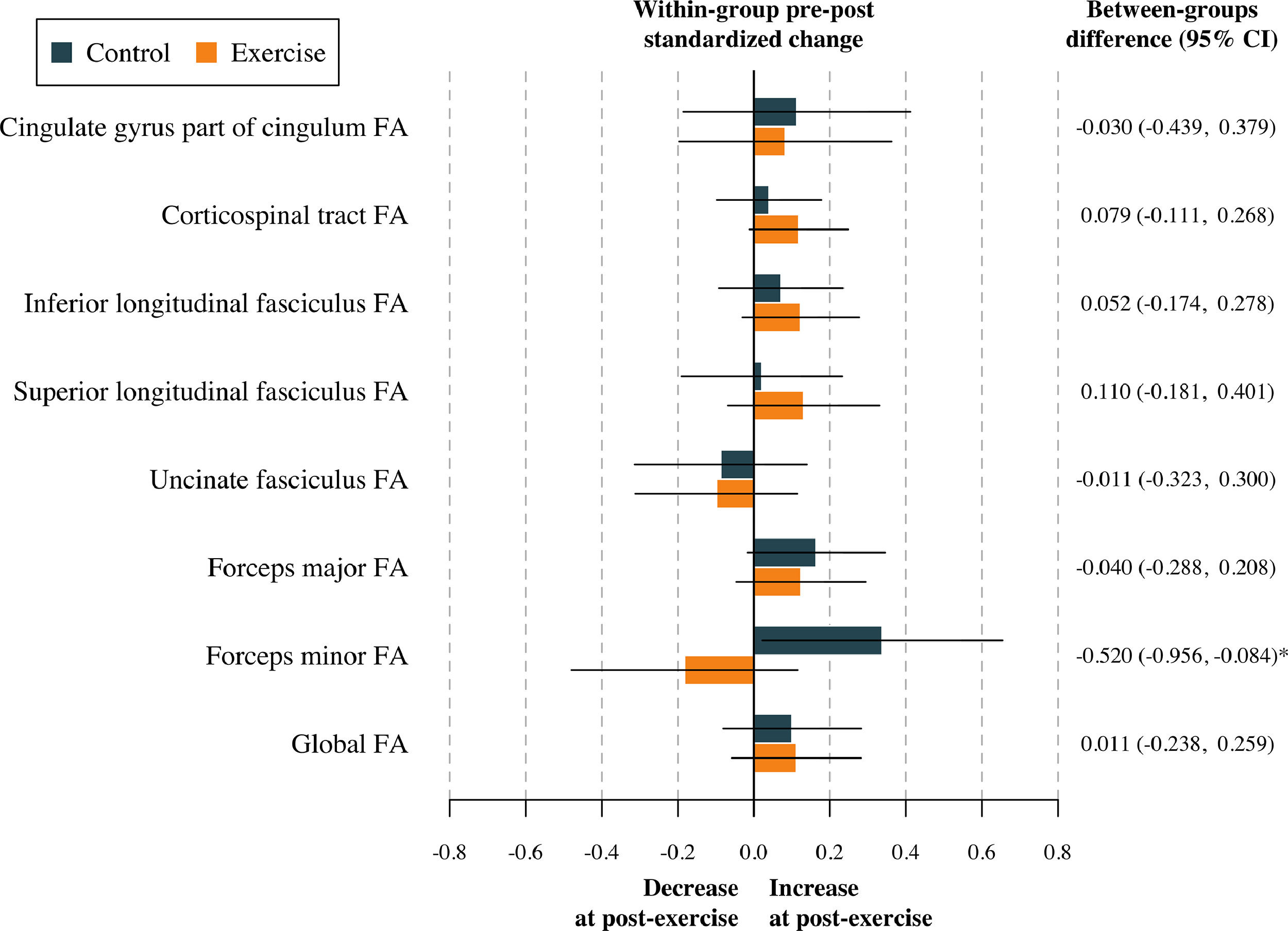
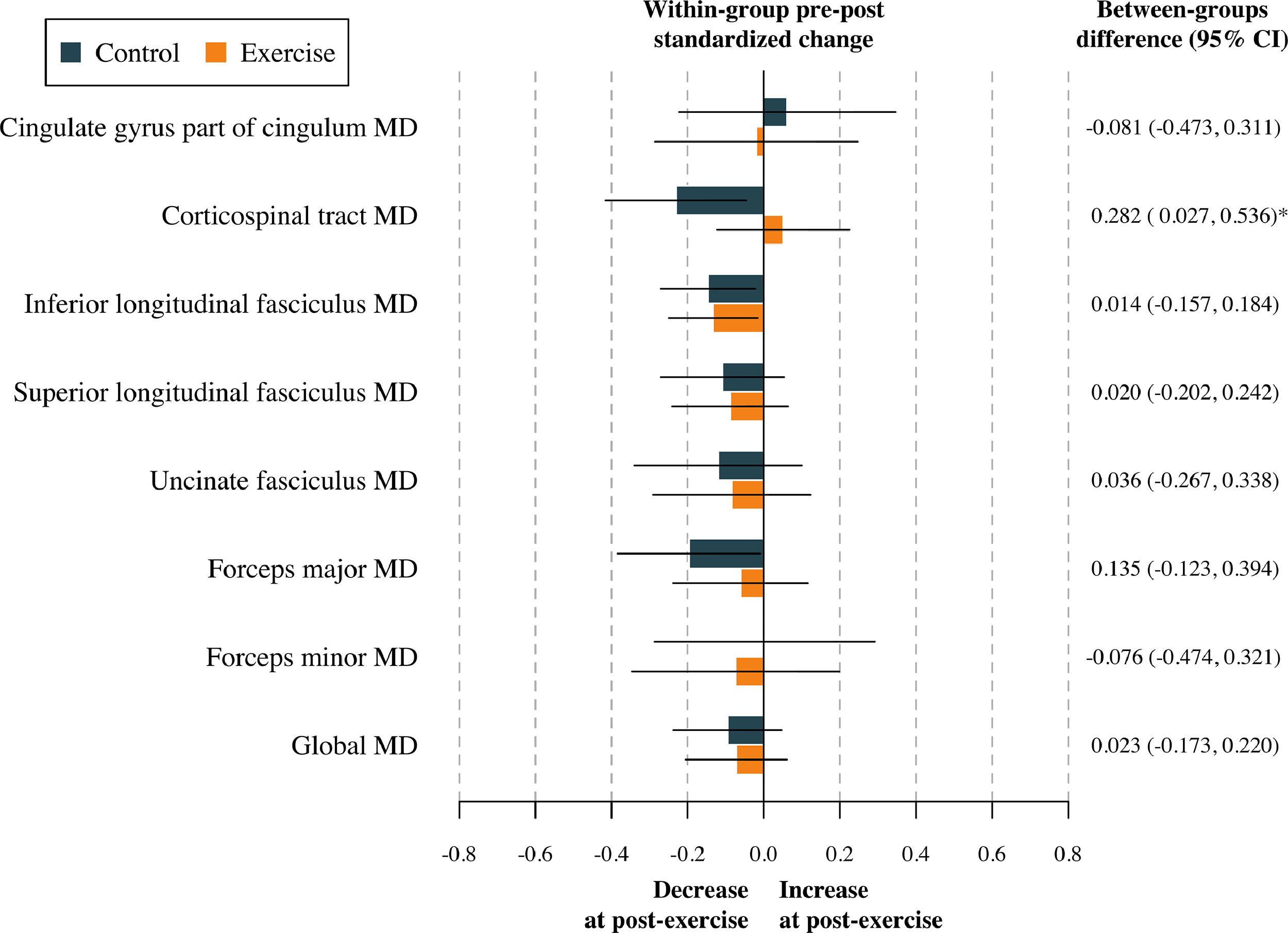
The effect of the physical activity program on global FA and global MD after adjustment for baseline values of the study outcomes according to the per-protocol analyses is shown in Table S3. No effect was found in terms of global DTI metrics (all p > 0.813). The effects of the physical activity program on tract-specific FA and MD are shown in Table S4, and represented in Figs. 2 and 3. To sum up, no effect of the physical activity program on tract-specific FA and MD was found in children with overweight or obesity (all pfdr>0.212). Per-protocol analyses (i.e., Tables S3 and S4) were replicated following the intention-to-treat principle (i.e., including participants not meeting the requirement of a minimum 70 % attendance to the physical activity program). These analyses have been presented in Tables S5 and S6. Overall, the effects shown in the intention-to-treat analyses were similar compared to the per-protocol effects. That is, no effect of physical activity was found on global (see Table S5) and tract-specific white matter microstructure (see Table S6).
Effects of the exercise program z score changes between groups in tract-specific and global fractional anisotropy. Data analyses were primarily conducted under the per-protocol principle—that is, participants attending at least 70 % of the sessions. Baseline z score of the outcomes was calculated by subtracting the mean value and dividing by the SD of each outcome. Post-exercise z scores were calculated by subtracting the baseline mean and dividing by the baseline SD, being a z score of the change in each outcome. CI= Confidence interval; FA= Fractional anisotropy. *= p < 0.05 before adjusting for multiple comparisons.
