A high percentage of patients relapse within months following an attempt to quit smoking. For this reason, greater understanding of the determinants of successful smoking cessation is needed. The present study assessed the effect of Contingency Management (CM) combined with Cognitive-Behavioral Treatment (CBT) on certain in-treatment behaviors (treatment retention, in-treatment smoking abstinence, and weekly decrease of cotinine levels) and examined the effects of these in-treatment behaviors on smoking status at a 6-month follow-up. A total of 154 treatment-seeking patients in a community setting were randomly assigned to a CBT, CBT plus CM for Abstinence (CMA) or to a CBT plus CM for Shaping cessation (CMS) group. Both CBT+CM procedures improved the in-treatment behaviors compared to CBT alone. These in-treatment behaviors (particularly in-treatment smoking abstinence) were associated with long-term abstinence. The effect of CM on in-treatment behaviors may partially explain the positive long-term outcomes of this procedure. Our findings extend previous knowledge about the effect of CM on smoking behavior.
Un alto porcentaje de pacientes recae en cuestión de meses después de un intento para dejar de fumar. Por esta razón, es necesaria una mayor comprensión de los determinantes del éxito para dejar de fumar. Este estudio evaluó el efecto del Manejo de Contingencias (MC) combinado con un Tratamiento Cognitivo-Conductual (TCC) sobre ciertas conductas intra-tratamiento (tasas de retención, abstinencia durante el tratamiento y reducción semanal de los niveles de cotinina) y examinó los efectos de estas conductas sobre el consumo de tabaco a los 6 meses de seguimiento. Un total de 154 pacientes que buscaban tratamiento en un entorno comunitario fueron asignados aleatoriamente a TCC, TCC más MC para Abstinencia (MCA) o TCC más MC con Moldeamiento (MCM). Ambos procedimientos de TCC+MC mejoraron las conductas intra-tratamiento en comparación con TCC solo. Estas conductas (particularmente abstinencia durante el tratamiento) se asociaron con la abstinencia a largo plazo. El efecto del MC sobre las conductas intra-tratamiento puede explicar parcialmente los resultados positivos a largo plazo de este procedimiento. Nuestros hallazgos amplían el conocimiento previo acerca del efecto del MC sobre la conducta de fumar.
Tobacco smoking continues to be the health problem that causes more mortality and morbidity in Spain (Díaz-Gete et al., 2013) and appears to be negatively associated with quality of life (Becoña et al., 2013). Despite significant progress being made in the field of smoking cessation treatments, a high percentage of patients relapse within months of a quit attempt (García-Rodríguez et al., 2013), so more effective intervention strategies containing specific long-term relapse prevention components are needed (Alessi, Petry, & Urso, 2008). In addition, identifying the predictors of long-term success is essential for improving treatments for smoking cessation.
Certain individual and environmental factors moderate both long-term abstinence and relapse in patients who have received treatment for smoking cessation. Being male or having received a higher level of education usually increases the likelihood of quitting (Dorner, Troestl, Womastek, & Groman, 2011; Ferguson et al., 2003; Higgins et al., 2009). Later initiation, lower nicotine dependence, a longer duration of prior abstinence and a higher stage of change are also related to better long-term outcomes (Dorner et al., 2011; Ferguson et al., 2003). Conversely, some factors are related to a lower likelihood of quitting, such as illicit substance use (Winhusen et al., 2014), having a social and family smoking context (García-Rodríguez, Suárez-Vázquez, Santonja-Gómez, Secades-Villa, & Sánchez-Hervás, 2011) or some psychopathological factors, such as previous history of depression or schizotypal personality (Burch & Hemsley, 2008; Dorner et al., 2011).
In-treatment variables have also been identified as predictors of long-term abstinence. Previous research has shown that prior smoking abstinence during treatment can directly influence subsequent efforts to abstain from smoking (Heil, Alessi, Lussier, Badger, & Higgins, 2004), suggesting that smoking treatment programs could be optimized by targeting this specific behavior (Romanowich & Lamb, 2010b). Furthermore, consecutive abstinence throughout and at end-of-treatment, and attending more sessions during the treatment are factors commonly related to a higher chance of success in quitting (Dorner et al., 2011; Romanowich & Lamb, 2010b). Other variables such as monitoring participants’ behavior (e.g., the proportion of negative samples submitted or attendance during the treatment), or the use of biochemical tests to verify abstinence, also increase the likelihood of success (McPherson, Packer, Cameron, Howell, & Roll, 2014; Petry, Alessi, & Ledgerwood, 2012).
One of the most efficacious treatment modalities for the treatment of addictive behaviors related to a wide range of drugs, including tobacco, is Contingency Management (CM), an approach that typically involves rewards contingent upon objective verification of self-reported status (Fernández-Artamendi, Fernández-Hermida, Godley, & Secades-Villa, 2014; Higgins, Silverman, & Heil, 2008; Secades-Villa, García-Rodríguez, López-Núñez, Alonso-Pérez, & Fernández-Hermida, 2014; Sigmon & Patrick, 2012). This empirically-supported behavioral treatment is based on the principle of operant conditioning, suggesting that substance-use behavior occurs within the context of environmental contingencies that make it more or less likely to occur (Higgins et al., 2008).
