Background/Objective: Cancer and its treatment can have a detrimental impact on psychological well-being. Acceptance as the basis of acceptance and commitment therapy (ACT) has shown beneficial effects on depression and anxiety. However, its relationship to fatigue and cognitive impairment has not been investigated. A protective effect of acceptance may open up a new target for psychological intervention.
Method: A cross-sectional postal survey was undertaken. 922 hematological cancer survivors (≥ 2.5 years post diagnosis) were recruited through two regional cancer registries in Germany. Acceptance (AAQ-II), fatigue (BFI) and subjective cognitive impairment (AFI) were assessed.
Results: Higher levels of acceptance were negatively associated with fatigue and subjective cognitive impairment (R2= .34 and R2= .26, respectively). The relationship between fatigue and fatigue-related impairment of daily life was weaker for survivors with high acceptance.
Conclusions: Acceptance is strongly associated with fatigue and subjective cognitive impairment. ACT may be useful to reduce symptoms of fatigue and subjective cognitive impairment in cancer survivors.
Antecedentes/Objetivo: El cáncer y su tratamiento pueden tener un impacto perjudicial sobre el bienestar psicológico. La aceptación, base de la terapia de aceptación y compromiso (ACT), ha mostrado efectos beneficiosos sobre la depresión y la ansiedad. Sin embargo, su relación con la fatiga y el deterioro cognitivo no ha sido investigada. Un efecto protector de la aceptación puede abrir un nuevo objetivo para la intervención psicológica.
Método: Se llevó a cabo un estudio transversal de encuesta por correo. Un total de 922 supervivientes al cáncer hematológico (≥ 2,5 años después del diagnóstico) fueron reclutados a través de dos registros regionales en Alemania. Se evaluaron la aceptación (AAQ-II), la fatiga (BFI) y el deterioro cognitivo subjetivo (AFI).
Resultados: Los niveles elevados de aceptación se asociaron negativamente con la fatiga y el deterioro cognitivo subjetivo (R2= 0,34 y R2= 0,26, respectivamente). La relación entre fatiga y deterioro ede la vida diaria relacionado con la fatiga fue más débil en supervivientes con una mayor aceptación.
Conclusiones: La aceptación se asocia fuertemente con la fatiga y el deterioro cognitivo subjetivo. La ACT puede ser útil para reducir los síntomas de fatiga y el deterioro cognitivo subjetivo en supervivientes al cáncer.
Hematologic cancer is highly distressing. Next to physical threat of the cancer disease, it influences all aspects of daily life including family, friends, work and financial situation (Mehnert, 2011; Weis & Faller, 2012). As such, it is not surprising that hematologic cancer and its treatment can have a detrimental impact on mental health and quality of life. Among the large variety of psychosocial symptoms that may occur, fatigue (Esser, Kuba, Mehnert et al., 2017;Kreissl et al., 2016; Yeo & Cannaday, 2015) and cognitive impairment (Dhillon et al., 2018; Scherwath et al., 2013) seem to be of particular interest: Fatigue has repeatedly been shown to be the most frequent and persistent symptom (Esser, Kuba, Scherwath et al., 2017; Yeo & Cannaday, 2015) and is experienced as strongly conflicting with daily life (Yeo & Cannaday, 2015). Similarly, cognitive impairment has been found to significantly impact quality of life and to interfere with daily functioning (Dhillon et al., 2018).
In order to tackle the complexity and diversity of psychological symptoms among cancer survivors, survivors need psychological strategies that are holistic and applicable to not only one diagnosis with a certain set of symptoms (transdiagnostic) (Dindo, van Liew, & Arch, 2017; Graham, Gouick, Krahe, & Gillanders, 2016; Hulbert-Williams, Storey, & Wilson, 2015; Kuba & Weißflog, 2017). A way of dealing with inner experiences that has been emphasized by practitioners and researchers throughout the last years is acceptance. Acceptance forms the target variable for acceptance and commitment therapy (ACT; Hayes, Strosahl, & Wilson, 1999), which is part of the third wave of cognitive and behavioral therapy. In the ACT-specific literature, acceptance is usually called ‘psychological flexibility’. However, since acceptance plays a central role in ACT and is a more intuitive description for the concept than psychological flexibility, we will use the term acceptance throughout this article. Acceptance is defined by staying in contact with the present moment, which means to accept any emotional, cognitive or physical experiences without regulating or controlling them. Furthermore, it entails commitment, which means that behavior persists or is changed in order to pursue personal values and goals (Hayes et al., 1999). Since acceptance involves facing (as opposed to avoiding) the present moment, which may also include symptoms, the primary goal of ACT is not to reduce symptoms but to live a valued life irrespective of unwanted bodily states, cognitions and emotions. As such, acceptance promotes mental health by reducing the impact of negative stimuli on well-being and by remaining committed to pursuing personal goals (Dindo et al., 2017; Hayes et al., 1999; Kuba & Weißflog, 2017). ACT and the underlying mechanism of acceptance have been advocated as especially useful for cancer patients because of their transdiagnostic and holistic approach, which makes it applicable to the wide range of symptoms of cancer survivors and facilitate symptom management. Its beneficial effect on depression and anxiety has been verified in a couple of studies among patients with cancer (Aguirre-Camacho et al., 2017; Feros, Lane, Ciarrochi, & Blackledge, 2013; Hulbert-Williams & Storey, 2016) as well as among non-cancer patient populations with mental health issues (A-Tjak et al., 2015). However, despite its transdiagnostic claim, no previous study has investigated its relationship to consequences of cancer such as fatigue and cognitive impairment.
It can be argued that accepting individuals tend to refrain from controlling negative emotions and other internal states and thus do not employ cognitive control when negative emotions occur (Hayes et al., 1999). This cognitive resource-saving benefit of acceptance has been studied scantly, but one study has shown that acceptance (as opposed to avoidance) during a sad mood induction task predicted a better performance on a subsequent cognitive task (Alberts, Schneider, & Martijn, 2012). Furthermore, two work psychological studies have shown that acceptance is associated with less emotional exhaustion (Biron & van Veldhoven, 2012) and less fatigue (Kuba & Scheibe, 2017). As such, acceptance seems to have a protective effect on cognitive resources, which may express in lower levels of fatigue and cognitive impairment.
