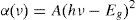Cadmium selenide nanoparticles (NPs-CdSe) were synthesized by colloidal route at room temperature and atmospheric pressure using cadmium chloride (CdCl2·2.5 hydrate) and elemental selenium (Se) as precursors. Sodium borohydride (NaBH4) was used as reducing agent to obtain Se2− ions and an aqueous solution with a NaOH and Penta sodium tripolyphosphate (STPP) was used to protect Cd2+ ions. To remove the by-products generated during the chemical reaction and to promote the precipitation of NPs-CdSe, a cleaning process with an aqueous solution of HCl was performed. The HCl volume was varied from 0.2 to 1.2ml during the cleaning process to study its effects on CdSe synthesis. The crystalline structure was analyzed by inspection of the high-resolution transmission electron microscope (HR-TEM) and X-ray diffraction (XRD). This analysis showed that crystals of CdSe exhibit a face-centered cubic structure (FCC). The calculated crystallite size is 3.5nm and increases to 4.5nm as the HCl volume increases. The morphologies of the products were observed by SEM and TEM techniques. HRTEM images showed that NPs-CdSe synthetized to 0.8ml are composed of a great number of homogeneous and smooth nanospheres which are not appreciable in SEM but are observable in TEM. By contrast, 0.2 and 1.2ml HCl samples are comprised of a great deal of rods of compounds of Se mixed with CdSe spheres nanostructures. This work, which did not require the use of surfactants complexes or specials environment, is considered to have advantages over other works.
Over recent decades, the development of the synthesis of low-dimensional semiconductor structures has been established by Brus (1984). Actually, alterations in nanostructures due to the size and morphology have a direct influence on the energy band distribution of the material; therefore, it is necessary to study the effects related to the nanometric scale. Structures of the type MX (M=Cd, Pb, Hg and X=S, Se, Te) have been characterized (Mansur & Mansur, 2011). However, in these works, the nanoparticles are synthesized using surfactants complexes like carboxylic-functionalized PVA (PVA–COOH), making the process expensive and a lot of times slow because it requires special environments. Other research had used chemical techniques such as sol–gel, chemical bath deposition, spray pyrolysis, colloidal route, among others (Cheng et al., 2003; Onwudiwe & Strydom, 2013) based on the coating of the precursor ions with a hydrophobic surfactant in order to ensure water solubility and long term stability of the nanostructures. In this way, colloidal chemistry has attracted increasing attention from the research community as an available option for producing high quality nanostructures with a relatively simple process. In this work, a morphological study of CdSe nanoparticles synthesized at room conditions by the colloidal method uses HCl solution in the cleaning process to ensure the quality of the nanoparticles.
2Material and methodsAnalytical chemical reagents such as cadmium chloride (CdCl2·2.5H2O, Baker, 99.9%), selenium metal powder (Aldrich, 99.5%), sodium borohydride (NaBH4, Merck, 98.0%), hydrochloric acid (HCl, 37% concentration), Extran® (Merck, MA01) (mix of sodium tripolyphosphate (STPP) and sodium hydroxide) with a pH of 11.6, and deionized (DI) water with 18MΩcm were used. The Cd2+ ions were obtained by mixing 1mmol of CdCl2·2.5H2O into 50ml of DI-water under moderate magnetic stirring of the solution (resulting in a homogeneous solution with this work condition), and stabilized by adjusting the pH to 8 with Extran® (aggregating dropwise until 8ml). An aqueous solution (20ml) of powdered selenium (Se, 1mM) and sodium borohydride (NaBH4, 2mM) was prepared using DI-water in an 80ml flask. The reaction mixture was magnetically stirred and heated at 95°C for 12min. CdSe nanoparticles were synthesized by the aqueous route in the reaction flask of cadmium by adding the reduced selenium precursors. The aqueous solution of CdSe was kept under moderate magnetic stirring for 30min. After this time, drops of HCl were slowly added to the solution in intervals of 15min, interspersing in the decantation process for extraction of the by-products. The process was developed at room temperature and atmospheric pressure. Structural, morphological and compositional characterizations were realized for the analysis and evaluation of the nanoparticles. X-ray diffraction (XRD) measurements were performed on a Bruker D8 Advanced diffractometer operating in the reflection mode with Cu-Kα radiation (45kV, 45mA) and diffracted beam monochromator, using a scan step mode with the step of 0.02° (2θ) and 2.5s by step. High resolution transmission electron microscopy (HRTEM), JEOL 2010 operating with 200kV as accelerating voltage, was used to confirm the formation of the CdSe NPs and to determine their size. The TEM samples were prepared by diluting the NP colloidal solutions with ethanol, and then a drop of the diluted solution was placed on one side of a TEM grid (Lacey Carbon or Cu–carbon coated TEM grid). SEM micrographs were obtained with a scanning electronic microscope FE-SEM JEOL7600F.