Although long-term smoking abstinence is the intended outcome of CM interventions (Higgins et al., 2006; Lamb, Morral, Kirby, Iguchi, & Galbicka, 2004), some studies have used incentives for improving in-treatment behaviors. These studies have shown that CM procedures improve both smoking reduction and abstinence during the treatment (Alessi, Badger, & Higgins, 2004; Alessi et al., 2008; Chivers, Higgins, Heil, Proskin, & Thomas, 2008; Dunn, Sigmon, Thomas, Heil, & Higgins, 2008; Higgins et al., 2004; Higgins et al., 2012; Lamb et al., 2007; Lussier, Higgins, & Badger, 2005; Romanowich & Lamb, 2010a; Tidey, Rohsenow, Kaplan, Swift, & Reid, 2011). CM has been shown to reduce carbon monoxide levels (Dallery, Raiff, & Grabinski, 2013) and to enhance early abstinence during the treatment (Heil et al., 2004; Higgins et al., 2006; Lamb et al., 2004; Romanowich & Lamb, 2010b; Yoon, Higgins, Bradstreet, Badger, & Thomas, 2009). The CM procedure is also associated with significantly higher rates of treatment completion (Volpp et al., 2006).
Despite previous knowledge, important questions remain about the effect of CM on in-treatment behaviors. Most of this previous work has been carried out in particular samples of smokers, such as residential substance abuse patients (Alessi et al., 2008), smokers with schizophrenia (Tidey et al., 2011), pregnant women (Higgins et al., 2006; Higgins et al., 2004; Higgins et al., 2012), methadone-maintained patients (Dunn et al., 2008) or low-income patients (Volpp et al., 2006). In addition, the generalizability of results is limited due to the small sample sizes of previous studies (Alessi et al., 2004; Alessi et al., 2008; Dunn et al., 2008; Heil et al., 2004) or the fact that samples were composed of patients without plans to quit (Alessi et al., 2004; Chivers et al., 2008; Heil et al., 2004; Lamb et al., 2007; Lussier et al., 2005; Romanowich & Lamb, 2010a; Yoon et al., 2009). Furthermore, most of the studies did not include a control group (Romanowich & Lamb, 2010b) or they compared a CM condition with another one that also provided incentives (Alessi et al., 2004; Dunn et al., 2008; Heil et al., 2004; Higgins et al., 2004; Lamb et al., 2004; Lamb et al., 2007; Lussier et al., 2005; Romanowich & Lamb, 2010a; Tidey et al., 2011). Finally, previous studies have focused either on retention or early abstinence and have not analyzed the effect of CM on other in-treatment behaviors, such as carbon monoxide (CO) or cotinine monitoring, which could also be related to long-term success of smoking cessation treatments.
In this study, we combined two different CM protocols with group-based Cognitive-Behavioral Treatment (CBT). The first CM protocol delivered incentives contingent upon smoking abstinence. The second protocol delivered incentives contingent upon gradual reductions in cotinine levels. Despite the fact that previous studies suggest that shaping procedures may help individuals to achieve abstinence (Lamb et al., 2004; Romanowich & Lamb, 2010a), this schedule of incentive delivery merits further investigation, since the evidence is still scarce. The aims of the present study were: (1) to assess whether adding two different CM protocols to CBT improved the main in-treatment variables that the literature has shown to predict long-term smoking abstinence; and (2) to analyze the effect of these in-treatment variables on patients’ smoking status (abstinent versus smoker) at a 6-month follow-up among treatment-seeking patients in a community setting.
MethodParticipantsThis study was developed at the Addictive Behaviors Clinic of the University of Oviedo (Spain). Participants were treatment-seeking smokers from the general population. Inclusion criteria for the study were being aged over 18, meeting the diagnostic criteria for nicotine dependence according to the Diagnostic and Statistical Manual of Mental Disorders (fourth ed., text rev.; DSM–IV–TR; American Psychiatric Association, 2000) assessed using the Structured Clinical Interview for DSM-IV (SCID), having smoked 10 or more cigarettes per day for the previous 12 months, and be willing to attend to the clinic twice a week. We excluded patients who displayed a severe psychiatric disorder (including substance use disorder) or who were receiving any other smoking cessation treatment.