The ACT model proposes that suffering is part of human life. By accepting painful physical or emotional experiences the way they are and by refraining from attempting to control them, focus can be diverted from the symptom (aversive experience) to important goals and values (Hayes et al., 1999). As such, impact of a symptom on daily life functioning should be lower in accepting individuals.
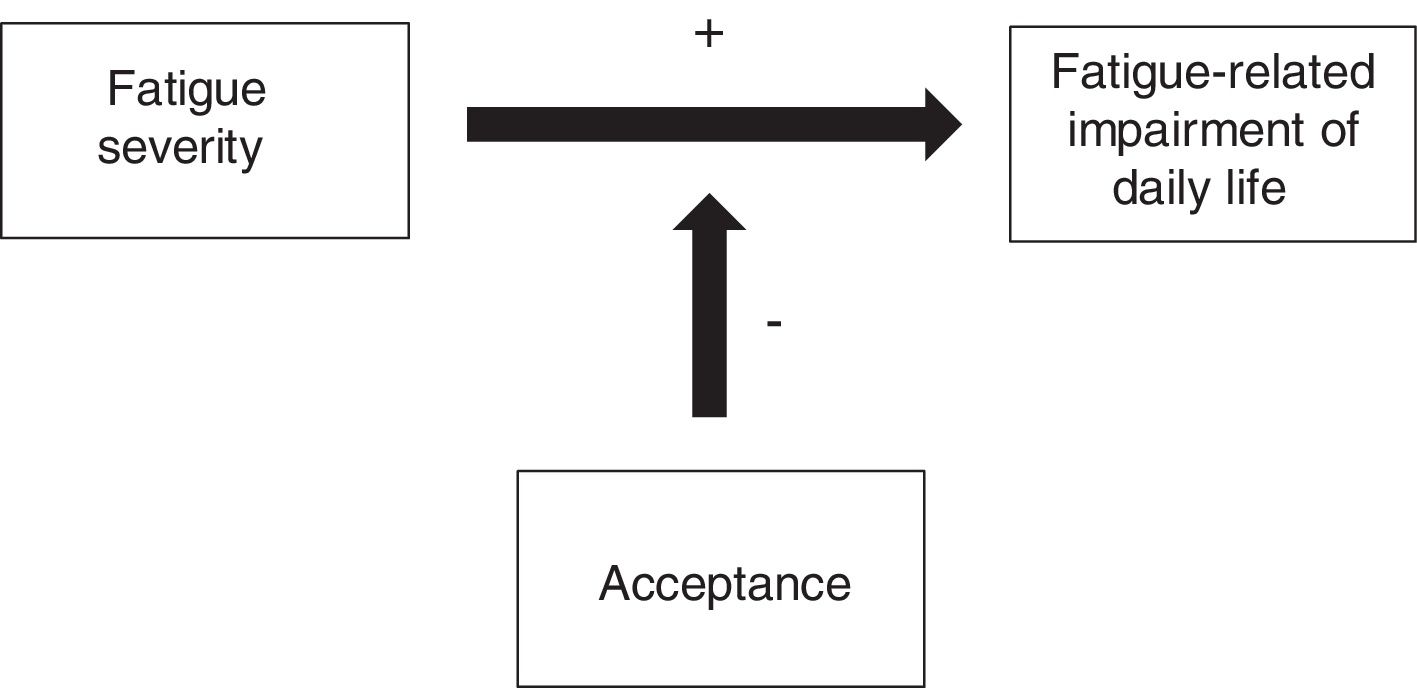
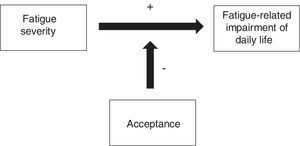
The present study aims to investigate the correlation between acceptance and fatigue and subjective cognitive impairment in hematologic cancer survivors. The goal is to identify a new target for psychological interventions, specifically ACT-based interventions. Based upon the described theoretical background we hypothesize the following: (H1) Acceptance is associated with less subjective cognitive impairment in hematologic cancer survivors; (H2) Acceptance is associated with less fatigue in hematologic cancer survivors; and (H3) The negative impact of fatigue on daily life is buffered by high levels of acceptance in hematologic cancer survivors. Figure 1 graphically displays this hypothesized effect.
