Green synthesis of metal nanoparticles is an important technique in the methods of eco-friendly nanoparticle production. The synthesis of silver nanoparticles was accomplished using Ocimum sanctum leaf extract at room temperature. These particles were then encapsulated with polyvinyl alcohol (PVA) polymer matrix. The presence of silver was confirmed by different characterization techniques such as UV–vis spectroscopy, Fourier transform infrared spectroscopy (FTIR) and X-Ray Diffraction (XRD). Scanning electron microscopic (SEM) images of the synthesized powder shows spherical shaped silver nanoparticles embedded in sponge-like polymer matrix. The energy dispersive X-ray analysis confirms the presence of elemental silver along with iron signal. Energy dispersive signal corresponding to elemental iron has been attributed to O. sanctum plant. The silver nanoparticles in PVA matrix thus obtained shows high antibacterial activity against gram positive Staphylococcus aureus (S. aureus) and gram negative Escherichia coli (E. coli) water borne bacteria. The inhibition zone against S. aureus and E. coli were also calculated.
Research on nanoparticles is currently an area of intense scientific interest due to its wide range of applications (Abdulrahman, Krajczewski, Aleksandrowska, & Kudelski, 2015; Park, Lee, & Lee, 2016; Taylor, Coulombe, et al., 2013; Yahyaei et al., 2016). In spite of being the size of the ultrafine particles individual molecules are usually not referred to as nanoparticles (Hewakuruppu et al., 2013). Nanoparticles form a bridge between bulk materials and atomic/molecular structures. Nanoparticles do not need to have constant physical properties, they may vary (Taylor, Otanicar, et al., 2013). The size dependent property such as quantum confinement can be observed in semiconductor particles, surface plasmon resonance is found in some metal particles and super magnetism is observed in magnetic materials (Taylor, Otanicar, & Rosengarten, 2012).
Nowadays, metallic nanoparticles are the focus of interest because of their huge potential in nanotechnology (Mody, Siwale, Singh, & Mody, 2010; Salunke, Sawant, Lee, & Kim, 2016). Metallic nanoparticles have been embraced by industrial sectors because of their applications in the field of electronic storage systems (Kang, Risbud, Rabolt, & Stroeve, 1996), biotechnology (Pankhurst, Connolly, Jones, & Dobson, 2003), magnetic separation and pre-concentration of target analysts, targeted drug delivery (Dobson, 2006; Rudge et al., 2001) and vehicles for gene and drug delivery. With a wide range of applications available, these particles have the potential to make a significant impact on society.
Silver nanoparticles have some advantages over other nanoparticles because they are reported to be non-toxic to human and most effective against bacteria, viruses and other eukararyolitic micro-organisms at a very low concentration, without any known side effects (Chauhan, Gupta, & Prakash, 2012). Many efforts have been taken to incorporate silver nanoparticles into a wide range of medical devices, such as bone cement, surgical instruments, surgical masks (Valente, Gaspar, Antunes, Countinho, & Correia, 2013); however, it has also been shown that ionic silver in the right quantities is suitable in treating wounds (Atiyeh, Costagliola, Hayek, & Dibo, 2007; Chen & Schluesener, 2008; Qin, 2005).
There are many approaches available for the synthesis of silver nanoparticles which include the chemical method (Sun, Yin, Mayers, Herricks, & Xia, 2002), the electrochemical method (Yin, Ma, Wang, & Chen, 2003), the radiation method (Dimitrijevic, Bartels, Jonah, Takahashi, & Rajh, 2001), the photochemical method (Callegari, Tonti, & Chergui, 2003), and the biological techniques (Huang et al., 2016; Naik, Stringer, Agarwal, Jones, & Stone, 2002). Among these methods for silver preparation, plant mediated biomimetic synthesis of silver nanoparticles is considered as widely acceptable technology for rapid production of silver nanoparticles for successfully meeting the excessive needs and current market demands. When a metal core is capped with a plant extract then these biomaterials will act as a more effective therapeutic agent, compared to the nanomaterials synthesized by any chemical method (Mittal, Chisti, & Banerjee, 2013). Phytochemicals, such as ursolic acid, flavonoids, saccharides and proteins, present in plant extract are responsible for the reduction of silver ions (Bhaumik et al., 2015); hence this bioinspired synthesis of nanomaterials is highly advantageous as a natural and cost effective resource.