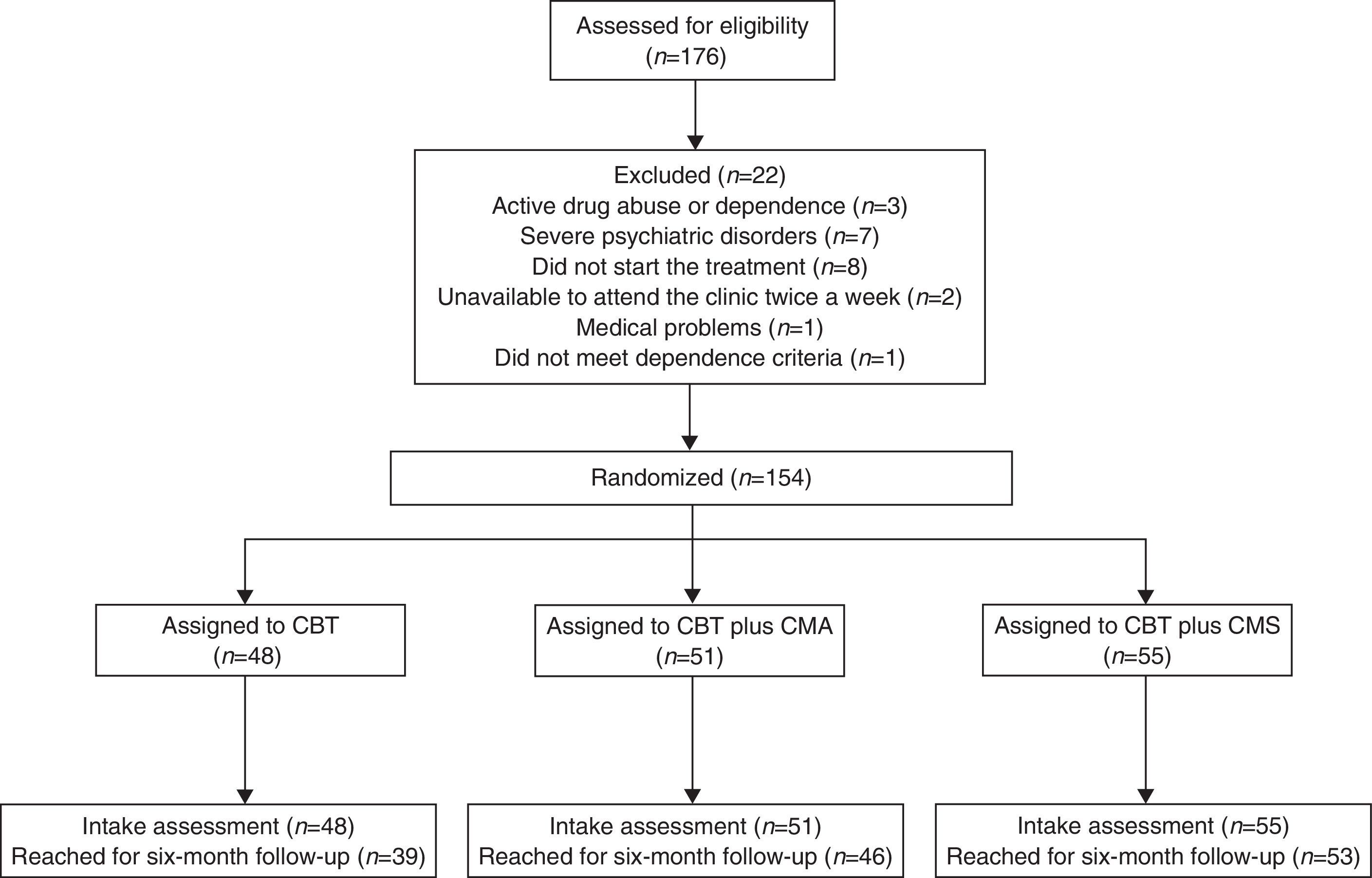
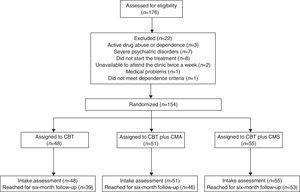
In order to report this randomized controlled trial (RCT) according to international standards, we followed the Consolidated Standards of Reporting Trials (CONSORT) (Moher et al., 2012). Participants provided informed consent, and the procedures followed were in accordance with the ethical standards of the institution. An a priori power analysis using G*Power 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007) was computed to ensure sufficient power (≥ 80%) for testing the aims of the present study. Out of a total of 176 people screened, 154 (38.3% men and 61.7% women) met the inclusion criteria and were enrolled in the study (Figure 1). The mean age was 44.58 years (SD=12.64), the mean number of cigarettes smoked per day at intake was 21.10 (SD=8.52) and mean score on the Fagerström Test for Nicotine Dependence (FTND) (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) was 5.53 (SD=1.91).
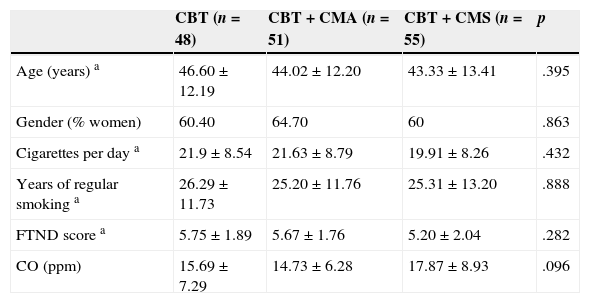
Eligible participants were randomly assigned to a CBT group (N=48), to a CBT plus CM for Abstinence (CMA) group (N=51) or to a CBT plus CM for Shaping cessation (CMS) group (N=55), according to a computer-generated randomization list. There were no significant differences (p<.05) in baseline characteristics between the three groups (Table 1). The selection process is described in a flow chart (Figure 1), as recommended by Hartley (2012).