3Results and discussion3.1Mechanism of reactionsThe precursor of cadmium was dissolved in deionized water, 1mmol of CdCl2·2.5H2O in 50ml DI water was put in 100ml flask and homogenized under moderate magnetic stirring. Cadmium ions stabilized and the pH adjusted to 8.00±0.02 with Extran®, which was slowly added drop wise until completing 8.0ml:
We found that STPP into Extran® functions as a complexing agent that binds strongly to Cd2+ for the formation of the single crystal of Na3CdP3O10 and the formation of NaCl takes places through NaOH.
Selenium ions were obtained from an aqueous solution (20ml) of powdered selenium (Se, 1mmol) and sodium borohydride (NaBH4, 2mmol) was prepared using DI-water in 80ml flask. The reaction mixture was magnetically stirred and heated at (75±5)°C for 12min:
CdSe nanoparticles were synthesized by the aqueous route adding flask of cadmium into the reaction, the precursors selenium reduced. The simplified reaction is shown in Eq. (3):
The aqueous solution of CdSe was kept under moderate magnetic stirring for 30min; after this; HCl was added slowly in drops Xml (X=0.2, 0.4, 0.6, 0.8, 1, 1.2, 1.4) in 15min intervals interspersing in processes decanted for extraction of the by-products.
By mixing the solutions Cd and Se as well as the CdSe nucleus, other by-products nucleate. The possibilities of high concentration of elemental cadmium and selenium can be avoided. When HCl is added in volume to 1.0ml, a large excess of Cl− increases the precipitate's solubility. Also, other compounds are formed such as NaCl, B(OH)3, among others and H+ ions are grouped around the CdSe thus:
However, while the addition of HCl has higher concentrations (<1ml) the sample is supersaturated and nucleation is faster than the growth of crystals CdSe, thus leading to the precipitation of primary particles of selenium in excess. It is believed that some HSe− ions tend to enclose the core [CdSe:H2]+, as these have a positive charge and need negatives charges for keeping the electric neutrality, these ions are called contra-ions and they have weak bonding. When the clean process with DI-water is performed, the impurities and contra-ions are removed after a second clean process is developed, leading to peptization of the solutions; the HCl added for second time allows the growth, nucleation and precipitation of CdSe free of impurities.