Recently many plants have gained importance because of their unique properties. These plants have versatile applicability in various developing fields of research and development. Among these medicinal plants, the tulsi leave (Ocimum sanctum) plant have high rate of medicinal value. Studies have shown that silver nanoparticles prepared from tulsi leaves were used in different applications (Sharma, Yngard, & Lin, 2009; Zhang, Wu, Chen, & Lin, 2009). The leaves have a long history of medicinal uses. O. sanctum leaves act as antifertility, anticancer, antidiabetic, antifungal & antimicrobial agents (Philip & Unni, 2011). Even though silver nanoparticles reduced by O. sanctum leaves exhibit high therapeutic potential, using silver alone is a challenging task for applications in food industry and pharmaceutical technology. Hence attention has been given to polymer metal composite to potentiate the protection of biological active compounds from degradation, control drug release, and improve absorption of the therapeutic agent (Ahmed & Aljaeid, 2016).
The aim of the present study is to synthesis eco-friendly silver nanoparticles by green synthesis from fresh leaves of O. sanctum and to encapsulate these silver nanoparticles in a PVA matrix. The synthesis of nanoparticles from plant extract is cost effective and does not require much equipment. The PVA encapsulated silver nanoparticles synthesized from such technique are stable for several months and can be stored at room temperature without any special attention. Different characterization techniques were carried out to confirm the existence of silver nanoparticles.
2Materials and methods2.1Preparation of leaf extractFresh leaves of O. sanctum were collected from local residence around Coimbatore, Tamil Nadu, India. The leaves were washed thoroughly several times with double distilled water. Leaf extract used for the synthesis was prepared by weighing 20g of fresh leaves. The freshly cut leaves were grained by mortar and pestle. The crushed leaves were added to 200ml of double distilled water and boiled at 100°C in an Erlenmeyer flask for 15min. The leaf extract was filtered through Whatman No.1 filter paper.
2.2Biosynthesis of silver nanoparticlesSilver nanoparticles from plant extract were synthesized by using 100ml of 1×10−3M aqueous silver nitrate (Himedia, Bangalore) with 20ml of leaf extract. Both were mixed at room temperature and stirred vigorously for 1h in a magnetic stirrer. It was observed that the color of the mixture changed from light green to dark brown indicating the formation of silver nanoparticles.
2.3Preparation of silver nanoparticles in PVA matrixPoly Vinyl Alcohol (PVA) (Himedia, Bangalore) of 0.14g was mixed with 100ml of double distilled water and stirred for 2h. The solution was then slowly added with 120ml of leaf extract AgNO3.
2.4UV–visible spectroscopy (UV)The formation of silver nanoparticles from the leaf extract was characterized by UV–visible Spectroscopy using a Cyber lab UV-100 double beam spectrophotometer in the wavelength range of 300–800nm. A comparative absorption spectrum was obtained between the pure silver nanoparticles and the PVA encapsulated silver nanoparticles.
2.5X-ray diffraction (XRD)Colloidal form of silver nanoparticles in the PVA matrix was coated on a well cleaned glass substrate by drop technique and was allowed to dry at room temperature for 24h. The glass substrate was then characterized using SHIMADZU Lab XRD 6000 with CuKα radiation monochromatic filter in the range 10°–80°. Debye-Scherer's equation was used to calculate the particle size of silver nanoparticles.
2.6Fourier transform infrared spectroscopy (FTIR)To identify the functional groups present in the colloidal form of the PVA embedded silver nanoparticles, the Fourier transform infrared (FTIR) analysis was carried out using IR-Affinity 1, Shimadzu make FTIR Spectrometer in a wavenumber range from 500 to 3500cm−1.
2.7Scanning electron microscopy (SEM)The PVA embedded silver nanoparticles coated on the glass substrate by drop method were characterized using a TESCAN make Scanning Electron Microscope. SEM images were taken for different magnifications. An elemental analysis was also done by energy dispersive X-ray analysis (EDAX) along with the scanning electron microscopy.