Sample characteristics.
| CBT (n=48) | CBT+CMA (n=51) | CBT+CMS (n=55) | p | |
|---|---|---|---|---|
| Age (years) a | 46.60±12.19 | 44.02±12.20 | 43.33±13.41 | .395 |
| Gender (% women) | 60.40 | 64.70 | 60 | .863 |
| Cigarettes per day a | 21.9±8.54 | 21.63±8.79 | 19.91±8.26 | .432 |
| Years of regular smoking a | 26.29±11.73 | 25.20±11.76 | 25.31±13.20 | .888 |
| FTND score a | 5.75±1.89 | 5.67±1.76 | 5.20±2.04 | .282 |
| CO (ppm) | 15.69±7.29 | 14.73±6.28 | 17.87±8.93 | .096 |
Note. a=Means±SD; CBT=Cognitive-Behavioral Treatment; CMA=Cognitive-Behavioral Treatment plus Contingency Management for Abstinence; CMS=Cognitive-Behavioral Treatment plus Contingency Management for Shaping cessation; FTND=Fagerström Test for Nicotine Dependence; CO (ppm)=carbon monoxide (parts per million).
During the intake session, which lasted for approximately one and a half hours, the participants’ clinical history was obtained in order to gather data on sociodemographic and smoking-related characteristics. The FTND was used to assess nicotine dependence, in addition to the DSM-IV-TR criteria. Participants also provided a baseline CO sample in expired air using a Micro Smokerlyzer (Bedfont Scientific Ltd., Rochester, UK) for objective verification of self-reported smoking status. The Micro Smokerlyzer has an accuracy level of±2 parts per million (ppm) or±2%. A BS-120 chemistry analyzer (Shenzhen Mindray Bio-medical Electronics Co. Ltd., Shenzhen, P. R. China) designed for in vitro determination of clinical chemistries was used to determine semi-quantitative urine cotinine levels through a homogeneous enzyme immunoassay system. According to the Technical Service of Quality Control at Spinreact (Spinreact SAU, St. Esteve d’en Bas, Girona, Spain), precision of analysis includes a between-day coefficient of variation from 5.2% to 7.6% at values equal to or less than 625 nanograms per milliliter (ng/ml). Concerning specificity, various potentially interfering substances were found to be non cross-reacting with the analyses (list is not shown but is available upon request). All cotinine specimens were obtained under direct supervision by a same-gender staff member and measured immediately.
With the aim of assessing the effect of the treatments on in-treatment behavior, the following outcome variables were analyzed: treatment retention, in-treatment smoking abstinence and weekly decrease of cotinine levels. Treatment retention was assessed as the number of sessions the participants attended during the 6 weeks of treatment (from 1 to 11 sessions: 6 CBT therapy sessions plus 5 sessions to collect CO and cotinine specimens). In-treatment smoking abstinence was defined as the total number of days without smoking during treatment until end-of-treatment (from zero to 36 days). The weekly decrease of cotinine levels was assessed taking into account the number of sessions (from 1 to 11) in which patients met the specified cotinine level criteria (participants were aware of a weekly reduction goal from the beginning of treatment). From the first to the fourth week, a weekly reduction of 30% of nicotine intake (validated by the corresponding decrease of cotinine levels) was required for all groups. From the fifth session onward, specimens collected should test negative for cotinine. Agreement was needed between cotinine and CO measurements, and self-report.
In-treatment smoking abstinence and weekly decrease of cotinine levels provide complementary information about participants’ smoking status throughout the treatment. The first variable refers exclusively to the number of days patients maintain abstinence, while decrease of cotinine levels also measure adherence to treatment. Therefore, the patient could meet the scheduled cotinine criteria and still continue smoking.
In order to describe the predictive value of in-treatment behavior on patients’ long-term smoking status, the outcome variable analyzed was abstinence at the 6-month follow-up (defined as abstinence for a minimum of seven days before the assessment) (Cavallo et al., 2007). Self-reported abstinence was validated by a negative result of CO (equal to or less than 4ppm) and a negative urine cotinine test (equal to or less than 80ng/ml). Agreement between all three measures was required.
Treatment interventionsTherapists were members of the staff at the institution, who were all masters-level psychologists with previous intensive training in the specific protocols. Each therapist practiced with two or three training cases before treating any study participant. To ensure the therapists’ adherence to the protocols and competence in implementing the techniques, all sessions were audio-recorded and there was a one-hour weekly supervision session for the entire duration of the treatment program.