Note: Higher FA values are thought to reflect a higher degree of neuronal organization.
Effects of the exercise program z score changes between groups in tract-specific and global mean diffusivity. Data analyses were primarily conducted under the per-protocol principle—that is, participants attending at least 70 % of the sessions. Baseline z score of the outcomes was calculated by subtracting the mean value and dividing by the SD of each outcome. Post-exercise z scores were calculated by subtracting the baseline mean and dividing by the baseline SD, being a z score of the change in each outcome. CI= Confidence interval; MD= Mean diffusivity. *= p < 0.05 before adjusting for multiple comparisons.
Note: Higher MD indicates relatively unimpeded diffusion (i.e., it is negatively correlated with FA).
In line with tractography, we did not observe any effects of our physical activity program on the voxel-wise DTI parameters (i.e., FA and MD) using the most restricted TBSS approach (i.e., per protocol analyses (n = 83) and p-corrected image with the display set p < 0.05 and p < 0.1). In the intention-to-treat sample (n = 89), we found that our physical activity program had a borderline effect on 7 different clusters when the p-corrected FA map was set to p < 0.1 (See Table S7). However, no significant cluster was found when the p-corrected FA map was set to p < 0.05. The identified clusters are represented in Fig. S1. Using the JHU ICBM-DTI-81 White-Matter Labels, we observed 22.9 % of the total voxels corresponding to the limb of the internal capsule, followed by 8.5 % in the corpus callosum, 8.5 % corona radiata, 3.4 % cerebral peduncle, 1.4 % external capsule, and 3.2 % right superior fronto-occipital fasciculus. 52.1 % of the total voxels remained unclassified. Using the JHU White-Matter Tractography Atlas, we classified previously unclassified voxels as part of the anterior thalamic radiation (see Table S7). Lastly, in post hoc exploratory analyses, we calculated the pre and post-mean difference of FA in the identified clusters, and we observed the intervention group showed significantly increased levels of FA compared to the control group (mean difference=0.012, 95 % CI= 0.008 to 0.015, p < 0.001).
No significant results were found when the p-corrected MD map was set up to p < 0.1 and p < 0.05 in the intention-to-treat sample.
DiscussionThe main aim of this study was to investigate the effects of a 20-week randomized clinical physical activity trial on global white matter microstructure in children with overweight or obesity. To determine whether the effect of physical activity on white matter microstructure was indeed global or focal, the effect of physical activity on FA and MD within individual tracts and white matter regions was also tested. Briefly, we found that a 20-week physical activity intervention had no significant effect on global and tract-specific white matter microstructure in children with overweight or obesity. Due to our small sample size, our whole-brain and restricted approach, and the observed borderline effects in line with previous less restricted regions of interest approaches, more research is needed to confirm whether physical activity positively affects specific white matter tracts (e.g., the corpus callosum) during childhood.
This intervention study was unable to demonstrate that physical activity affects global and local white matter microstructure during childhood. These results do not align with those from previous cross-sectional studies (Rodriguez-Ayllon et al., 2020b; Rodriguez-Ayllon et al., 2020a). For instance, in over 2500 children from the Generation R Study, it was found that total self-reported physical activity was positively associated with global FA and negatively associated with global MD (Rodriguez-Ayllon et al., 2020a). These results were partially confirmed in a sample of children overweight or obese from the ActiveBrains study, where self-reported and objectively measured physical activity was positively associated with global FA (Rodriguez-Ayllon et al., 2020b). There are several possible explanations for these discrepancies. First, it is unclear whether the reverse relationship could be explaining the positive association observed in previous cross-sectional studies (i.e., those children with greater global white matter microstructure practice more physical activity over time). In this line, Hofman et al. (2022) found that greater global white matter microstructure is associated with higher levels of walking among older people. However, the reverse association was not observed (i.e., higher levels of walking were not associated with global white matter microstructure). Hence, more prospective cohort studies are needed to explore the bidirectional relationship between physical activity and white matter microstructure during childhood. Other possible explanations could be that a 20-week physical activity intervention might not be enough to induce global changes in white matter microstructure during childhood or that short-term physical activity interventions affect only certain white matter regions instead of the whole brain (Chaddock-Heyman et al., 2018; Krafft et al., 2014b; Schaeffer et al., 2014). Accordingly, a previous 8-month physical activity intervention showed that, compared to controls, children with overweight in the physical activity group increased FA in sections of the uncinate fasciculus and superior longitudinal fasciculus (Krafft et al., 2014b; Schaeffer et al., 2014). In addition, another 9-month physical activity intervention showed that, compared to controls, those children who participated in the physical activity program showed increased FA in the genu of the corpus callosum (Chaddock-Heyman et al., 2018). In an exploratory voxel-wise analysis, we found that physical activity had a borderline non-significant effect on 7 tracts including the corpus callosum. Therefore, more intervention studies with larger sample sizes are needed to confirm whether the effects of physical activity on white matter microstructure could be restricted to local changes in particular white matter tracts during childhood.