3Results and discussions3.1UV–visible spectroscopic analysisThe absorption spectra of pure silver nanoparticles, and the PVA encapsulated silver nanoparticles were carried out. The reduction of silver nanoparticles using tulsi (O. sanctum) leaves was evidenced visually by a change in color, from light green to dark brown. Color intensity increases with increase in time. Initially when adding 1×10−3M aqueous silver nitrate with 20ml of leaf extract, the color of the solution was found to be light green; after one hour, the color changed from reddish brown to dark brown (Fig. 1). This effect may be due to Surface Plasmon Vibration (Krasovskii & Karavanskii, 2008; Sun & Xia, 2002).
SPR peaks for pure silver nanoparticles were recorded for different time intervals (Fig. 2). The absorption peak at 480nm appears because of the presence of silver nanoparticles, and the results are in good correlation between the earlier reported studies (Sharma, Vendamani, Pathak, & Tiwari, 2015). The intensity of the absorption peak increases steadily as a function of time and was highest for 1h. The increase in intensity indicates the increase in concentration of the silver nanoparticles (Cheng, Hung, Chen, Liu, & Young 2014). The UV-spectra recorded after 2h does not show any increase in the intensity of the absorption spectrum, which shows that the reaction was completed within 2h where the silver ions gets separated and settled down at the bottom of the tube from the supernatant mother liquid leaving it as a colorless solution.
A broad SPR peak was observed for the PVA encapsulated silver nanoparticles solution and the absorption spectra was observed from 450nm to 480nm and (Fig. 2a). The broad nature of the band might be due to the presence of polymer in the solution. The reaction mixture was stable for 3 months at room temperature without any special attention.