Cognitive-Behavioral Treatment (CBT)This consisted of an intervention based on previous studies (Becoña & Vázquez, 1997; Secades-Villa, Alonso-Pérez, García-Rodríguez, & Fernández-Hermida, 2009), implemented in group-based sessions of five or six patients. Each session took about one hour and was carried out once a week over 6 weeks. The components of the CBT program were highly structured and included: information about tobacco, behavioral contract through which the patients pledged to attend the sessions and quit smoking, self-monitoring and graphical representation of cigarette smoking, nicotine fading (a weekly reduction of 30% of nicotine intake from the first to the fourth week, and abstinence from the fifth session onwards), stimulus control, strategies for controlling nicotine withdrawal symptoms, physiological feedback consumption (measured by CO and cotinine), training in alternative behaviors, social reinforcement of objectives completion and abstinence, and relapse prevention strategies. CO and cotinine specimens were collected twice a week. One of the measures coincided with the weekly CBT session and the other was scheduled midweek between sessions. A total of eleven samples were collected for each participant during the treatment. Participants were informed of their CO level and urinalysis results (cotinine) immediately after submitting their specimens, but received no type of reward in exchange for achieving or maintaining abstinence.
CBT plus CM for Abstinence (CMA)The CBT plus CMA was provided as in the above CBT condition, but with the addition of a CM procedure. CO and cotinine samples were collected in accordance with the procedure explained above. The number of sessions (6 CBT therapy sessions plus 5 sessions to collect CO and cotinine specimens) was also the same as in the previous condition. The CM protocol included a vouchers program through which smoking abstinence was reinforced. In order to reinforce patients’ behavior, we checked cotinine specimens collected in the fifth CBT session (the first session after the patient was required to be abstinent), between the fifth and sixth CBT sessions and in the sixth CBT session. Participants that tested negative for cotinine earned points exchangeable for rewards on a schedule of escalating magnitude of reinforcement (the first cotinine-negative specimen earned 80 points, with a 20-point increase for each subsequent and consecutive cotinine-negative specimen) with a reset contingency (i.e., cotinine-positive specimens or failure to submit a scheduled specimen set the value back to the initial 80 points). It is noteworthy that this protocol delivered rewards contingent upon smoking abstinence and not only for attending the scheduled appointments. In the fifth CBT session a negative urine cotinine result was defined as equal to or less than 80ng/ml in order to avoid residual effects. With the aim of ensuring that rewards worked as reinforcers for participants’ behavior, a negative result of CO and self-reported abstinence were also required. Failure to submit a urine specimen as scheduled rendered it cotinine positive unless the patient provided some sort of official justification (job-related or medical) and attended the clinic the following day to submit a specimen. The schedule of reward delivery did not allow participants to return to the value they had obtained prior to the reset. However, points could not be lost once earned. Points were frequently accumulated throughout the treatment and exchanged at the end of the program (sixth CBT session). The maximum amount that patients could earn was 300 points, which were exchangeable for rewards with a variety of uses, including leisure activities, cinema, theatre, museums, sports events, gyms, adventure sports, meals in restaurants, training, purchases in department stores, bookshops, clothes shops and art shops, and spa and beauty services.
CBT plus CM for Shaping Cessation (CMS)Patients in this group received the same treatment as the CBT plus CMA group, with just one difference. The CMS procedure reinforced both the closer approximations to smoking abstinence (from the first to the fourth session) and smoking abstinence (from the fifth session onward). The specimens collected from the first to the fourth session that tested progressive reductions in cotinine according to an individualized percentile schedule earned points. The first weekly reduction of 30% of nicotine intake (checked at the session between the first and second CBT sessions and corroborated by a comparable reduction in urine cotinine levels) earned 12 points, with a 4-point increase for both each subsequent nicotine reduction of 30% and abstinence after the fifth CBT session (a maximum of 300 points could be earned). As explained above, failure to submit a urine specimen as scheduled rendered it cotinine positive if the patient did not provide official justification or did not attend the clinic the following day. Points could not be lost once earned, but cotinine-positive specimens or failure to submit a scheduled specimen set the value back to the initial 12 points. However, submission of two consecutive cotinine-negative specimens returned the value to its level before the reset. Points were exchangeable for the same type of rewards that were available for patients included in the CBT plus CMA group.
Data analysesVarious descriptive and frequency analyses were carried out with regard to the participants’ characteristics. Comparisons between the treatment groups for baseline characteristics were performed using a one-way between-groups analysis of variance (ANOVA) for the continuous variables and the χ2 test for the dichotomous variables. An ANOVA was performed in order to assess the effect of CM on the in-treatment behavior. Effect sizes of principal comparisons were calculated using eta squared (η2), taking into account values for small, medium and large effects (.01, .06 and .14) (Cohen, 1988). Discriminant analyses were calculated with the aim of analyzing the predictive value of the in-treatment variables (treatment retention, in-treatment smoking abstinence and weekly decrease of cotinine levels) affecting the patients’ condition (abstinent versus smoker) at a follow-up assessment 6 months after treatment completion. The outcomes are reported in two ways, one in which missing urine samples at 6-month follow-up were considered positive (following an intent-to-treat approach) and a second one in which missing samples were considered as missing data. The confidence level was 95%, and the statistical package used was the SPSS (V19; SPSS, Inc., Chicago, IL).