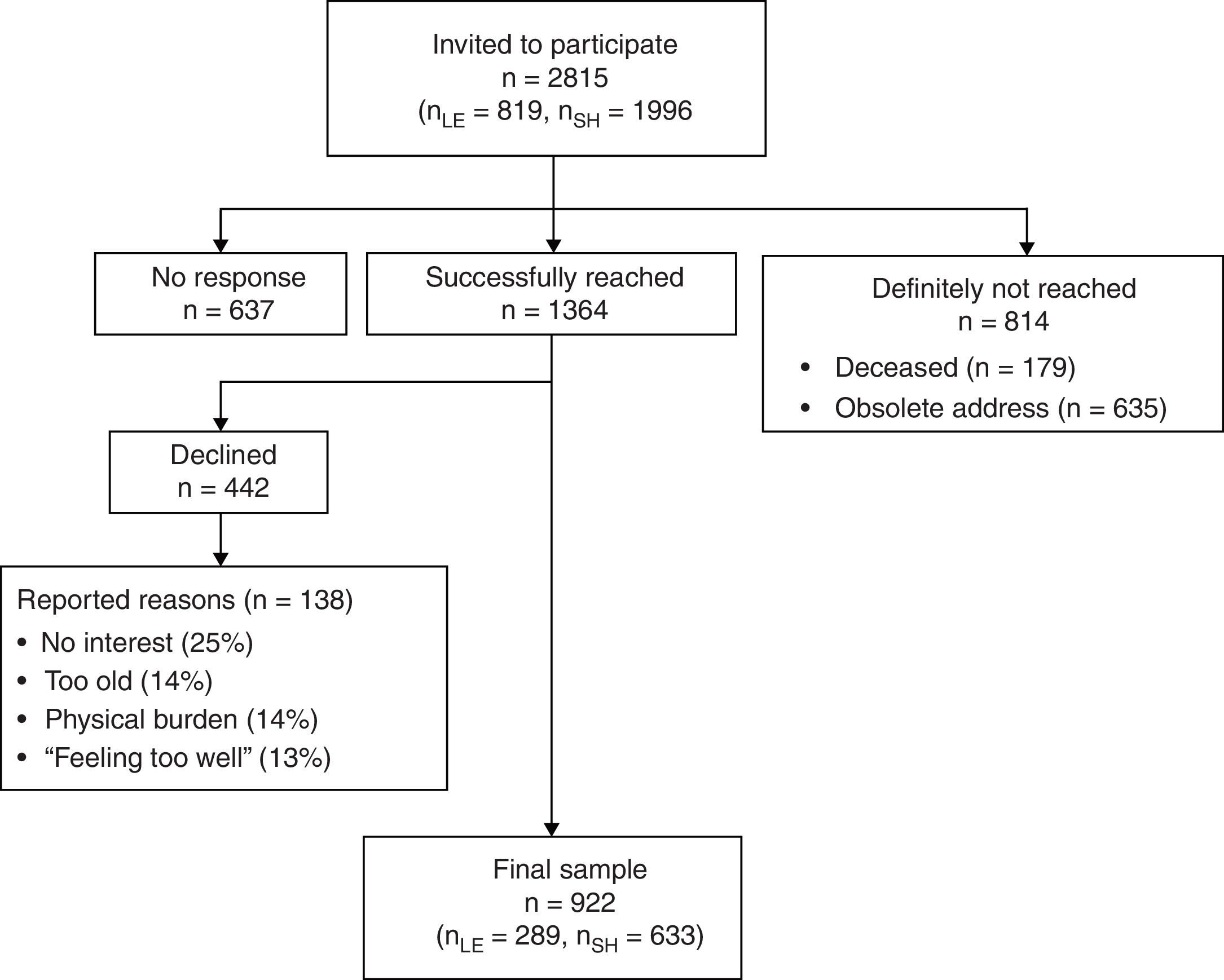
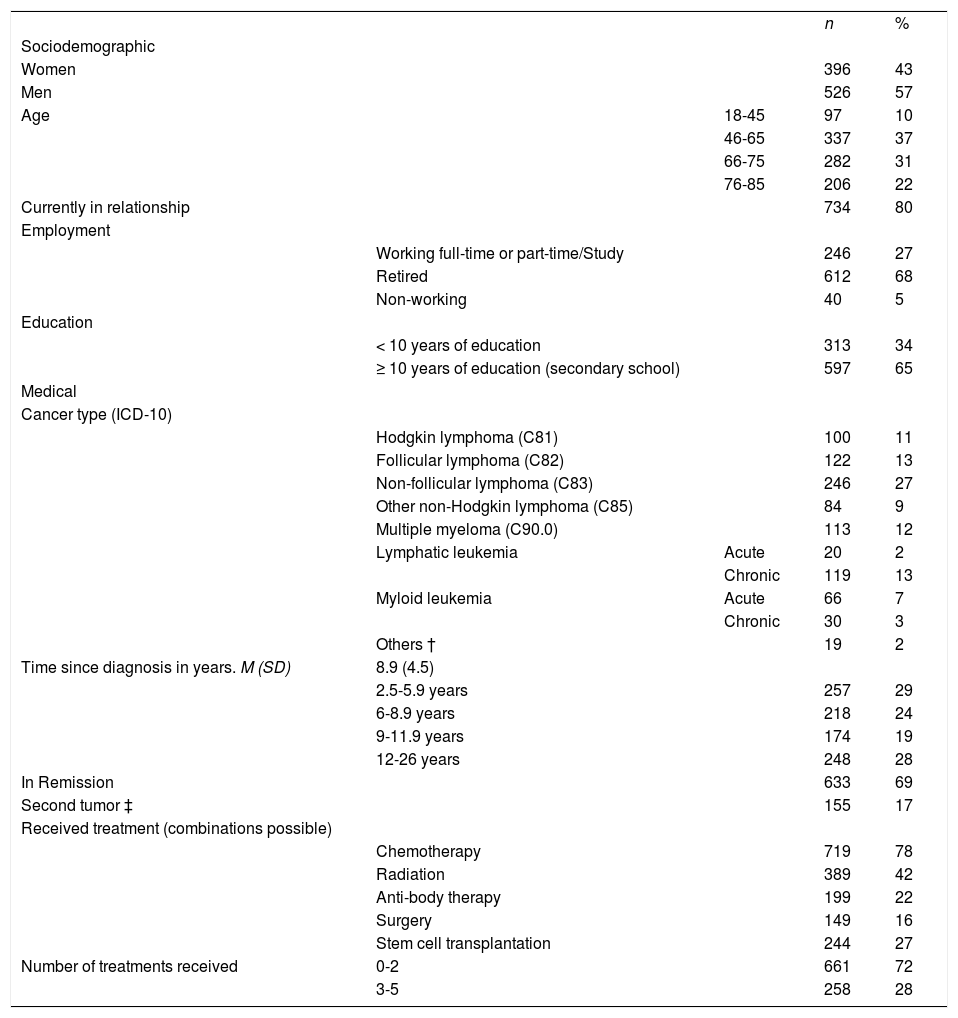
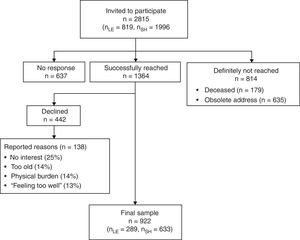
Nine hundred and twenty two survivors participated in the study. Mean age of the survivors was 64 years (SD = 13.4) and 43% of the participants were female. Detailed demographic and medical information of the sample can be found in Table 1. Non-responders (n=1,074) were significantly younger than responders (n=922) (p=.001) but did not differ in terms of gender, diagnosis or years since diagnosis. Three patients had more than 80% of missing data and were excluded from all calculations.