3.2X-ray diffraction analysisFigure 3 shows the XRD pattern for the synthesized silver nanoparticles in PVA matrix and it is observed that the particles are crystalline in nature. Strong peaks from Braggs reflection was observed in the XRD pattern at 2θ=12°, 32° and 38°. It is well known that the peaks at 2θ less than 20° are due to the crystalline nature of the PVA polymer molecule. The lattice planes [101] corresponding to 2θ=12° may be formed as a result of strong intermolecular and intramolecular hydrogen bonding between the PVA chains. The X-ray diffraction peak corresponding to 38° is due to the silver nanoparticles corresponding to the lattice plane [111]. The peaks at 32° might be due to the presence of certain impurities while bonding between the PVA and the silver molecule (Kim, Kim, Lee, & Kim, 1992; Guirguis & Moselhey, 2012). The grain size of silver nanoparticles were calculated using Debye-Scherer's equation D=Kλ/βcosθ, where the average grain size was found to be 20nm.
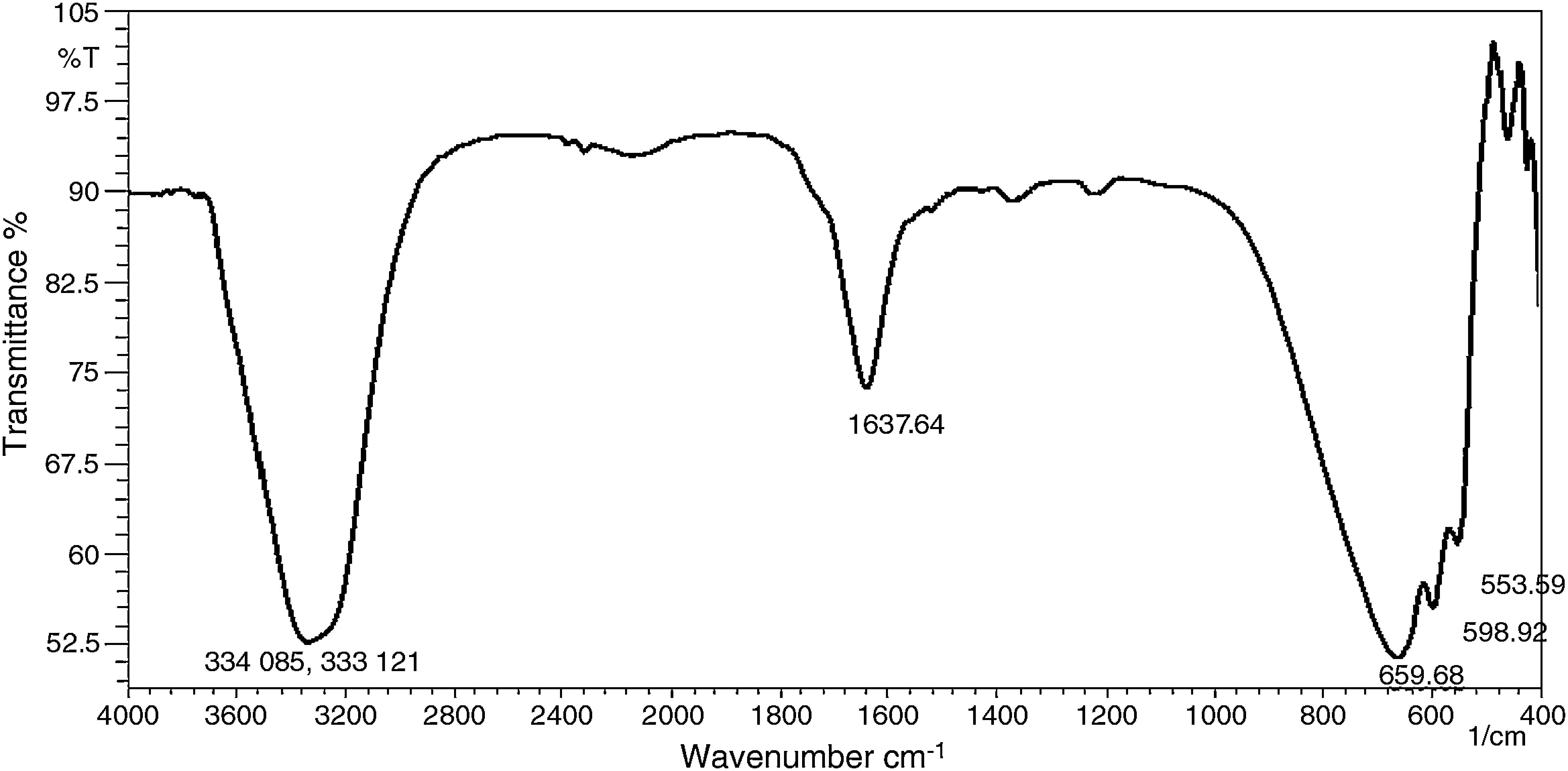
3.3FTIR analysisFTIR spectroscopy has been proved to be a very powerful technique to study the internal structure of polymeric material and intra-molecular interaction between polymeric material and filler. The FTIR spectrum of the synthesized Ag nanoparticles in PVA matrix is shown in Figure 4. Prominent peaks were observed at 3340cm−1, 1637cm−1, 659cm−1 to 553cm−1. The peak observed at 3340cm−1 indicates the presence of a hydrogen bond between the PVA polymer and leaf causing OH/NH2 stretching. The peak at 1637cm−1 may be attributed to CC, stretching mode. The peaks from 659cm−1 to 553cm−1 might be caused by wagging mode of OH groups (Agnihotri, Mukherji, & Mukherji, 2012; Cheng et al., 2014). The FTIR results confirm that the PVA, as a capping agent, plays an important role in the formation of silver nanoparticles.