Previous intervention studies observed a positive effect of physical activity on cognition during childhood (Davis et al., 2011; Ortega et al., 2022). However, the neurobiological mechanisms through which physical activity benefits cognitive health are not clear and could vary across the lifespan. For instance, Yotsumoto et al. (2014) indicated that significant changes in FA in the white matter only occurred with older individuals after training on a texture discrimination task for 3 daily sessions. They suggested that mechanisms for visual perceptual learning were different between the younger and older groups. In particular, they hypothesized that while in young people the improvement might be explained by the strengthening of synaptic efficacy (Schwartz et al., 2002) in terms of activity/activation, in older adults changes might be reflected by FA values, in terms of axonal transmission related to myelination, axon caliber or crossing fibers in white matter. Supporting this hypothesis, a few studies found evidence that physical activity may influence brain networks and task-evoked activation patterns in children (Chaddock-Heyman et al., 2013; Davis et al., 2011; Krafft et al., 2014a; Naidoo et al., 2014). However, those studies showed inconsistent activation patterns and had small sample sizes, which precludes the ability to make definitive conclusions. In addition, this hypothesis was not supported by our recently published paper where we observed that physical activity affected cognition and academic achievement during childhood but not the functional connectivity between specific regions associated with cognition (i.e., hippocampal and prefrontal cortex subregions) (Ortega et al., 2022). Overall, future studies with larger sample sizes and defined connectivity patterns using fMRI and DTI data are needed to understand how physical activity interventions could affect brain connectivity during childhood.
Lastly, not only the duration of the program (i.e., 4.5 months vs. more than 8 months) might be important to induce changes in the white matter during childhood, but also the type of intervention (e.g., aerobic training vs. muscular training). Findings from the ActiveBrains project revealed that those children with higher muscular fitness showed greater white matter in terms of volume (Esteban-Cornejo et al., 2019) and microstructure (Rodriguez-Ayllon et al., 2020a). While cardiorespiratory fitness and motor fitness were also related to white matter volume, these associations were weaker (Esteban-Cornejo et al., 2019). In addition, no association was found between cardiorespiratory fitness and motor fitness with white matter microstructure in those children (Rodriguez-Ayllon et al., 2020a). Consistent with the literature, a network meta-analysis found that high-intensity and frequent resistance exercises may be the most effective, followed by exergames, aerobic exercises, and mind–body exercises (Wang et al., 2019) to improve cognition in adults. Our physical activity intervention, which consisted of 60 min of aerobic exercise and 20 min muscular training, improved cardiorespiratory fitness but not muscular fitness (Migueles et al., 2023), and it could be one of the reasons we did not find any significant effect on white matter microstructure. Therefore, muscular training interventions to improve muscular fitness are needed to test if muscular fitness influences white matter in young people.
Limitations and strengthsSome limitations need to be considered. First, our effects are limited to a sample of children with overweight or obesity. Second, the sample size of this study might seem relatively small (n = 83), however, it is the second largest trial (after the FITitKid2 trial with n = 143 (Chaddock-Heyman et al., 2018)) examining the effects of physical activity on white matter microstructure. Third, it is unknown whether the effects observed would have been larger if the intervention would have lasted longer. Lastly, the voxel size was a 4-mm-section nonisotropic voxel (1.8 × 1.8 × 4 mm3). Therefore, FA could be underestimated in regions containing crossing fibers (i.e., superior longitudinal fasciculus), although the FA measured in regions without crossing fibers (i.e., corticospinal tract) is not prone to underestimation (Oouchi et al., 2007).
Strengths of the present randomized controlled trial were to be one of the few examining the chronic effects of physical activity on white matter microstructure in children, the intention-to-treat exploratory analyses, and the design, which allows causal inferences (i.e. it is the strongest empirical evidence of a treatment's efficacy).
ConclusionsWe found that a 20-week physical activity intervention did not affect global and local white matter microstructure in children with overweight or obesity. However, the effects of physical activity on white matter microstructure could be restricted to specific white matter microstructure regions (e.g., the body of the corpus callosum), where we found a borderline non-significant effect. Then, future work using different approaches is needed to explore whether physical activity definitively has a positive effect on white matter microstructure during childhood.