Sample characteristics of 922 hematologic cancer survivors.
| n | % | |||
| Sociodemographic | ||||
| Women | 396 | 43 | ||
| Men | 526 | 57 | ||
| Age | 18-45 | 97 | 10 | |
| 46-65 | 337 | 37 | ||
| 66-75 | 282 | 31 | ||
| 76-85 | 206 | 22 | ||
| Currently in relationship | 734 | 80 | ||
| Employment | ||||
| Working full-time or part-time/Study | 246 | 27 | ||
| Retired | 612 | 68 | ||
| Non-working | 40 | 5 | ||
| Education | ||||
| < 10 years of education | 313 | 34 | ||
| ≥ 10 years of education (secondary school) | 597 | 65 | ||
| Medical | ||||
| Cancer type (ICD-10) | ||||
| Hodgkin lymphoma (C81) | 100 | 11 | ||
| Follicular lymphoma (C82) | 122 | 13 | ||
| Non-follicular lymphoma (C83) | 246 | 27 | ||
| Other non-Hodgkin lymphoma (C85) | 84 | 9 | ||
| Multiple myeloma (C90.0) | 113 | 12 | ||
| Lymphatic leukemia | Acute | 20 | 2 | |
| Chronic | 119 | 13 | ||
| Myloid leukemia | Acute | 66 | 7 | |
| Chronic | 30 | 3 | ||
| Others † | 19 | 2 | ||
| Time since diagnosis in years. M (SD) | 8.9 (4.5) | |||
| 2.5-5.9 years | 257 | 29 | ||
| 6-8.9 years | 218 | 24 | ||
| 9-11.9 years | 174 | 19 | ||
| 12-26 years | 248 | 28 | ||
| In Remission | 633 | 69 | ||
| Second tumor ‡ | 155 | 17 | ||
| Received treatment (combinations possible) | ||||
| Chemotherapy | 719 | 78 | ||
| Radiation | 389 | 42 | ||
| Anti-body therapy | 199 | 22 | ||
| Surgery | 149 | 16 | ||
| Stem cell transplantation | 244 | 27 | ||
| Number of treatments received | 0-2 | 661 | 72 | |
| 3-5 | 258 | 28 |
Note. † International Classification of Diseases. version 10; C 90.2; Extramedullary plasmacytoma; C 91.5. Adult T-cell leukemia; C 93 Monocytic leukemia; C 94. other leukemias of specified cell type; C 95. other leukemias of unspecified cell type. ‡ before or after hematological malignancy.
Acceptance was measured with the Acceptance and Action Questionnaire (AAQ-II; Bond et al., 2011), which is translated in German and has shown good psychometric properties (Gloster, Klotsche, Chaker, Hummel, & Hoyer, 2011).The scale contains 7 items that are rated on a seven-point Likert scale ranging from 1 (never true) to 7 (always true). Examples of items are “I am afraid of my feelings” or “Worries get in the way of my success”. Cronbach's alpha in our sample indicated excellent reliability (α=.93). Scores are reverse-coded ranging from 1 to 7 with high scores representing high acceptance.
The validated Attentional Function Index (AFI; Cimprich, Visovatti, & Ronis, 2011) assesses subjective cognitive impairment in common daily life activities focusing on attention and working memory that are thought to be most commonly impaired in cancer survivors. We translated this instrument following the state-of-the-art procedure applying forward-translation and back-translation. 13 items are rated on a visual analogue scale ranging from 0 (not at all) to 10 (extremely well/a great deal). Cronbach's alpha in the present sample was .90.
The Brief Fatigue Inventory (BFI; Mendoza et al., 1999) is validated in German (Radbruch et al., 2003) and assesses fatigue in clinical populations. Patients rate the intensity of fatigue on 3 items (at the moment, on average, strongest in the last 24h.) and its impact on 6 items (general activity, mood, walking ability, normal work, relations with others and enjoyment of life). Statements can be rated on a 10-point scale ranging from 0 (no fatigue/does not interfere) to 10 (as bad as you can imagine/completely interferes). Next to an overall fatigue score including all items, scores for severity und impairment can be created by building the means of the respective items. Reliability was .94 for the total score.
Depressive symptomatology was assessed using the Patient Health Questionnaire for Depression (PHQ-9; Spitzer, Kroenke, Williams, & Löwe, 2006; Spitzer, Kroenke, Williams, & Patient Health Questionnaire Primary Care Study Group, 1999), which is a widely-used self-report measure that has been validated in different populations (Gräfe, Zipfel, Herzog, & Löwe, 2004; Hinz et al., 2016). The module scores the occurrence during the last two weeks of each of the 9 DSM-IV criteria of depression on a 4-point Likert scale from not at all to nearly every day. In the current study, reliability was excellent with Cronbach's α of .95.
We assessed anxious symptoms using the Generalized Anxiety Disorder Screener (GAD-7; Spitzer et al., 2006). The one-dimensional scale has been validated in German and shows good psychometric properties (Löwe et al., 2008; Spitzer et al., 2006). For 7 items participants rated on a four-point Likert scale how often symptoms occurred during the last 2 weeks ranging from not at all to nearly every day. The GAD-7 shows good reliability in the present study (α = .89).