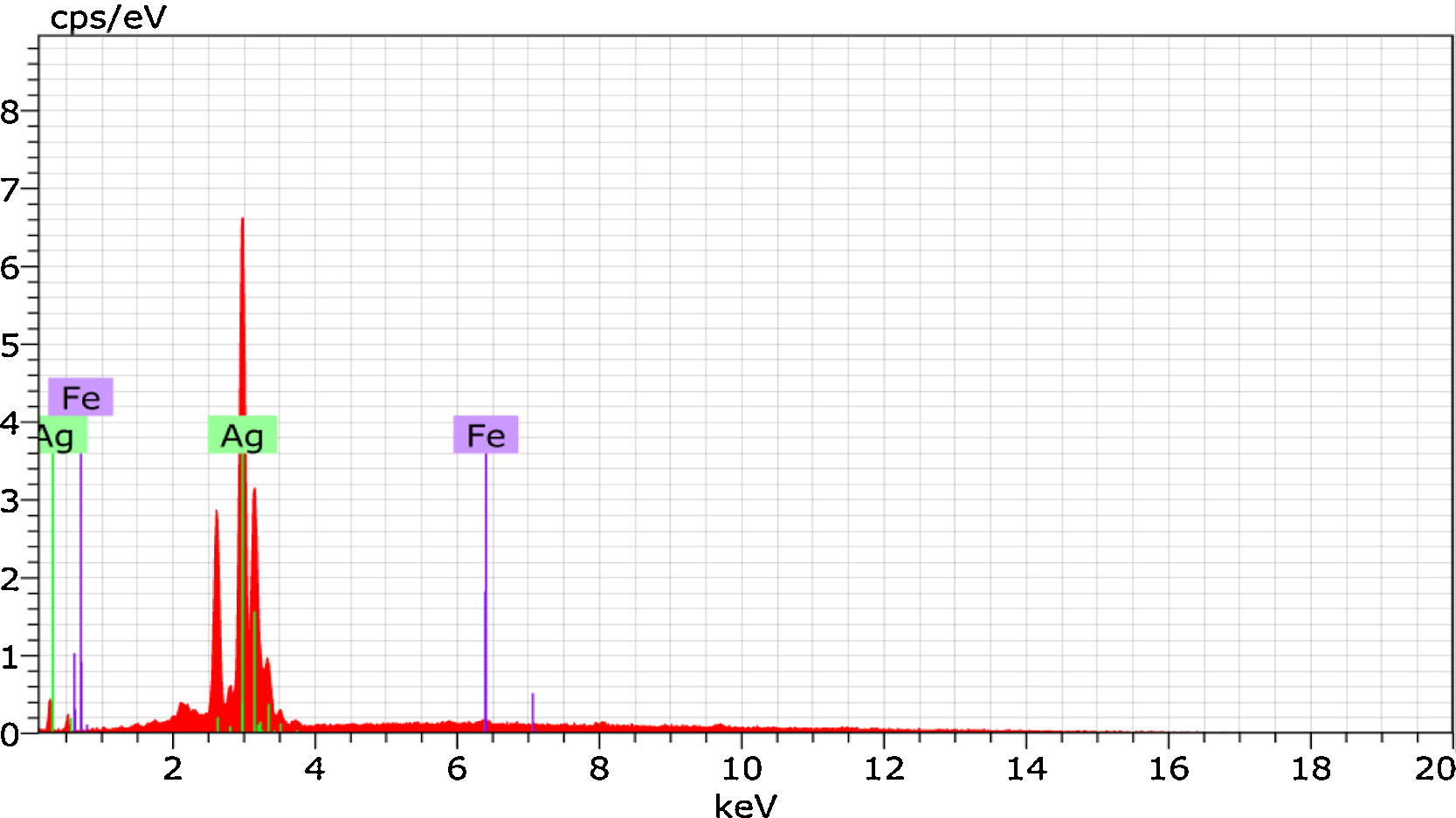
3.4SEM analysisMorphology of the synthesized silver nanoparticles in the PVA matrix was observed from the SEM micrographs. Figure 5 shows the SEM images for different magnifications. From the graph, it is found that the spherical shaped silver nanoparticles were embedded in a sponge-like PVA matrix. The energy dispersive X-ray analysis (EDAX) reveals strong signal in the silver region and confirms the presence of silver nanoparticles (Fig. 6). Generally silver nanoparticles show optical absorption peak approximately at 3keV due to surface plasmon resonance (Ahmad et al., 2003). Along with silver, Fe nanoparticles were also found in the graph which might be caused by the iron content present in the O. sanctum leaves. The atomic weight percentage of the elements present was tabulated and is shown in Table 1.