Drying constitutes the last step of the synthesis and its objective is to eliminate the excess of solvent, volatile substances and recrystallize the NCs-CdSe. Structural, morphological and compositional characterizations were used for the analysis and evaluation of nanoparticles.
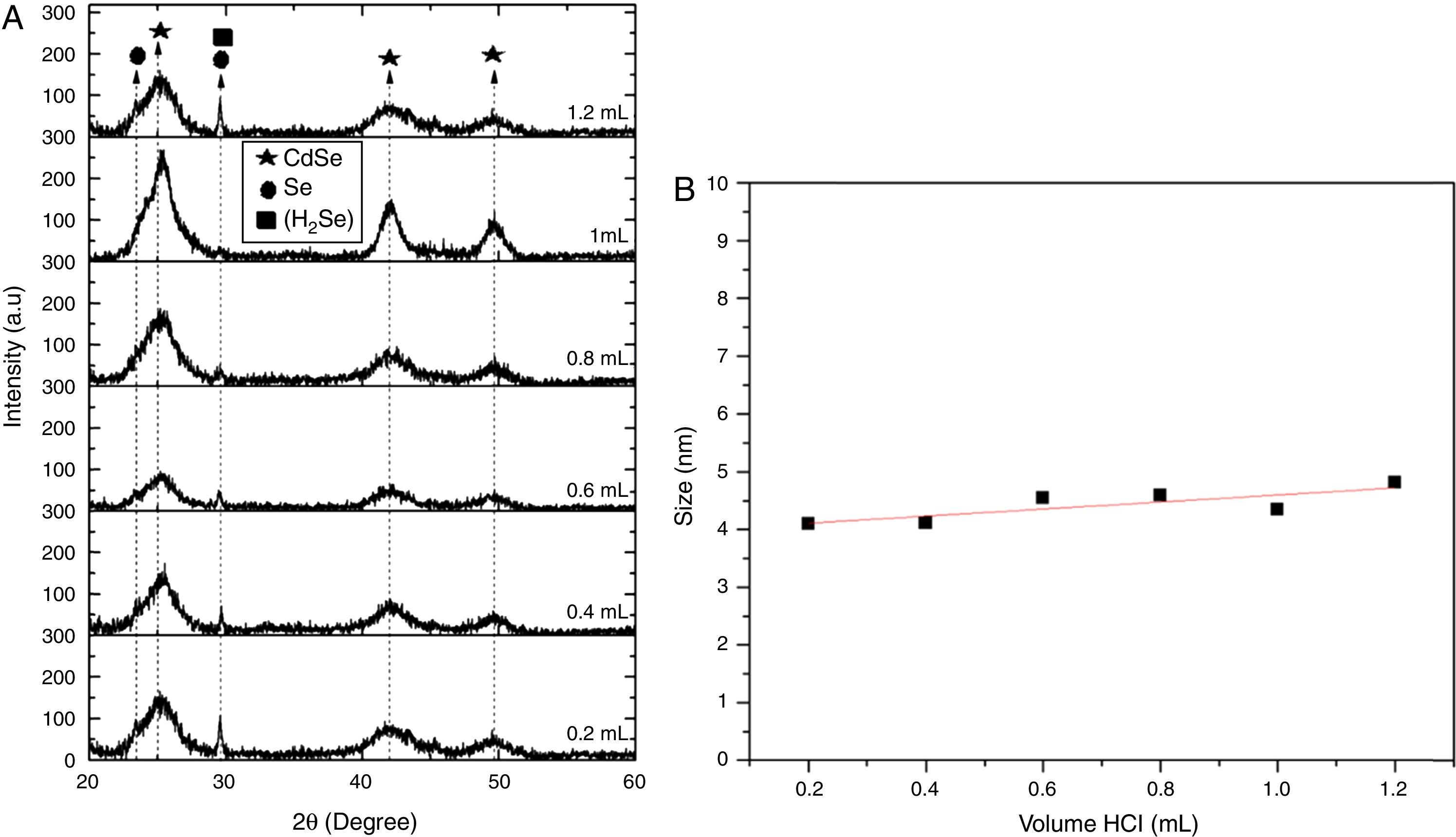
3.2Structural analysisFigure 1A shows the XRD patterns of the samples prepared with different volume ratios of HCl for cleaning the solution. The peaks in 2θ=25.35°, 42.01° and 49.69°, that exhibit well-defined orientations, are assigned to (111), (220) and (311) plains and are associated with cubic CdSe (JCPDS-ICDD No. 19-01191). Also, the (101) reflection has been detected which is assigned to hexahonal selenium (JCPDS-ICDD No. 01-0848). Figure 1A shows the change in the XRD pattern for chemical reactions carried out from 0.2 to 1.2ml of HCl concentration in the cleaning process for a 1:1 Cd:Se ratio, in a preliminary work, a study CdSe with different precursors and concentrations, using for the cleaning process a volume of HCl of 1.0ml (Arellano et al., 2014). As from the results obtained, 1:1 (Cd:Se) is considered as the best concentration to obtain CdSe NPs. In this work, a study of influence of HCl volume in the cleaning process for optimizing the process of synthesis was done. No diffraction peaks corresponding to by-products were detected, just diffraction peaks at 23.83° and 29.65° corresponding to selenium composites were found.
When a bit amount of HCl was added in the CdSe solution (Fig. 1A), by-products can be eliminated; however, these H+ and Cl− ions are not enough to remove HSe− contra-ions. Planes corresponding to selenium show well defined in X-ray diffraction pattern from 0.2ml. An increase in the amount of HCl added (between 0.4 and 0.8ml) improves the solubility and increase the hydronium H3O+ that transform the contra-ions HSe−. The strong effect of HCl can be summarized by the following reaction sequence:
Selenhydric acid, H2Se is water soluble and is visible in the X-ray diffraction pattern with the peak (220) in 29.65° for volume ranging from 0.4 to 0.8ml. Selenium as an element can be recovered as from H2Se adding more HCl (1–1.2ml). Allowing nucleation of news crystals of CdSe and the elimination of Selenium in excess.