ProcedureThe present study was part of a large cross-sectional observational survey on hematologic cancer survivors in Germany. A detailed description of the study has been described in a study protocol (Esser, Kuba, Götze, & Mehnert, 2017). Results on quality of life, depression and anxiety have been published elsewhere (Esser et al., 2018). We received the addresses of all registered individuals that were diagnosed with hematologic cancer through the Clinical Cancer Registry of Leipzig and the Cancer Registry of the federal state of Schleswig-Holstein. Eligible patients had been diagnosed with hematological cancer (ID-10: C81-C96) at least 2.5 years ago, had a minimum age of 18 years at time of diagnosis and maximum age of 85 at time of assessment. Suitable patients were contacted by mail. When they agreed to participate, they signed the declaration of consent, completed the questionnaire and sent back both documents in a postage-paid envelope. Alternatively, patients could participate online using the software LimeSurvey (LimeSurvey GmbH, 2015). All data were collected between June 2015 and August 2017. Of 2001 patients that were reached, 922 participated, which resulted in a response rate of 46% (see recruitment flowchart in Figure 2). Study approval was obtained from the ethics committee of the Medical Faculty at the University of Leipzig (File number: 292-15-24082015).
AnalysesParticipants and non-responders were compared in terms of age and years since diagnosis by t-tests for independent groups and in terms of gender and diagnosis by chi-square tests. First, the relationship between acceptance and subjective cognitive impairment (H1) and fatigue (H2) was calculated by two hierarchical regression models using a three-block entry method. In each first block, the relationship between the acceptance and the outcome variables were assessed without the impact of any covariates (unconditional models). In the second block, demographic and medical variables were added and in a third step depression and anxiety (conditional models). Second, in order to test hypothesis 3, we investigated the association between fatigue severity and impairment by fatigue at different levels of acceptance (moderation analysis). The interaction term of fatigue severity and acceptance, which constitutes the moderation effect of acceptance on the relationship of fatigue and fatigue-related impairment, was included in a second step in order to assess its incremental effect for the model. To avoid potentially problematic multicollinearity with the interaction term and to facilitate interpretation, variables for the interaction (acceptance and fatigue severity) were centered by their mean. Simple slopes were investigated between fatigue severity and fatigue impairment for individuals with high (one SD above the mean) and low (one SD below the mean) acceptance using a tool developed by Preacher, Curran and Bauer (2006). For all models,R2 and the squared semi-partial correlation (sr2) were reported to determine the contribution of the variables to the explained variance. The squared semi-partial correlation can be interpreted as the improvement in model fit (R2-change) if only that respective variable had been added to the model. The control variables gender, education (≥ 10 years, i.e., secondary school or higher), having a partner, and time since diagnosis were consistently non-significant across all models and thus were excluded for reasons of parsimony. Age, working status (employment/education vs. non-working) and number of treatments were included as covariates. We performed the analyses with the Statistical Package for the Social Sciences (SPSS) (IBM Corp., Released 2011) version 20.
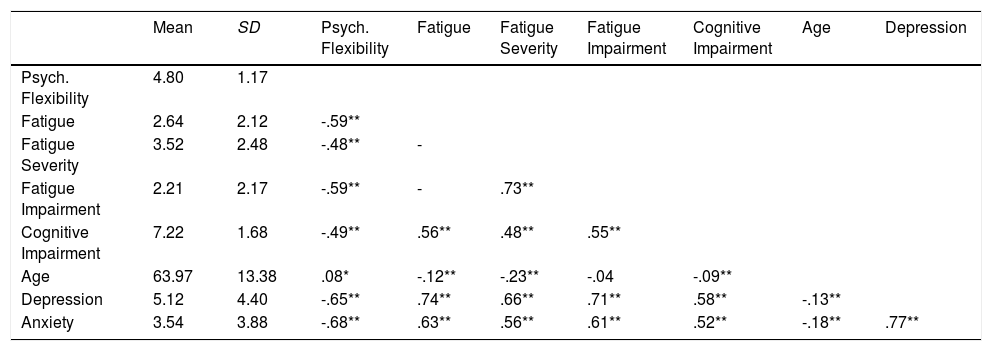
ResultsAcceptance was strongly negatively related to cognitive impairment, to the fatigue variables and to depression and anxiety. Descriptive information and correlations between the test variables are presented in Table 2.
Descriptive and correlational information of the variables.
| Mean | SD | Psych. Flexibility | Fatigue | Fatigue Severity | Fatigue Impairment | Cognitive Impairment | Age | Depression | |
|---|---|---|---|---|---|---|---|---|---|
| Psych. Flexibility | 4.80 | 1.17 | |||||||
| Fatigue | 2.64 | 2.12 | -.59** | ||||||
| Fatigue Severity | 3.52 | 2.48 | -.48** | - | |||||
| Fatigue Impairment | 2.21 | 2.17 | -.59** | - | .73** | ||||
| Cognitive Impairment | 7.22 | 1.68 | -.49** | .56** | .48** | .55** | |||
| Age | 63.97 | 13.38 | .08* | -.12** | -.23** | -.04 | -.09** | ||
| Depression | 5.12 | 4.40 | -.65** | .74** | .66** | .71** | .58** | -.13** | |
| Anxiety | 3.54 | 3.88 | -.68** | .63** | .56** | .61** | .52** | -.18** | .77** |
Note. Pearson correlations are reported. Significance tests are two-sided. **p<.01, *p<.05.