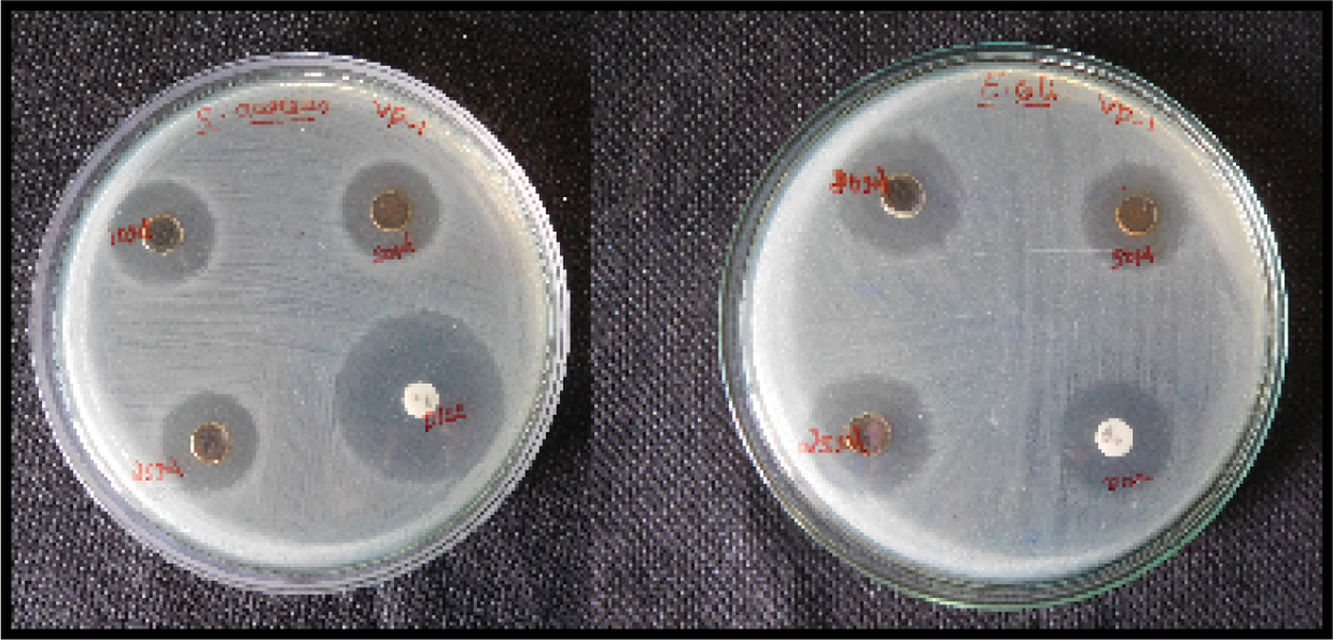
3.5Antibacterial activityThe antibacterial potential of silver has been known for many years. Antibacterial activity of silver nanoparticles was evaluated by agar disk diffusion method using Muller Hinton agar. The antibacterial effect of PVA encapsulated silver was tested against gram positive Staphylococcus aureus (S. aureus) and gram negative Escherichia coli (E. coli) bacteria for different concentrations (Fig. 7). Eugenol (1-hydroxy-2-methoxy-4-allylbenzene), the active constituent present in O. sanctum leaves has been found to be largely responsible for the plants therapeutic potential (Prakash & Gupta, 2005). Its antibacterial effect was evidenced by the value of diameter of zone of inhibition. The inhibition zone for different concentrations is shown in Table 2. From the table, it is found that E. coli and S. aureus are sensitive to silver nanoparticles. The observed value from the inhibition zone is in good agreement with previous reported studies (Abishek & Amruthaa, 2013).
The reduction of silver to silver nanoparticles from green synthesis using tulsi leaves was carried out at room temperature. The green synthesis method from plant extract provides simple, efficient and good control over synthesized nanoparticles. Biosynthesized silver nanoparticles in an organic polymer (PVA) matrix were characterized using UV–vis, XRD, FTIR and SEM spectroscopic techniques. The presence of silver was evidenced using UV spectrum. From XRD studies, the peak reveals the presence of organic substance and silver nanoparticles, which was again confirmed by the FTIR spectrum. The SEM images show the presence of spherical shaped silver present inside a sponge-like PVA matrix. The presence of silver and iron was observed from the EDAX studies. The investigation on the antibacterial effect of nano-sized silver against E. coli and S. aureus reveals PVA encapsulated silver nanoparticles as a strong antibacterial agent. The synthesized silver nanoparticles can show new pathways in various fields like water purification, anticancer studies and drug delivery systems.
Conflict of interestThe authors have no conflicts of interest to declare.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.