The crystallite size L was calculated from the full width at half maximum (β) value of the diffraction peak, by using the Scherrer formula, L=Kλ/β cos θ where λ is the X-ray wavelength in nanometers, β is the FWHM value in radians, θ is the diffraction angle, and K is a constant related to the crystallite shape, the shape factor varies from 0.89 for spherical to 0.94 for cubic particles (Monshi, Foroughi, & Monshi, 2012; Onwudiwe & Strydom, 2013). In this work, we used 0.89. The estimated crystallite size ranged from between 3.8 and 4.6nm (Fig. 1B), increasing when the volume of HCl added in the cleaning process is increased.
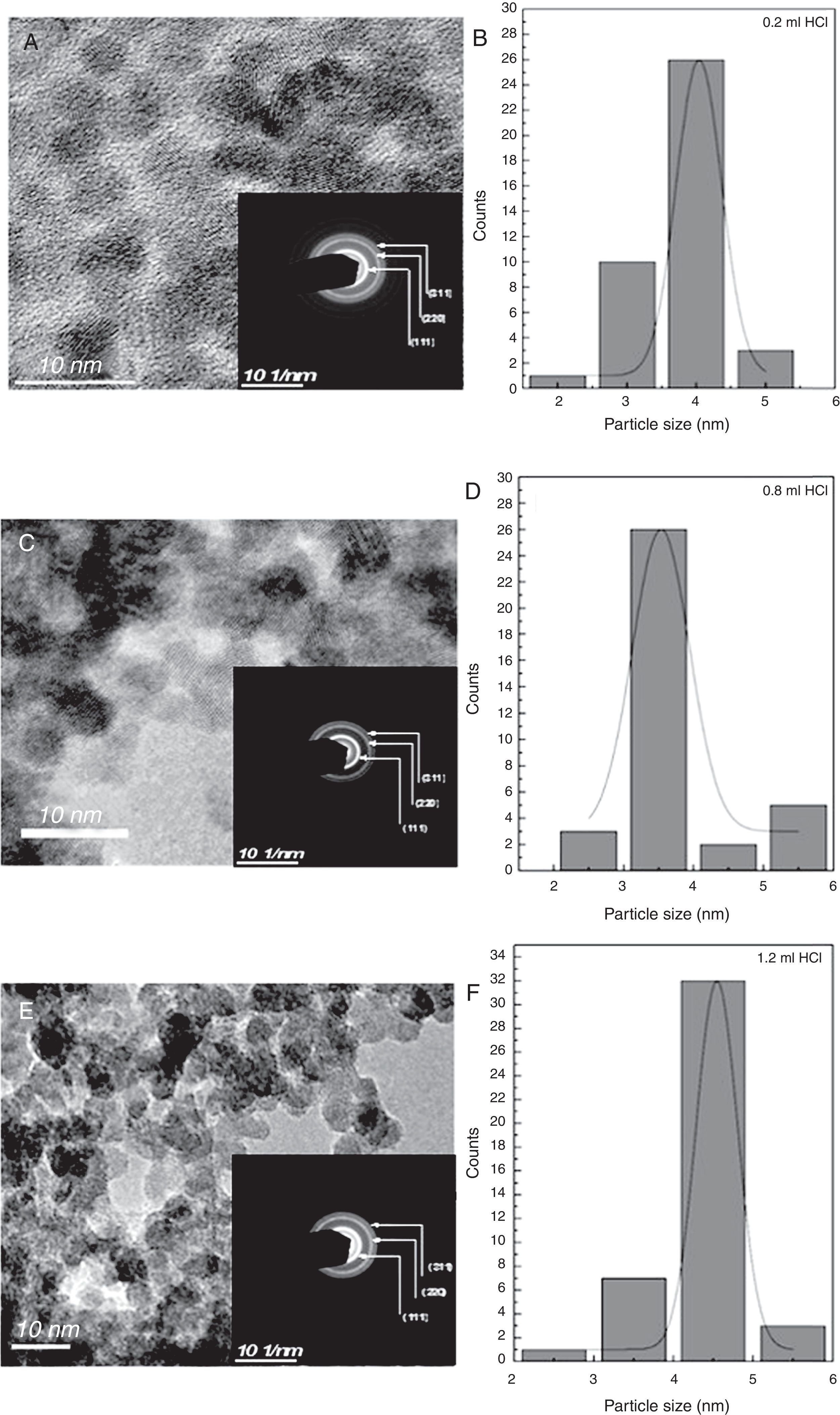
The crystalline structure can be analyzed by inspection of the high-resolution transmission electron microscope (HR-TEM), images and selected area electron diffraction (TEM-SAED) for the sample with 0.2, 0.8 and 1.0ml of HCl are shown in Figure 2A, C and E. A plot of size dispersion can be observed in Figure 2B, D and F for the Se samples. The average diameter of the nanocrystals obtained is between 3.0 and 4.5nm, as shown in the histograms. This crystallite size value obtained from the HR-TEM is consistent with the value estimated by X-ray diffraction. For calculating the size, we focused on the nanoparticle agglomerates. Electron diffraction (ED) patterns confirm that the particles have a single crystalline feature (insets in Fig. 2A, C and E).