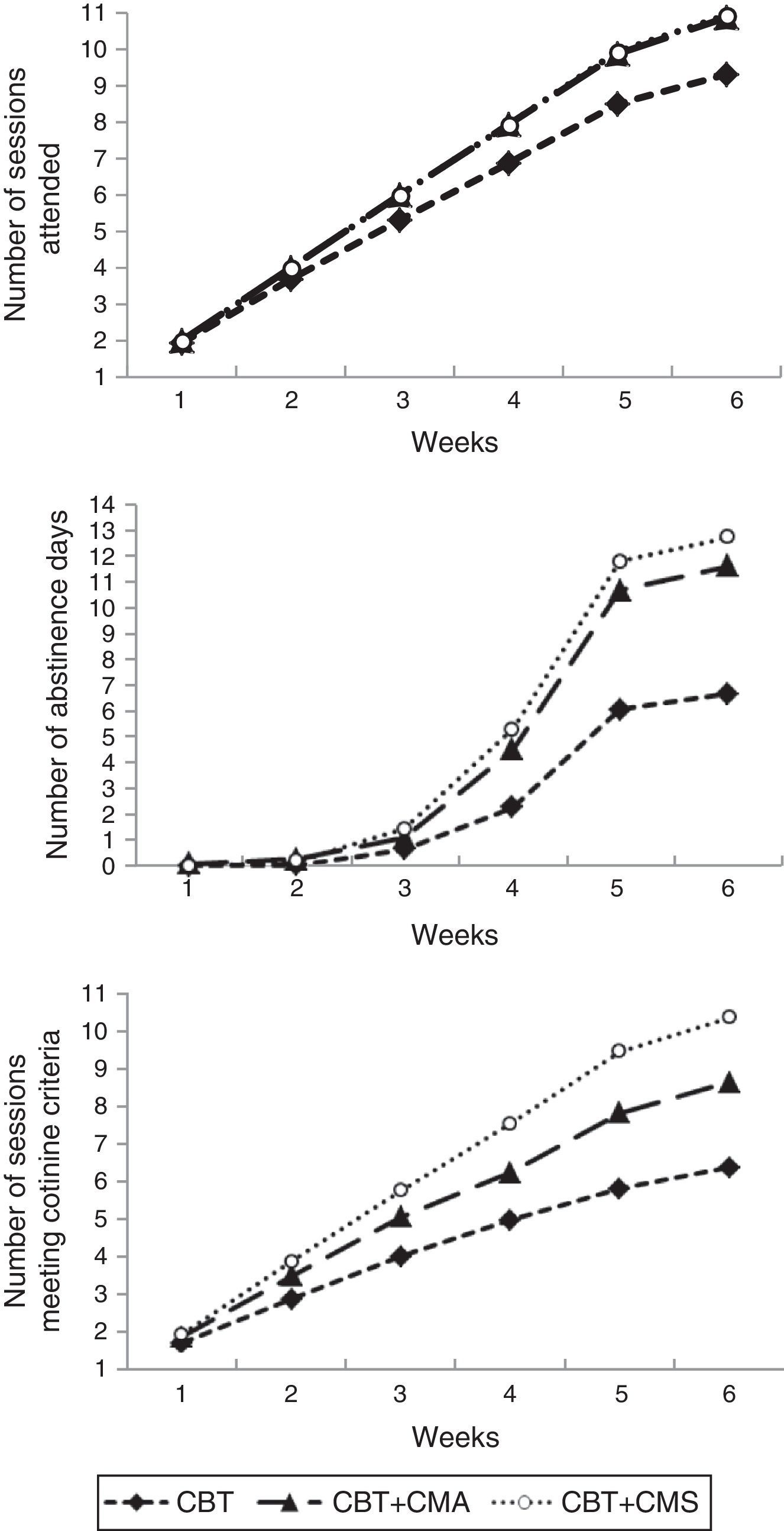
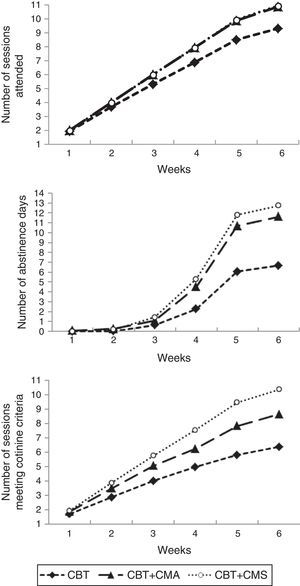
ResultsTreatment retentionThere was a statistically significant difference in treatment retention for the three conditions (F (2, 151)=13.04, p<.01). The mean number of sessions attended among patients included in the CBT group (M=9.29; SD=3.23) was significantly lower than the mean number of sessions attended in the CBT plus CMA group (M=10.86; SD=0.53; p<.01) and in the CBT plus CMS group (M=10.96; SD=0.19; p<.01). CBT plus CMA did not differ significantly from CBT plus CMS (p=.957). Despite the fact that the data hardly differ among the three groups, the magnitude of the differences in the number of sessions was large (η2=.15). The week-by-week progression of treatment retention outcomes is shown in Figure 2.