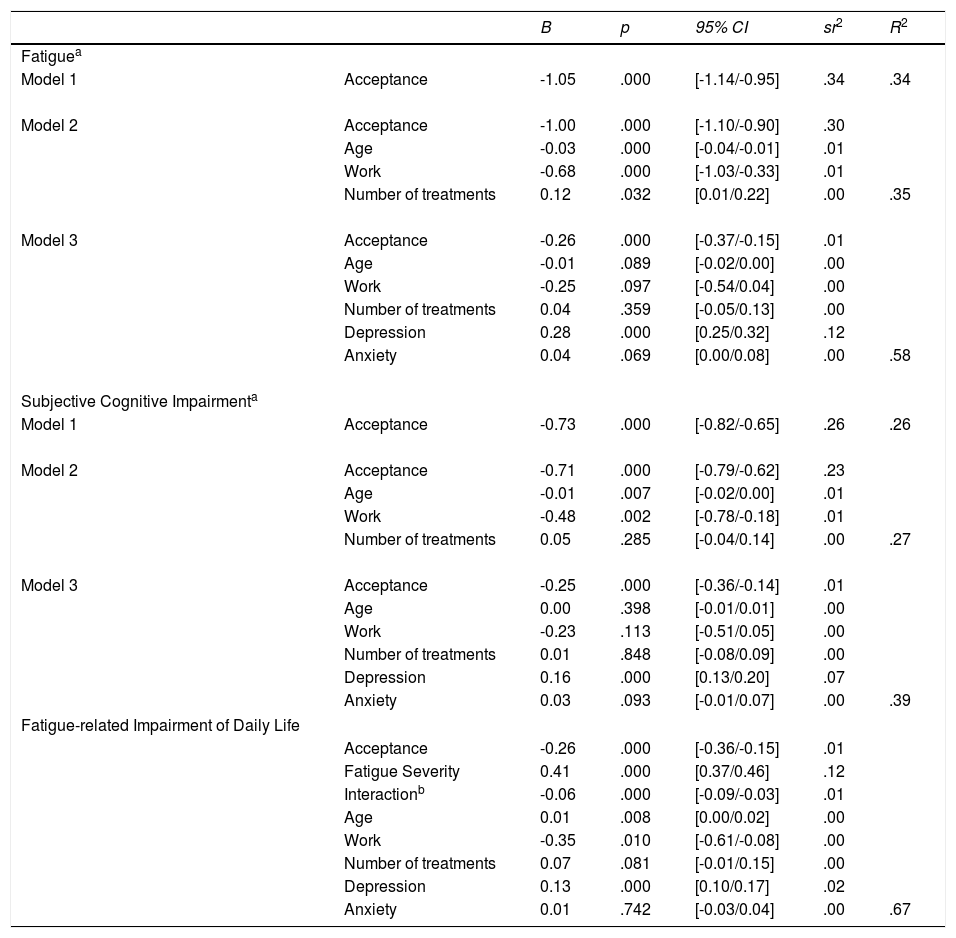
Multiple regression models revealed that hypothesis 1 and 2 could be confirmed (see Table 3). In detail, there was a significant negative relationship between acceptance and subjective cognitive impairment (H1) and fatigue (H2). Acceptance explained 26% of the variance of subjective cognitive impairment and 34% of the variance in fatigue. Both effects remained significant when control variables were entered into the model, but the unique effect of acceptance was reduced to 12% and 10%, respectively. Including depression and anxiety further reduced the uniquely shared variance to 1% in both models.
Hierarchical regression analyses of acceptance on fatigue, subjective cognitive impairment and impairment of daily life with confidence intervals.
| B | p | 95% CI | sr2 | R2 | ||
|---|---|---|---|---|---|---|
| Fatiguea | ||||||
| Model 1 | Acceptance | -1.05 | .000 | [-1.14/-0.95] | .34 | .34 |
| Model 2 | Acceptance | -1.00 | .000 | [-1.10/-0.90] | .30 | |
| Age | -0.03 | .000 | [-0.04/-0.01] | .01 | ||
| Work | -0.68 | .000 | [-1.03/-0.33] | .01 | ||
| Number of treatments | 0.12 | .032 | [0.01/0.22] | .00 | .35 | |
| Model 3 | Acceptance | -0.26 | .000 | [-0.37/-0.15] | .01 | |
| Age | -0.01 | .089 | [-0.02/0.00] | .00 | ||
| Work | -0.25 | .097 | [-0.54/0.04] | .00 | ||
| Number of treatments | 0.04 | .359 | [-0.05/0.13] | .00 | ||
| Depression | 0.28 | .000 | [0.25/0.32] | .12 | ||
| Anxiety | 0.04 | .069 | [0.00/0.08] | .00 | .58 | |
| Subjective Cognitive Impairmenta | ||||||
| Model 1 | Acceptance | -0.73 | .000 | [-0.82/-0.65] | .26 | .26 |
| Model 2 | Acceptance | -0.71 | .000 | [-0.79/-0.62] | .23 | |
| Age | -0.01 | .007 | [-0.02/0.00] | .01 | ||
| Work | -0.48 | .002 | [-0.78/-0.18] | .01 | ||
| Number of treatments | 0.05 | .285 | [-0.04/0.14] | .00 | .27 | |
| Model 3 | Acceptance | -0.25 | .000 | [-0.36/-0.14] | .01 | |
| Age | 0.00 | .398 | [-0.01/0.01] | .00 | ||
| Work | -0.23 | .113 | [-0.51/0.05] | .00 | ||
| Number of treatments | 0.01 | .848 | [-0.08/0.09] | .00 | ||
| Depression | 0.16 | .000 | [0.13/0.20] | .07 | ||
| Anxiety | 0.03 | .093 | [-0.01/0.07] | .00 | .39 | |
| Fatigue-related Impairment of Daily Life | ||||||
| Acceptance | -0.26 | .000 | [-0.36/-0.15] | .01 | ||
| Fatigue Severity | 0.41 | .000 | [0.37/0.46] | .12 | ||
| Interactionb | -0.06 | .000 | [-0.09/-0.03] | .01 | ||
| Age | 0.01 | .008 | [0.00/0.02] | .00 | ||
| Work | -0.35 | .010 | [-0.61/-0.08] | .00 | ||
| Number of treatments | 0.07 | .081 | [-0.01/0.15] | .00 | ||
| Depression | 0.13 | .000 | [0.10/0.17] | .02 | ||
| Anxiety | 0.01 | .742 | [-0.03/0.04] | .00 | .67 | |
Note. CI = Confidence Interval; LB = lower bound; UB = upper bound; sr2 = squared semi-partial correlation. The following covariates were consistently non-significant and removed from the models: gender, education (≥ 10 years), having a partner, time since diagnosis.