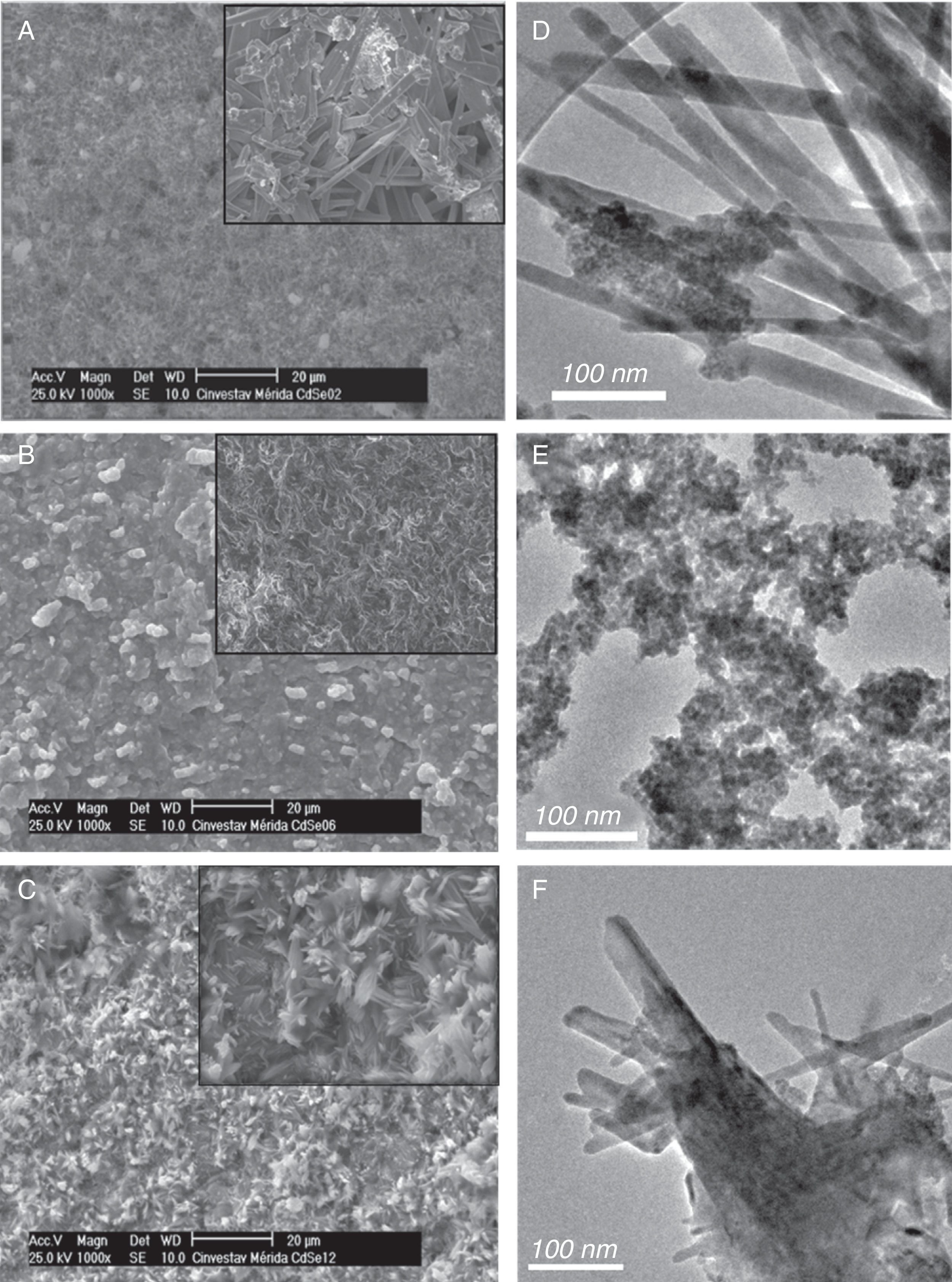
3.3Morphology analysisThe morphologies of the obtained products were observed by SEM and TEM techniques. Figure 3A–C depicts representative SEM images of the 0.2, 0.8 and 1.2ml samples treated with HCl, respectively. For 0.8ml, morphology and size are obviously different than for 0.2 and 1.2ml of HCl.