In-treatment smoking abstinenceA statistically significant difference was found for in-treatment smoking abstinence for the three groups (F (2, 151)=15.46, p<.01). The mean number of days without smoking during the treatment achieved by participants in CBT group (M=6.67; SD=6.77) was significantly lower than the mean days of abstinence achieved by patients included in both the CBT plus CMA (M=11.59; SD=5.84; p<.01) and CBT plus CMS (M=12.84; SD=5.01; p<.01) groups. No statistically significant differences were found between the CBT plus CMA and CBT plus CMS (p=.520) groups. The magnitude of the differences in the total number of days of abstinence was large (η2=.17). The week-by-week progression of in-treatment smoking abstinence outcomes is shown in Figure 2.
Weekly decrease of cotinine levelsThere were statistically significant differences among treatment groups in the number of sessions of weekly reduction of cotinine (F (2, 137)=22.94, p<.01). Participants included in the CBT group achieved the scheduled weekly reduction of cotinine with fewer sessions (M=6.28; SD=3.71) than patients in the CBT plus CMA group (M=8.38; SD=2.76; p=.002) and patients in the CBT plus CMS group (M=10.11; SD=1.65; p<.01). Statistically significant differences were also found between CBT plus CMA and CBT plus CMS (p=.005). The magnitude of the differences in the weekly reduction of cotinine was large (η2=.25). Figure 2 shows the week-by-week progression of the number of sessions in which patients met the weekly decrease of cotinine levels.
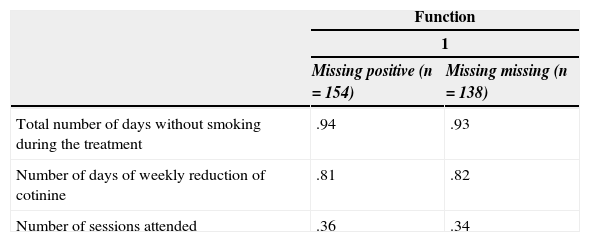
Predictive value of the in-treatment variables at 6-month follow-upAnalysis of the structure matrix (Table 2) indicates the relative importance of the predictors, showing the correlations of each variable with each discriminative function. Both discriminant analyses (missing urine samples at 6-month follow-up as positive and as missing data) revealed that in-treatment smoking abstinence (total number of days without smoking during the treatment) is the best predictor of long-term abstinence (.94 and .93, respectively).
Correlations of each in-treatment variable with each discriminative function (Structure Matrix)a.
| Function | ||
|---|---|---|
| 1 | ||
| Missing positive (n=154) | Missing missing (n=138) | |
| Total number of days without smoking during the treatment | .94 | .93 |
| Number of days of weekly reduction of cotinine | .81 | .82 |
| Number of sessions attended | .36 | .34 |
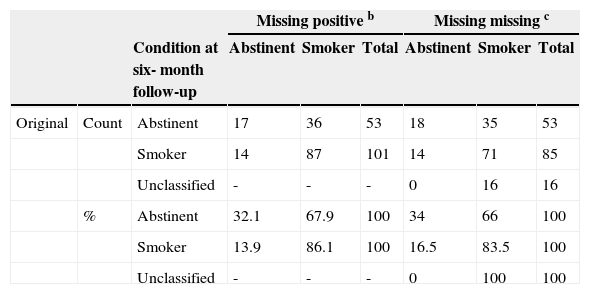
The classification results (Table 3) show that 67.5% of respondents for whom statistical analyses were conducted including missing urine samples at the 6-month follow-up as positive results (missing as positive) were classified correctly into abstinent or smoker groups. Classification results also show that 64.5% of respondents for whom missing urine samples at the 6-month follow-up were not included in the statistical analysis (missing as missing) were classified correctly in both groups. Overall, 16 participants failed to provide urine samples at the 6-month follow-up assessment (CBT=9, CBT plus CMA=5 and CBT plus CMS=2). Considering the first approach, smokers were classified with better accuracy (86.1%) than abstainers (32.1%). The second approach also showed the same results (83.5% of smokers were correctly classified vs. 34% of abstainers).
Percentage of smokers classified correctly into abstinent or smoker groups (Classification Results) a.
| Missing positive b | Missing missing c | |||||||
|---|---|---|---|---|---|---|---|---|
| Condition at six- month follow-up | Abstinent | Smoker | Total | Abstinent | Smoker | Total | ||
| Original | Count | Abstinent | 17 | 36 | 53 | 18 | 35 | 53 |
| Smoker | 14 | 87 | 101 | 14 | 71 | 85 | ||
| Unclassified | - | - | - | 0 | 16 | 16 | ||
| % | Abstinent | 32.1 | 67.9 | 100 | 34 | 66 | 100 | |
| Smoker | 13.9 | 86.1 | 100 | 16.5 | 83.5 | 100 | ||
| Unclassified | - | - | - | 0 | 100 | 100 | ||
a=Intent-to-treat analysis; b=Correctly classified 67.5% of original grouped cases; c=Correctly classified 64.5% of original grouped cases.