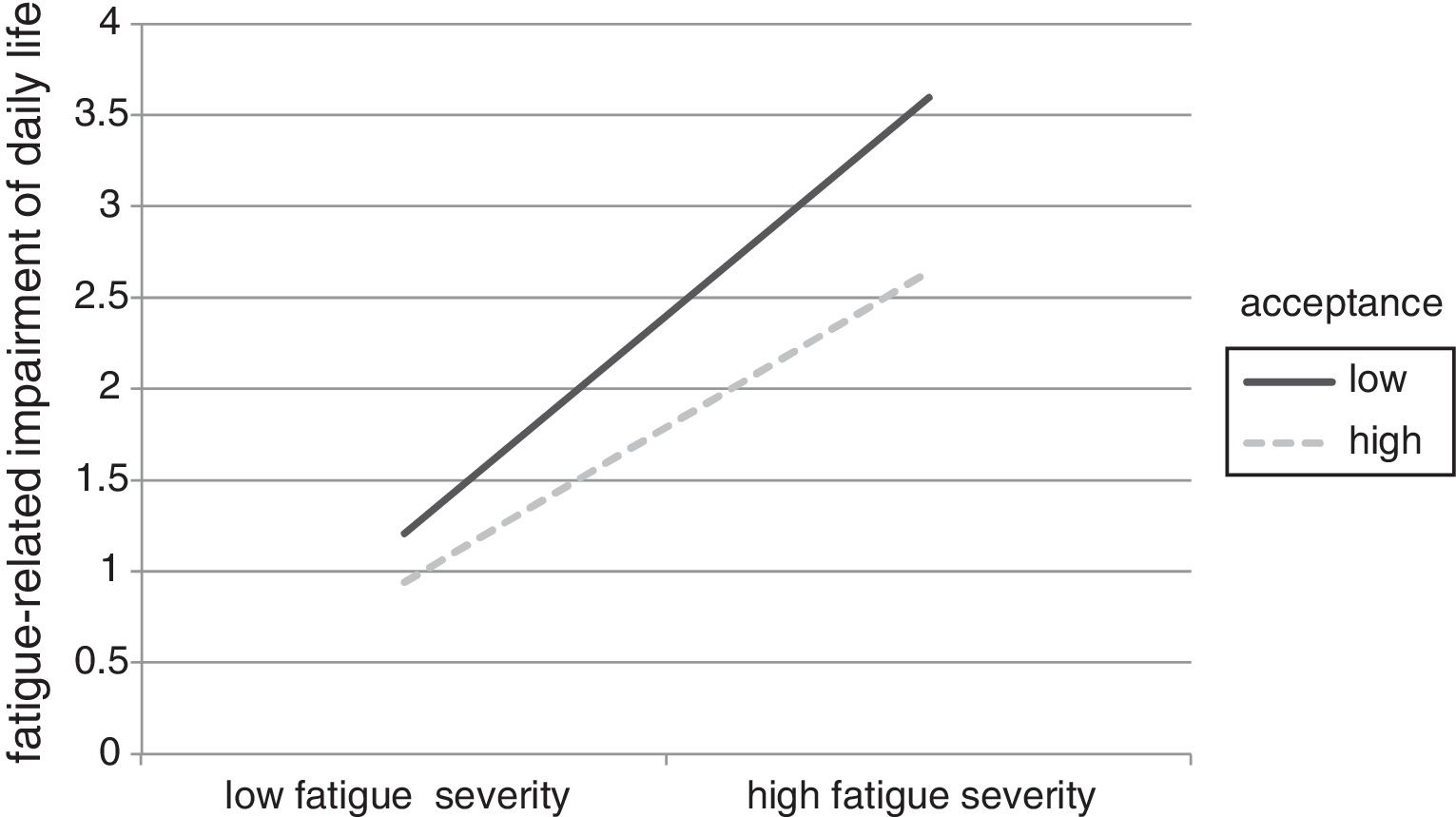
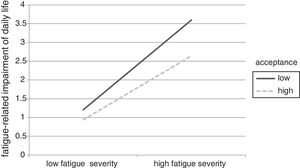
The moderation analyses revealed that the level of acceptance significantly affected the relationship between fatigue severity and fatigue-related impairment (see lower part of Table 3). In detail, main effects of acceptance and fatigue severity were significant. Including the interaction term in the analysis improved the model significantly. Investigating the simple slopes, we found that the relationship between fatigue severity and impairment is significantly weaker when acceptance is high (β=.34, 95%CI [.338/.342]) than when it is low (β=.48, 95%CI [.478/.482]). This moderation effect is displayed graphically in Figure 3.
DiscussionWhile the affective benefit of acceptance has been established in a large number of empirical studies, its benefit regarding cognitive resources has been poorly studied to date. This is the first study examining and supporting the resource-saving benefit of acceptance in a clinical sample.
Confirming hypothesis 1, our results show that acceptance is strongly negatively associated with subjective cognitive impairment. Specifically, one fourth of the variation in subjective cognitive impairment is explained by acceptance. We are aware of one study assessing the impact of emotional acceptance (resembling the construct of acceptance) on subjective cognitive performance (Alberts et al., 2012). Participants were instructed to either accept or to suppress emotions during a sad mood induction task. Accepting individuals performed better on a subsequent cognitive task than individuals suppressing emotions. Merging Albert and colleagues’ study (Alberts et al., 2012) with ours, one may carefully postulate that acceptance requires less cognitive resources and thus is associated with less subjective cognitive impairment. A second argument commending to the usefulness of acceptance for cognitive functioning is that a bunch of studies has shown that practicing meditation (a concept closely related to acceptance) improves cognitive functioning (for review, see Biegler, Chaoul, & Cohen, 2009). This is first evidence pointing to the potential usefulness of ACT-based interventions in order to manage subjective cognitive impairment in cancer survivors. Future research is needed to replicate findings and to investigate the causal effects and underlying mechanisms.