The 0.8ml sample is composed of a great number of homogeneous and smooth nanospheres which are not visible by SEM but visible by TEM. By contrast, the 0.2 and 1.2ml HCl samples are comprised of a great number of rods of “Se” mixed with CdSe sphere nanostructures. Obviously, the HCl causes the growth of Se rods, as explained in the structural study. A high-magnification SEM image is shown (inset) for each sample; in Figure 3A rods are observables; in Figure 3B, the magnification is not enough to see some kind of morphology; and in Figure 3C, rod nanostructures made of abundant nanoflakes can be clearly seen. TEM observations clearly show the presence of nanospheres of CdSe in the agglomerates for the 0.8ml sample, and rods and spheres in the agglomerates for 0.2ml and 1.2ml of HCl. This mix of wires and nanospheres, can be explained by considering that, at low and high HCl concentrations, Se ions and their compounds are less affected than the Se rich facets along the 〈220〉 axis are more highly reactive. Alternatively, due to the significant length of the Se nanowires relative to the size of the CdSe particles, each tip may act as an isolated nucleation site independent of the other tip.
In the proposed chemical reaction for the formation of CdSe nanoparticles, besides the desired product, by-products can be also obtained such as borates, sodium, phosphates, among others. Some of these ions come from the reduction of selenium borohydride (Eq. (3)). The equilibrium constant for this reaction is less than 1, indicating that the HSe− solution formation is >99% and Se2− is <5% (Klayman & Griffin, 1973). These ions are just affected to 1.0ml of HCl. Thus, in these concentrations, at which the rods do not experience growth, the nucleation will most likely occur on only the CdSe.
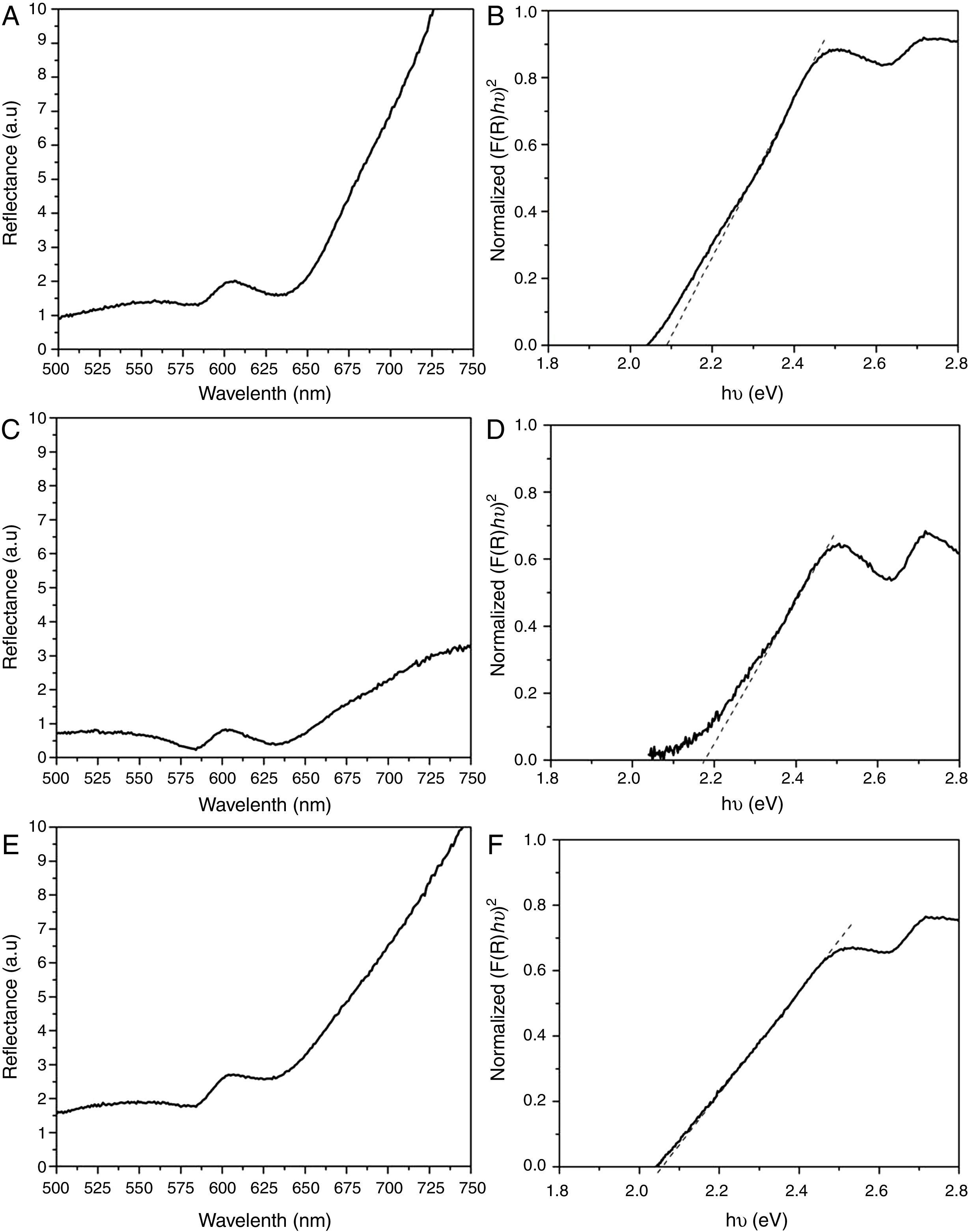
3.4Optical analysisWe have measured the diffusion reflection spectra of CdSe nanoparticles in order to understand their excitonic or interband (valence conduction band) transitions, which allow us to calculate their band gap energy. Figure 4A, C and E depicts the diffuse reflection spectra of the CdSe nanoparticles with 0.2, 0.8 and 1.0ml of HCl, respectively. An estimate of the optical band-gaps is obtained using the following equation (Murphy, 2007):