The goals of the present study were to analyze whether adding two different CM procedures to CBT improved three in-treatment behaviors (treatment retention, in-treatment smoking abstinence and weekly decrease of cotinine levels) and to identify the predictive effect of these in-treatment behaviors on smoking abstinence at long-term follow-up. The results showed that both CM protocols improved the three in-treatment behaviors and that these in-treatment behaviors (particularly in-treatment smoking abstinence) were associated with smoking abstinence at 6-month follow-up.
Both CM procedures (reinforcing smoking abstinence and closer approximations to smoking abstinence) improved treatment retention, in-treatment smoking abstinence and weekly decreased of cotinine levels in comparison with CBT alone. In particular, the number of days abstinent at the end-of-treatment in both CBT+CM groups was almost double the figure for the CBT alone group. It is noteworthy that despite rewards in CMA group being contingent on smoking abstinence at the end-of-treatment, this protocol also improved in-treatment behaviors. It seems that rewards increased motivation to change (Higgins et al., 2008) and due to this, reinforced the in-treatment behaviors among participants in their alignment with the target behavior (weekly reductions of nicotine and abstinence at the end of the treatment) (Lamb et al., 2004). The description of future consequences can influence current behavior when they are stated verbally (Strathman, Gleicher, Boninger, & Edwards, 1994). In this group, the expectation of reinforcement at the end of the treatment may shape participants’ behavior before reaching abstinence; in other words, in-treatment behaviors (particularly in-treatment smoking abstinence) could be analyzed as conditioned responses governed by the final reinforcement at end-of-treatment, which is indeed derived from compliance with such in-treatment behaviors (Chivers et al., 2008).
It is noteworthy that, comparing CBT alone to the two groups with CM added, the number of days abstinent during the treatment differed by 5 to 6 days. We believe this result is clinically meaningful taking into consideration the influence of early abstinence over long-term success (Heil et al., 2004).
Despite the effectiveness of CM to improve the treatment behaviors, adding a CM protocol to standard care leads to an increase in costs, which could be an obstacle to the expansion of this program in community settings. Future studies should investigate the cost-effectiveness of such an evidence-based CM protocol in representative settings and populations in order to make policy decisions about CM implementation for smoking cessation in the broader community.
The in-treatment behaviors (particularly in-treatment smoking abstinence) were associated with patients’ smoking status at a 6-month follow-up. In accordance with previous research, in-treatment abstinence (Alessi et al., 2004; Heil et al., 2004; Higgins et al., 2006; Lamb et al., 2004; Yoon et al., 2009) and treatment retention (Dorner et al., 2011) predicted better long-term abstinence outcomes.
Participants who adhered to the scheduled weekly decrease of cotinine levels during the treatment also achieved better results at 6-month follow-up. However, to our knowledge, no previous study has compared the differential effect of these three in-treatment behaviors on long-term abstinence in a smoking cessation program that includes treatment-seeking patients in a community setting. Our results extend previous findings by indicating that particularly the factors related to early abstinence (total number of days without smoking during the treatment and weekly decrease of cotinine levels) are strongly associated with abstinence at 6-month follow-up.
The relationship between these in-treatment behaviors and long-term abstinence could be explained as a consequence of the experimental control over smoking behavior exerted during the treatment (Alessi et al., 2004; Chivers et al., 2008). The fulfillment of these in-treatment behaviors usually encourages patients to achieve and maintain their abstinence, and to adhere to the guidelines for quitting, which in turn reduce both nicotine withdrawal and future risk of relapse (Alessi et al., 2004; Yoon et al., 2009).
Taking together, these results suggest that the in-treatment behaviors, specially early initiation of abstinence, should be crucial goals for effective smoking cessation treatments in order to increase long-term smoking abstinence (Higgins et al., 2006; Romanowich & Lamb, 2010b).
Some limitations of the study merit mention. Firstly, the study enrolled more women than men, which could limit the sample's representativeness of the smoking population. However, previous research has shown that females are more likely than males to attempt to quit (Rafful et al., 2013). Secondly, our study assesses abstinence at 6-month follow-up after the end of the treatment. It would be useful to evaluate the effect of the in-treatment variables on longer-term abstinence, for example at 12-month follow-up. On the other hand, there was significant variability among participants in the number of cigarettes smoked. However, our smoking cessation program established 30% of nicotine reduction for all patients, so that weekly nicotine fading was higher for heavy smokers in comparison to medium smokers. In addition, all participants could earn the same amount of rewards. Thus, it would be interesting to adjust the requirement for accessing rewards taking into account nicotine fading among participants with different pre-treatment consumption levels.
In spite of these limitations, our findings indicate that CM protocols improve the in-treatment behaviors among smokers who want to quit and that these in-treatment behaviors (particularly in-treatment smoking abstinence) are associated with long-term smoking abstinence.
FundingWork on this manuscript was supported by the Spanish Ministry of Science and Innovation (MICINN) Grant (PSI2011-22804) and by the Predoctoral Grants BP12-037 and BP11-031, from the Foundation for the Promotion of Applied Scientific Research and Technology in Asturias (FICYT) and BES-2012-053988, from the Spanish Ministry of Economy and Competitiveness.