Regarding hypothesis 2, we have shown that acceptance is negatively related to fatigue. Importantly, acceptance explains about one third of the variation in fatigue, which represent large effect sizes according to Cohen's classification (Cohen, 1992). To our knowledge, two social and work psychological studies have shown this effect before (Biron & van Veldhoven, 2012; Kuba & Scheibe, 2017), which were longitudinal studies establishing a causal relationship between acceptance and fatigue. Transferring this association into the population of cancer patients sheds light on new potential targets for intervention. A large number of studies has shown that acceptance can be increased by ACT-based interventions (Graham et al., 2016), which in turn seems to be the crucial mediator for better well-being (Aguirre-Camacho et al., 2017; Feros et al., 2013). Few ACT-based intervention studies have investigated changes in fatigue: very recent pilot results of a telephone-delivered intervention with cancer patients showed moderate reductions in fatigue after the intervention and at 12-months-follow-up compared to small reductions in the active control group (Mosher et al., 2018). Conversely, however, results of an earlier telephone-delivered ACT-based intervention study did not find an intervention effect on fatigue at 6 and 12 months (Hawkes, Pakenham, Chambers, Patrao, & Courneya, 2014). There is one multiple single-case designed study (Roche, Dawson, Moghaddam, Abey, & Gresswell, 2017) and one intervention trial (Jacobsen, Kallestad, Landrø, Borchgrevink, & Stiles, 2017) assessing the effectiveness of an ACT-based intervention specifically to reduce fatigue in non-cancer patients with chronic fatigue syndrome. Even though in both studies patients had a large reduction in fatigue-scores, Jacobsen et al. (2017) reported no association between acceptance and fatigue. Beyond the scope of ACT-based interventions, cognitive behavioral therapy seems effective in reducing fatigue (Abrahams et al., 2017; Gielissen et al., 2007). However, a long-term follow-up up to 14 years after intervention has shown that half of the patients who had recovered during the intervention phase reported severe fatigue at follow-up (van Gessel et al., 2018). This high rate of relapse has been found in an earlier study with more than 500 fatigue-patients (Janse et al., 2017). Van Gessel et al. (2018) suggest that mindfulness-based interventions may help those patients that experience a relapse. We emphasize the importance of conducting randomized controlled trials to test the effects of ACT on fatigue.
Combining the results of these earlier studies with our findings, acceptance may be a strong target for a psychological intervention in order to reduce subjective cognitive impairment and fatigue in cancer patients. Given that interventions are urgently needed for patients and survivors to deal with these persistent and constraining symptoms (Kreissl et al., 2016), further research and especially randomized controlled trials are needed to test the effectiveness of ACT-based interventions in reducing subjective cognitive impairment and fatigue in cancer patients.
The postulation that psychologically flexible survivors would be less impaired by fatigue severity (H3) was supported in the current sample. This finding supports the theoretical foundations of acceptance, which postulate that refraining from attempting to control unwanted internal states reduces their impact on daily life and mental well-being (Hayes et al., 1999). The findings dovetail earlier research showing that participants with higher levels of acceptance were less affected by increased life stress (Kuba & Scheibe, 2017; Shallcross, Troy, Boland, & Mauss, 2010, study 2).
The results of the present study should be interpreted in lights of its limitations: First, the moderate response rate may have led to a biased participant group, which may for example be more or less burdened than the complete population of hematologic cancer survivors. However, a subsample of participants (N=138) provided reasons for study denial including physical burden (13%) and “feeling too well” (14%). It thus seems that particularly burdened patients as well as particularly well patients refrained from study participation at a same rate leveling each other out. A second limitation is the cross-sectional study design. Even though due to the nature of the statistical tests we used directive language in the results section, we cannot draw conclusions about the causal effect of acceptance. Future research my overcome this limitation by implementing longitudinal and experimental studies.
We have shown that acceptance is strongly negatively associated with fatigue and subjective cognitive impairment. Because consequences of cancer like fatigue and subjective cognitive impairment cannot always be avoided or treated medically, we showed that a protecting factor may be the manner in which emotions and cognitions are processed. Survivors who habitually have higher levels of acceptance seem to show fewer symptoms of fatigue and subjective cognitive impairment. Given that acceptance can be increased by ACT-based interventions, a new potential target for a psychological intervention has opened up in order to deal with these symptoms.
A second important finding is that survivors who have a habitual tendency to accept emotions and cognitions and maintain current goal pursuit seem well-equipped to maintain functioning in daily life even when confronted with symptoms of fatigue. Findings highlight the suitability of ACT- interventions for medical conditions when suffering can be limited even when the symptom itself may not be reduced.
ACT-based interventions may be tailored to the special circumstances of patients with fatigue and subjective cognitive impairment. However, given that ACT follows a holistic and transdiagnostic approach, future research may investigate whether a general ACT-approach may already be effective for the wide range of psychosocial consequences following cancer. Finally, while ACT has been delivered in face-to-face consultations (Feros et al., 2013), it has also shown effective in group-settings (Aguirre-Camacho et al., 2017), in self-help formats (Wersebe, Lieb, Meyer, Hofer, & Gloster, 2018), and newer formats like web-based or telephone-based programs (Hawkes et al., 2014; Mosher et al., 2018; Pots et al., 2016). These are often economically more cost-efficient, have a lower threshold for participation and can be delivered to patients at home (Heber et al., 2017). As such, ACT may constitute an effective and efficient intervention to increase levels of acceptance and thus reduce suffering from fatigue and subjective cognitive impairment. While its effect on fatigue has been investigated in a few attempts, its effect on subjective cognitive impairment has never been studied. We emphasize the importance of conducting randomized controlled trials that assess the impact of this format on (long-term) effects of cancer like fatigue and subjective cognitive impairment.
Acknowledgements and fundingWe thank all survivors who participated and Philipp Göbel for helping us to collect the data. We acknowledge support from the German Research Foundation (DFG) and Leipzig University within the program of Open Access Publishing. The work was supported by the Deutsche José Carreras Leukämie-Stiftung e.V. [grant number DJCLS R 14/18]. The funding source had no involvement in the study design, collection, analysis and interpretation of data.