where α is the absorption coefficient. The energy intercept of a plot of (F(R)×hν)2 versus hν gives Eg for a direct allowed transition when the linear region is extrapolated to the zero ordinate (Begum, Hussain, & Rahman, 2012). Herein, F(R) is Kubelka-Munk functions. Using this method, the band gaps of the CdSe nanoparticles obtained are 2.09, 2.17 and 2.05eV, for 2.0, 0.8 and 1.2ml of HCl, respectively. Figure 4 B, D and F gives the normalized plots deriving from the calculated data of curves Figure 4A, C and E), respectively. In all cases, a band gap of more than 1.74eV is obtained, better than that of the bulk crystalline CdSe (the room temperature bulk band gaps for hexagonal CdSe and cubic CdSe are 1.73 and 1.74eV (Ninomiya & Adachi, 1995), respectively). Our calculated band gap fits previous values obtained for particle size between 3.5 and 4.5nm where the corresponding band gaps are 1.90 and 1.88eV, respectively (Mastai, Polsky, Koltypin, Gedanken, & Hodes, 1999).4ConclusionsIn summary, we prepared nanoparticles of CdSe by colloidal synthesis at room conditions by cleaning them with an HCl solution. As far as we know, hydrochloric acid is a good precipitator but it also reacts with the by-products in the solution for eliminating them. When the volume of HCl was 0.2ml, shaped-wire particles with a triangular cross-section with an average size of 200nm enveloped the NPs CdSe. While the volume of HCl increased to 0.8ml, only clusters of CdSe were obtained. By using 1.2ml of HCl in the cleaning process, the morphology consisted of stacked sheets, these results are visible by SEM and TEM. It was found that the volume of HCl used in the cleaning process of the CdSe nanoparticles plays a very important role in the morphological formation of the final products and disposal of the by-products. By using DRX, the estimated crystallite size ranged between 3.8 and 4.6nm, increasing when the volume of HCl added in the cleaning process was increased.
Conflict of interestThe authors have no conflicts of interest to declare.
Peer Review under the responsibility of Universidad Nacional Autónoma de México.